Abstract
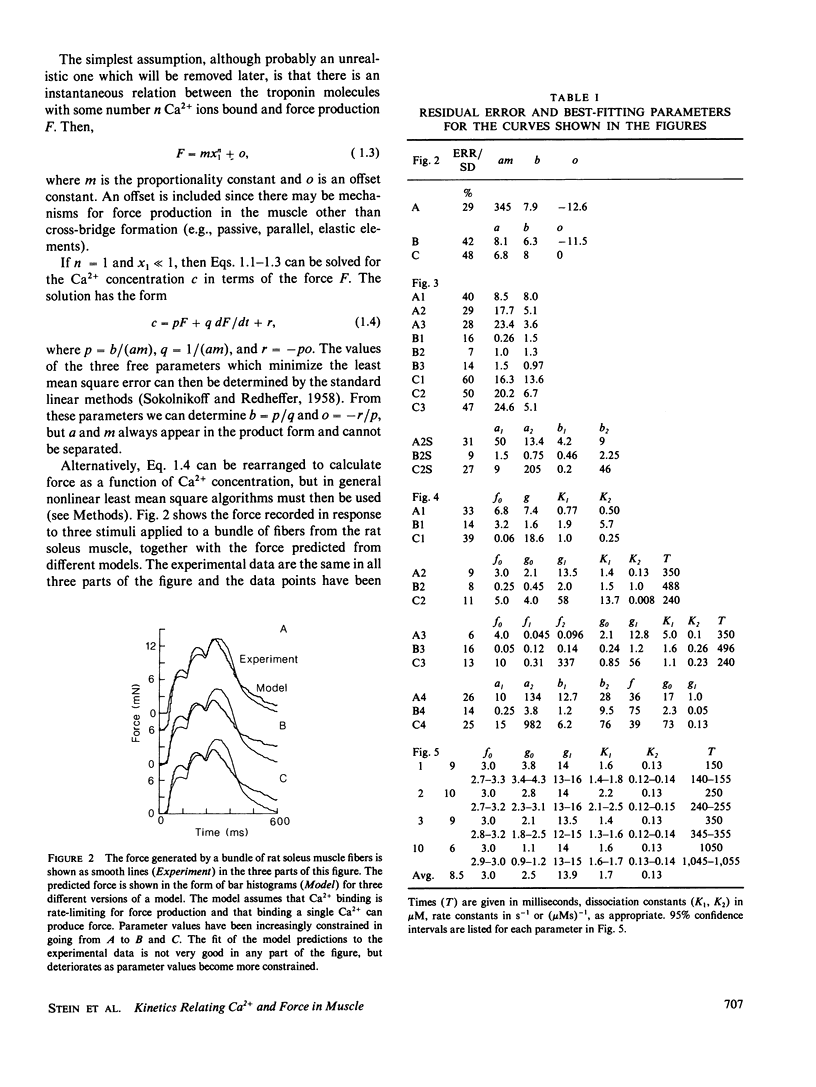
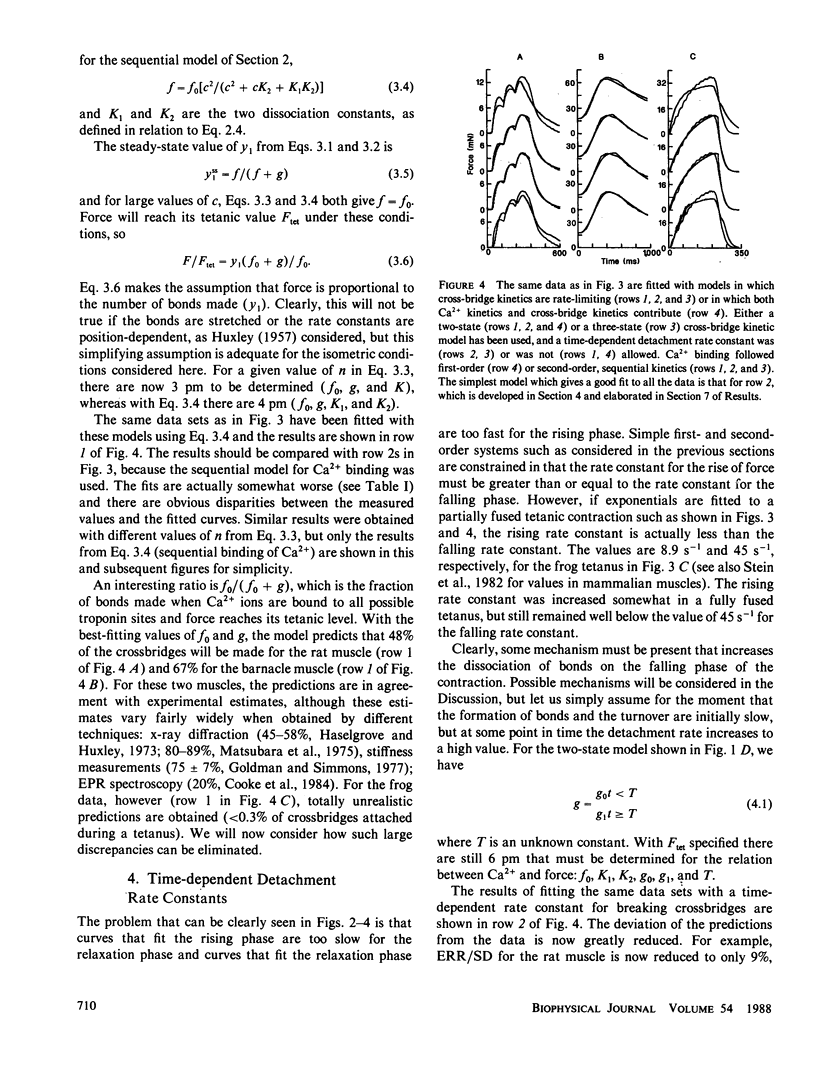
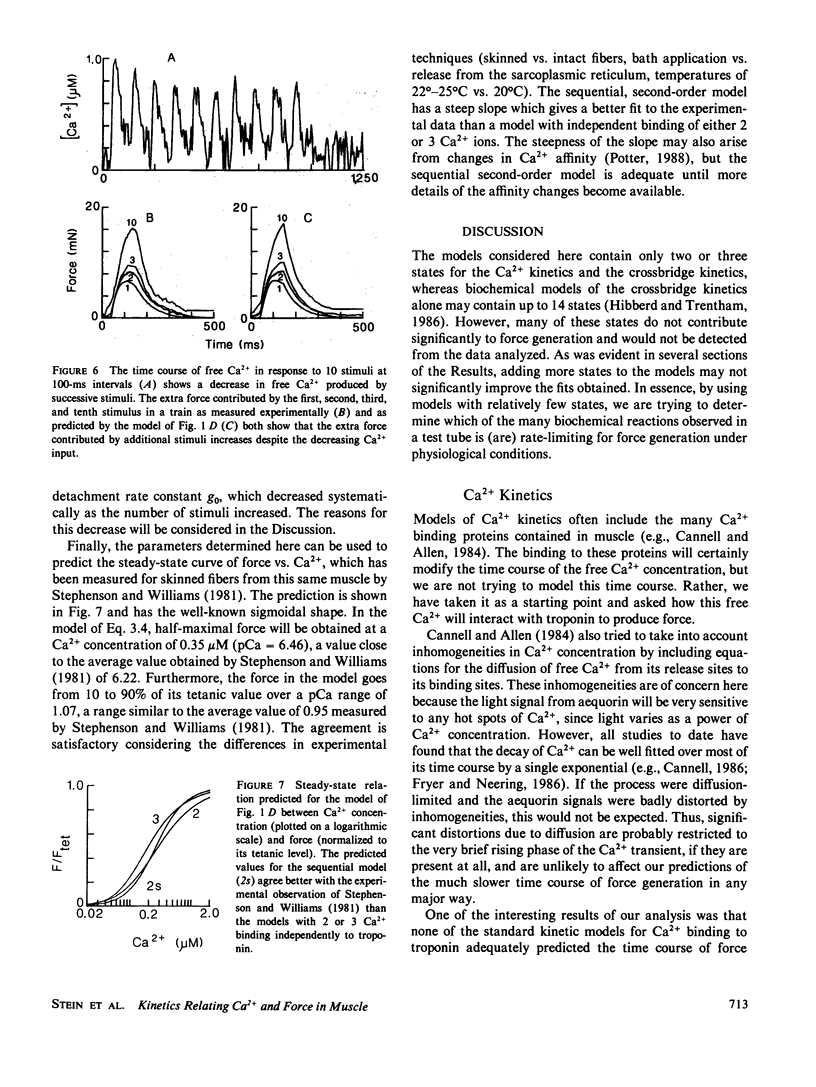
The kinetics relating Ca2+ transients and muscle force were examined using data obtained with the photoprotein aequorin in skeletal muscles of the rat, barnacle, and frog. These data were fitted by various models using nonlinear methods for minimizing the least mean square errors. Models in which Ca2+ binding to troponin was rate limiting for force production did not produce good agreement with the observed data, except for a small twitch of the barnacle muscle. Models in which cross-bridge kinetics were rate limiting also did not produce good agreement with the observed data, unless the detachment rate constant was allowed to increase sharply on the falling phase of tension production. Increasing the number of cross-bridge states did not dramatically improve the agreement between predicted and observed force. We conclude that the dynamic relationship between Ca2+ transients and force production in intact muscle fibers under physiological conditions can be approximated by a model in which (a) two Ca2+ ions bind rapidly to each troponin molecule, (b) force production is limited by the rate of formation of tightly bound cross-bridges, and (c) the rate of cross-bridge detachment increases rapidly once tension begins to decline and free Ca2+ levels have fallen to low values after the last stimulus. Such a model can account not only for the pattern of force production during a twitch and tetanus, but also the complex, nonlinear pattern of summation which is observed during an unfused tetanus at intermediate rates of stimulation.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley C. C. Calcium ion regulation in barnacle muscle fibers and its relation to force development. Ann N Y Acad Sci. 1978 Apr 28;307:308–329. doi: 10.1111/j.1749-6632.1978.tb41959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley C. C., Moisescu D. G. Model for the action of calcium in muscle. Nat New Biol. 1972 Jun 14;237(76):208–211. doi: 10.1038/newbio237208a0. [DOI] [PubMed] [Google Scholar]
- Blinks J. R., Rüdel R., Taylor S. R. Calcium transients in isolated amphibian skeletal muscle fibres: detection with aequorin. J Physiol. 1978 Apr;277:291–323. doi: 10.1113/jphysiol.1978.sp012273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinks J. R., Wier W. G., Hess P., Prendergast F. G. Measurement of Ca2+ concentrations in living cells. Prog Biophys Mol Biol. 1982;40(1-2):1–114. doi: 10.1016/0079-6107(82)90011-6. [DOI] [PubMed] [Google Scholar]
