Abstract
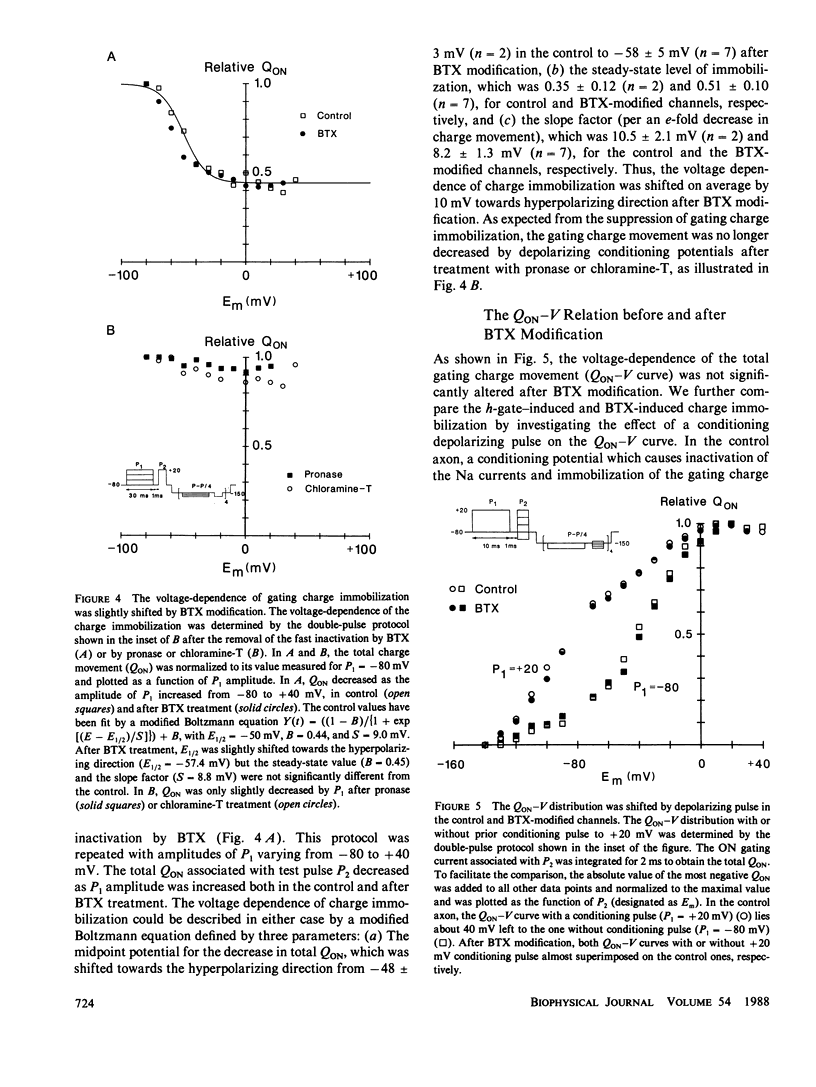
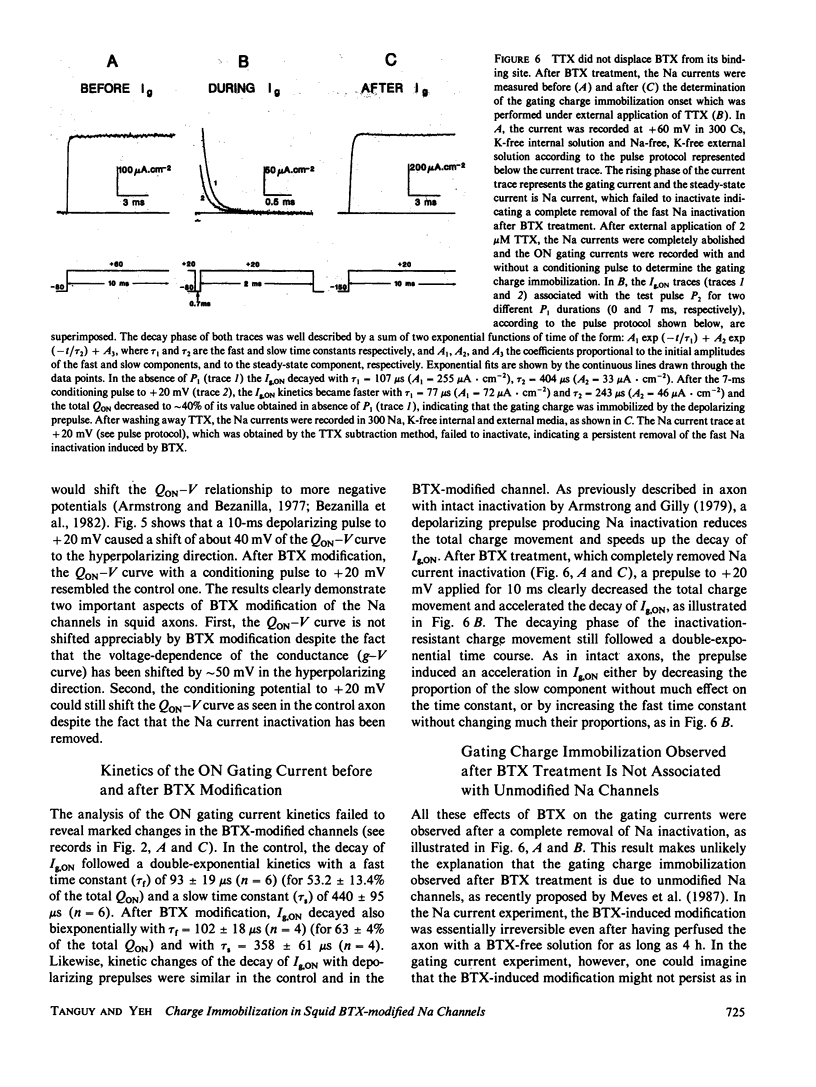
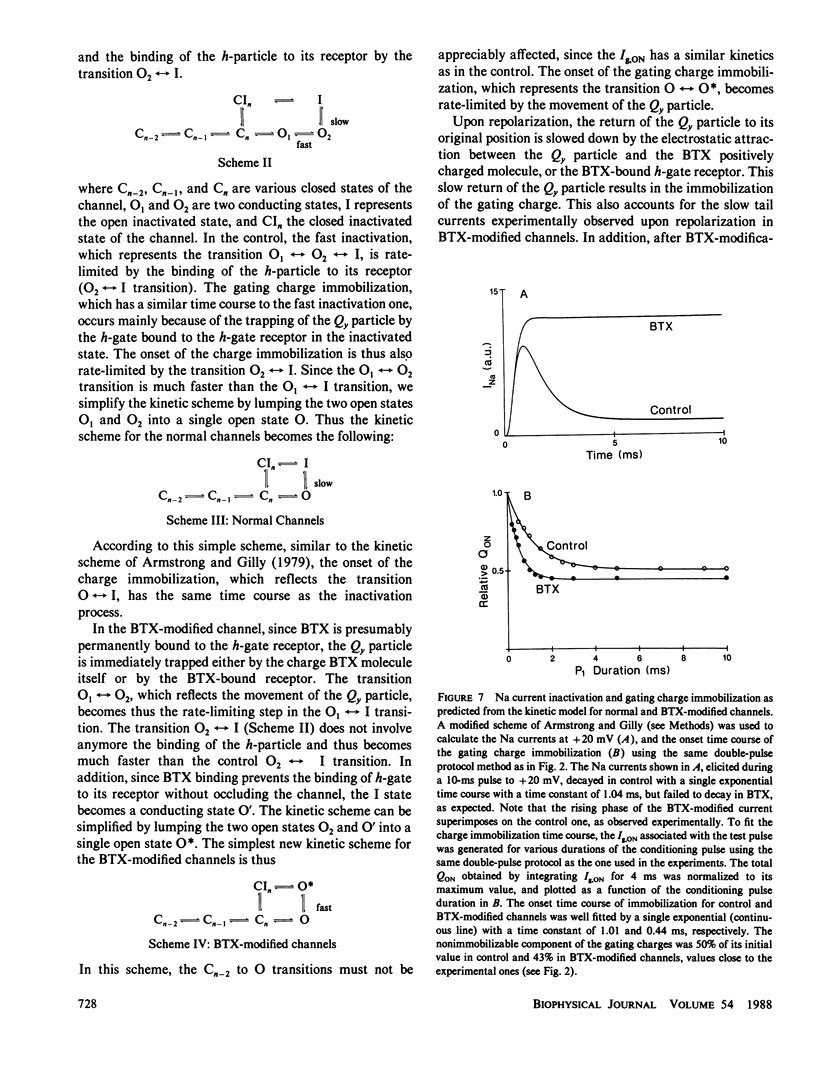
The fast inactivation of sodium currents and the immobolization of sodium gating charge are thought to be closely coupled to each other. This notion was tested in the squid axon in which kinetics and steady-state properties of the gating charge movement were compared before and after removal of the Na inactivation by batrachotoxin (BTX), pronase, or chloramine-T. The immobilization of gating charge was determined by measuring the total charge movement (QON) obtained by integrating the ON gating current (Ig,ON) using a double pulse protocol. After removal of the fast inactivation with pronase or chloramine-T, the gating charge movement was no longer immobilized. In contrast, after BTX modification, the channels still exhibited an immobilization of the gating charge (QON) with an onset time course and voltage dependence similar to that for the activation process. These results show that BTX can uncouple the charge immobilization from the fast Na inactivation mechanism, suggesting that the Na gating charge movement can be immobilized independently of the inactivation of the channel.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong C. M., Bezanilla F. Charge movement associated with the opening and closing of the activation gates of the Na channels. J Gen Physiol. 