Abstract
A fluorescence method has been developed for accurate and instantaneous measurement of transepithelial diffusional water permeability (Pd) in perfused kidney tubules based on the sensitivity of the fluorophore aminonapthelane trisulfonic acid (ANTS) to solution H2O/D2O content. The fluorescence of ANTS was 3.2-fold lower in an H2O buffer than in a D2O buffer. The response of ANTS fluorescence to a change in solution H2O/D2O content occurred in less than 1 ms and was due to a collisional quenching mechanism. Isolated cortical (CCT) and outer medullary (OMCT) collecting tubules from rabbit were perfused with an isosmotic D2O buffer at specified lumen flow rates (2-100 nl/min); tubules were bathed in isosmotic H2O or D2O buffers in which vasopressin (VP) could be added rapidly. Lumen fluorescence was monitored by quantitative epifluorescence microscopy at 380 +/- 5 nm excitation and greater than 530 emission wavelengths. Pd was determined from tubule geometry, lumen flow, ANTS fluorescence, and ANTS fluorescence vs. H2O/D2O calibration relation. The instrument response time for a change in bath H2O/D2O content was less than 4 s. At 37 degrees C, Pd values (mean +/- SE in cm/s x 10(4] were 6.4 +/- 1.0 (-VP, n = 9) and 14.3 +/- 1.1 (+250 microU/ml bath VP, n = 9) in the CCT, and 5.8 +/- 1.0 (-VP, n = 6) and 15.3 +/- 2.0 (+VP, n = 6) in the OMCT; at 23 degrees C, Pd was 5.1 +/- 0.6 (-VP, n = 4) and 7.8 +/- 0.6 (+VP, n = 4) in the CCT. In response to rapid addition of 250 micro U/ml vasopressin to the bath, CCT Pd remained unchanged for 71 +/- l0s (n = 9, 37 degree C) and 170 +/- 45 s (n = 4, 23 degree C); this was followed by a slow increase in Pd(TI/2 = 91 +/- 17 s, 37 degree C; 119 +/- 31 s, 23 degree C) to the new steady-state value. These results provide a new approach for study of transepithelial water transport in kidney tubules. Compared with 3H20 methods, the fluorescence method is superior in technical simplicity, time resolution, and accuracy. The improved time resolution is important for examination of the pre-steady-state kinetics of vasopressin-induced signalling events resulting in the hydroosmotic response.
Full text
PDF

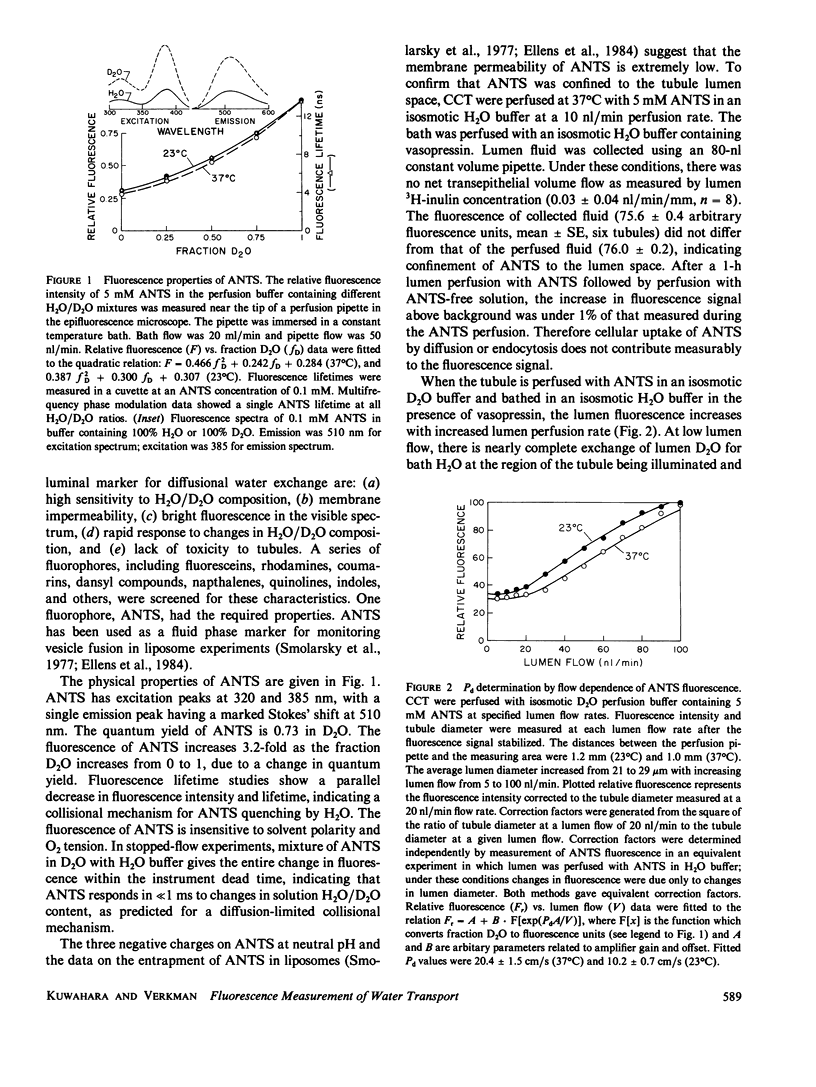
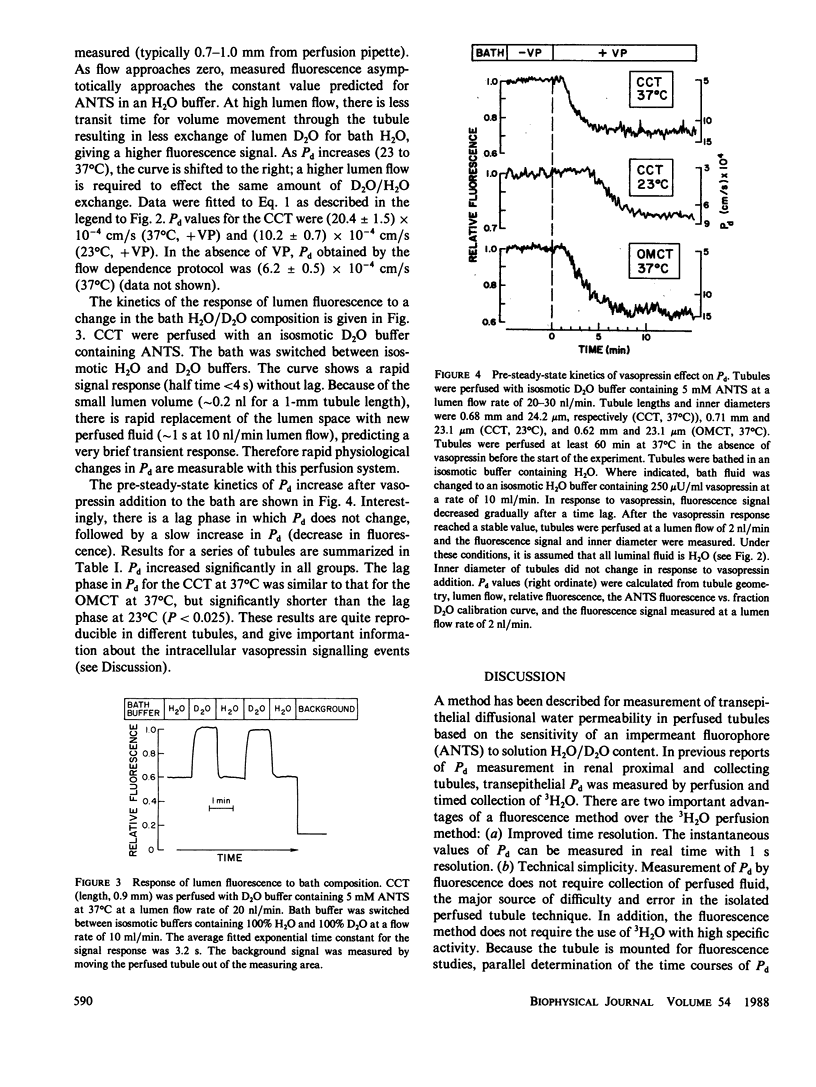