- Bremel R. D., Weber A. Cooperation within actin filament in vertebrate skeletal muscle. Nat New Biol. 1972 Jul 26;238(82):97–101. doi: 10.1038/newbio238097a0. [DOI] [PubMed] [Google Scholar]
- Brenner B., Eisenberg E. The mechanism of muscle contraction. Biochemical, mechanical, and structural approaches to elucidate cross-bridge action in muscle. Basic Res Cardiol. 1987;82 (Suppl 2):3–16. doi: 10.1007/978-3-662-11289-2_1. [DOI] [PubMed] [Google Scholar]
- Calancie B., Stein R. B. Measurement of rate constants for the contractile cycle of intact mammalian muscle fibers. Biophys J. 1987 Feb;51(2):149–159. doi: 10.1016/S0006-3495(87)83320-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell M. B., Allen D. G. Model of calcium movements during activation in the sarcomere of frog skeletal muscle. Biophys J. 1984 May;45(5):913–925. doi: 10.1016/S0006-3495(84)84238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell M. B. Effect of tetanus duration on the free calcium during the relaxation of frog skeletal muscle fibres. J Physiol. 1986 Jul;376:203–218. doi: 10.1113/jphysiol.1986.sp016149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchi G., Colomo F., Lombardi V. Force-velocity relation in deuterium oxide-treated frog single muscle fibres during the rise of tension in an isometric tetanus. J Physiol. 1981 Aug;317:207–221. doi: 10.1113/jphysiol.1981.sp013821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleworth D. R., Edman K. A. Changes in sarcomere length during isometric tension development in frog skeletal muscle. J Physiol. 1972 Dec;227(1):1–17. doi: 10.1113/jphysiol.1972.sp010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson M. J., Gadian D. G., Wilkie D. R. Mechanical relaxation rate and metabolism studied in fatiguing muscle by phosphorus nuclear magnetic resonance. J Physiol. 1980 Feb;299:465–484. doi: 10.1113/jphysiol.1980.sp013137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchateau J., Hainaut K. Nonlinear summation of contractions in striated muscle. II. Potentiation of intracellular Ca2+ movements in single barnacle muscle fibres. J Muscle Res Cell Motil. 1986 Feb;7(1):18–24. doi: 10.1007/BF01756198. [DOI] [PubMed] [Google Scholar]
- Eisenberg E., Hill T. L. Muscle contraction and free energy transduction in biological systems. Science. 1985 Mar 1;227(4690):999–1006. doi: 10.1126/science.3156404. [DOI] [PubMed] [Google Scholar]