1974 May;63(5):533–552. doi: 10.1085/jgp.63.5.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M., Bezanilla F. Inactivation of the sodium channel. II. Gating current experiments. J Gen Physiol. 1977 Nov;70(5):567–590. doi: 10.1085/jgp.70.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M., Bezanilla F., Rojas E. Destruction of sodium conductance inactivation in squid axons perfused with pronase. J Gen Physiol. 1973 Oct;62(4):375–391. doi: 10.1085/jgp.62.4.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M., Croop R. S. Simulation of Na channel inactivation by thiazine dyes. J Gen Physiol. 1982 Nov;80(5):641–662. doi: 10.1085/jgp.80.5.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAKER P. F., HODGKIN A. L., SHAW T. I. Replacement of the protoplasm of a giant nerve fibre with artificial solutions. Nature. 1961 Jun 3;190:885–887. doi: 10.1038/190885a0. [DOI] [PubMed] [Google Scholar]
- Bezanilla F., Armstrong C. M. Gating currents of the sodium channels: three ways to block them. Science. 1974 Feb 22;183(4126):753–754. doi: 10.1126/science.183.4126.753. [DOI] [PubMed] [Google Scholar]
- Bezanilla F., Taylor R. E., Fernández J. M. Distribution and kinetics of membrane dielectric polarization. 1. Long-term inactivation of gating currents. J Gen Physiol. 1982 Jan;79(1):21–40. doi: 10.1085/jgp.79.