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry C. A. Characteristics of water diffusion in the rabbit proximal convoluted tubule. Am J Physiol. 1985 Nov;249(5 Pt 2):F729–F738. doi: 10.1152/ajprenal.1985.249.5.F729. [DOI] [PubMed] [Google Scholar]
- Brahm J. Diffusional water permeability of human erythrocytes and their ghosts. J Gen Physiol. 1982 May;79(5):791–819. doi: 10.1085/jgp.79.5.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D., Orci L. Vasopressin stimulates formation of coated pits in rat kidney collecting ducts. Nature. 1983 Mar 17;302(5905):253–255. doi: 10.1038/302253a0. [DOI] [PubMed] [Google Scholar]
- Burg M., Grantham J., Abramow M., Orloff J. Preparation and study of fragments of single rabbit nephrons. Am J Physiol. 1966 Jun;210(6):1293–1298. doi: 10.1152/ajplegacy.1966.210.6.1293. [DOI] [PubMed] [Google Scholar]
- Ellens H., Bentz J., Szoka F. C. pH-induced destabilization of phosphatidylethanolamine-containing liposomes: role of bilayer contact. Biochemistry. 1984 Mar 27;23(7):1532–1538. doi: 10.1021/bi00302a029. [DOI] [PubMed] [Google Scholar]
- Fabry M. E., Eisenstadt M. Water exchange between red cells and plasma. Measurement by nuclear magnetic relaxation. Biophys J. 1975 Nov;15(11):1101–1110. doi: 10.1016/S0006-3495(75)85886-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham J. J., Burg M. B. Effect of vasopressin and cyclic AMP on permeability of isolated collecting tubules. Am J Physiol. 1966 Jul;211(1):255–259. doi: 10.1152/ajplegacy.1966.211.1.255. [DOI] [PubMed] [Google Scholar]
- Hall D. A., Grantham J. J. Temperature effect on ADH response of isolated perfused rabbit collecting tubules. Am J Physiol. 1980 Dec;239(6):F595–F601. doi: 10.1152/ajprenal.1980.239.6.F595. [DOI] [PubMed] [Google Scholar]
- Hebert S. C., Andreoli T. E. Interactions of temperature and ADH on transport processes in cortical collecting tubules. Am J Physiol. 1980 Jun;238(6):F470–F480. doi: 10.1152/ajprenal.1980.238.6.F470. [DOI] [PubMed] [Google Scholar]
- Kirk K. L., Schafer J. A., DiBona D. R. Quantitative analysis of the structural events associated with antidiuretic hormone-induced volume reabsorption in the rabbit cortical collecting tubule. J Membr Biol. 1984;79(1):65–74. doi: 10.1007/BF01868527. [DOI] [PubMed] [Google Scholar]