- Eusebi F., Miledi R., Takahashi T. Calcium transients in mammalian muscles. Nature. 1980 Apr 10;284(5756):560–561. doi: 10.1038/284560a0. [DOI] [PubMed] [Google Scholar]
- Fryer M. W., Neering I. R. Relationship between intracellular calcium concentration and relaxation of rat fast and slow muscles. Neurosci Lett. 1986 Feb 28;64(2):231–235. doi: 10.1016/0304-3940(86)90106-0. [DOI] [PubMed] [Google Scholar]
- Gillis J. M., Thomason D., Lefèvre J., Kretsinger R. H. Parvalbumins and muscle relaxation: a computer simulation study. J Muscle Res Cell Motil. 1982 Dec;3(4):377–398. doi: 10.1007/BF00712090. [DOI] [PubMed] [Google Scholar]
- Goldman Y. E., Hibberd M. G., Trentham D. R. Initiation of active contraction by photogeneration of adenosine-5'-triphosphate in rabbit psoas muscle fibres. J Physiol. 1984 Sep;354:605–624. doi: 10.1113/jphysiol.1984.sp015395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- Haselgrove J. C., Huxley H. E. X-ray evidence for radial cross-bridge movement and for the sliding filament model in actively contracting skeletal muscle. J Mol Biol. 1973 Jul 15;77(4):549–568. doi: 10.1016/0022-2836(73)90222-2. [DOI] [PubMed] [Google Scholar]
- Hibberd M. G., Trentham D. R. Relationships between chemical and mechanical events during muscular contraction. Annu Rev Biophys Biophys Chem. 1986;15:119–161. doi: 10.1146/annurev.bb.15.060186.001003. [DOI] [PubMed] [Google Scholar]
- Hill D. K. Tension due to interaction between the sliding filaments in resting striated muscle. The effect of stimulation. J Physiol. 1968 Dec;199(3):637–684. doi: 10.1113/jphysiol.1968.sp008672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. L., Eisenberg E., Chalovich J. M. Theoretical models for cooperative steady-state ATPase activity of myosin subfragment-1 on regulated actin. Biophys J. 1981 Jul;35(1):99–112. doi: 10.1016/S0006-3495(81)84777-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. L., Eisenberg E., Greene L. Theoretical model for the cooperative equilibrium binding of myosin subfragment 1 to the actin-troponin-tropomyosin complex. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3186–3190. doi: 10.1073/pnas.77.6.3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971 Oct 22;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- JEWELL B. R., WILKIE D. R. An analysis of the mechanical components in frog's striated muscle. J Physiol. 1958 Oct 31;143(3):515–540. doi: 10.1113/jphysiol.1958.sp006075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M., Brandt P. W. Sinusoidal analysis: a high resolution method for correlating biochemical reactions with physiological processes in activated skeletal muscles of rabbit, frog and crayfish. J Muscle Res Cell Motil. 1980 Sep;1(3):279–303. doi: 10.1007/BF00711932. [DOI] [PubMed] [Google Scholar]
- Kress M., Huxley H. E., Faruqi A. R., Hendrix J. Structural changes during activation of frog muscle studied by time-resolved X-ray diffraction. J Mol Biol. 1986 Apr 5;188(3):325–342. doi: 10.1016/0022-2836(86)90158-0. [DOI] [PubMed] [Google Scholar]
- Mannard A., Stein R. B. Determination of the frequency response of isometric soleus muscle in the cat using random nerve stimulation. J Physiol. 1973 Mar;229(2):275–296. doi: 10.1113/jphysiol.1973.sp010138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara I., Yagi N., Hashizume H. Use of an X-ray television for diffraction of the frog striated muscle. Nature. 1975 Jun 26;255(5511):728–729. doi: 10.1038/255728a0. [DOI] [PubMed] [Google Scholar]
- Melzer W., Ríos E., Schneider M. F. The removal of myoplasmic free calcium following calcium release in frog skeletal muscle. J Physiol. 1986 Mar;372:261–292. doi: 10.1113/jphysiol.1986.sp016008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein R. B., Gordon T. Nonlinear stiffness--force relationships in whole mammalian skeletal muscles. Can J Physiol Pharmacol. 1986 Sep;64(9):1236–1244. doi: 10.1139/y86-209. [DOI] [PubMed] [Google Scholar]
- Stein R. B., Gordon T., Shriver J. Temperature dependence of mammalian muscle contractions and ATPase activities. Biophys J. 1982 Nov;40(2):97–107. doi: 10.1016/S0006-3495(82)84464-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein R. B., Parmiggiani F. Nonlinear summation of contractions in cat muscles. I. Early depression. J Gen Physiol. 1981 Sep;78(3):277–293. doi: 10.1085/jgp.78.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson D. G., Williams D. A. Calcium-activated force responses in fast- and slow-twitch skinned muscle fibres of the rat at different temperatures. J Physiol. 1981 Aug;317:281–302. doi: 10.1113/jphysiol.1981.sp013825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zot A. S., Potter J. D. Structural aspects of troponin-tropomyosin regulation of skeletal muscle contraction. Annu Rev Biophys Biophys Chem. 1987;16:535–559. doi: 10.1146/annurev.bb.16.060187.002535. [DOI] [PubMed] [Google Scholar]
- el-Saleh S. C., Warber K. D., Potter J. D. The role of tropomyosin-troponin in the regulation of skeletal muscle contraction. J Muscle Res Cell Motil. 1986 Oct;7(5):387–404. doi: 10.1007/BF01753582. [DOI] [PubMed] [Google Scholar]