- Kuwahara M., Berry C. A., Verkman A. S. Rapid development of vasopressin-induced hydroosmosis in kidney collecting tubules measured by a new fluorescence technique. Biophys J. 1988 Oct;54(4):595–602. doi: 10.1016/S0006-3495(88)82994-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawaczeck R. Water permeability through biological membranes by isotopic effects of fluorescence and light scattering. Biophys J. 1984 Mar;45(3):491–494. doi: 10.1016/S0006-3495(84)84184-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S. D., Jacoby M., Finkelstein A. The water permeability of toad urinary bladder. II. The value of Pf/Pd(w) for the antidiuretic hormone-induced water permeation pathway. J Gen Physiol. 1984 Apr;83(4):543–561. doi: 10.1085/jgp.83.4.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif M. C., Troutman S. L., Schafer J. A. Sustained response to vasopressin in isolated rat cortical collecting tubule. Kidney Int. 1984 Nov;26(5):725–732. doi: 10.1038/ki.1984.208. [DOI] [PubMed] [Google Scholar]
- Schafer J. A., Andreoli T. E. Cellular constraints to diffusion. The effect of antidiuretic hormone on water flows in isolated mammalian collecting tubules. J Clin Invest. 1972 May;51(5):1264–1278. doi: 10.1172/JCI106921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolarsky M., Teitelbaum D., Sela M., Gitler C. A simple fluorescent method to determine complement-mediated liposome immune lysis. J Immunol Methods. 1977;15(3):255–265. doi: 10.1016/0022-1759(77)90063-1. [DOI] [PubMed] [Google Scholar]
- Verkman A. S., Lencer W. I., Brown D., Ausiello D. A. Endosomes from kidney collecting tubule cells contain the vasopressin-sensitive water channel. Nature. 1988 May 19;333(6170):268–269. doi: 10.1038/333268a0. [DOI] [PubMed] [Google Scholar]
- Verkman A. S., Wong K. R. Proton nuclear magnetic resonance measurement of diffusional water permeability in suspended renal proximal tubules. Biophys J. 1987 May;51(5):717–723. doi: 10.1016/S0006-3495(87)83398-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade J. B., Stetson D. L., Lewis S. A. ADH action: evidence for a membrane shuttle mechanism. Ann N Y Acad Sci. 1981;372:106–117. doi: 10.1111/j.1749-6632.1981.tb15464.x. [DOI] [PubMed] [Google Scholar]