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan M. D., Almers W. Interactions between quaternary lidocaine, the sodium channel gates, and tetrodotoxin. Biophys J. 1979 Jul;27(1):39–55. doi: 10.1016/S0006-3495(79)85201-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drews G. Effects of chloramine-T on charge movement and fraction of open channels in frog nodes of Ranvier. Pflugers Arch. 1987 Jul;409(3):251–257. doi: 10.1007/BF00583473. [DOI] [PubMed] [Google Scholar]
- Dubois J. M., Schneider M. F., Khodorov B. I. Voltage dependence of intramembrane charge movement and conductance activation of batrachotoxin-modified sodium channels in frog node of Ranvier. J Gen Physiol. 1983 Jun;81(6):829–844. doi: 10.1085/jgp.81.6.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greeff N. G., Keynes R. D., Van Helden D. F. Fractionation of the asymmetry current in the squid giant axon into inactivating and non-inactivating components. Proc R Soc Lond B Biol Sci. 1982 Jun 22;215(1200):375–389. doi: 10.1098/rspb.1982.0048. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J. M., Tanguy J., Yeh J. Z. Removal of sodium inactivation and block of sodium channels by chloramine-T in crayfish and squid giant axons. Biophys J. 1987 Aug;52(2):155–163. doi: 10.1016/S0006-3495(87)83203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodorov B. I. Batrachotoxin as a tool to study voltage-sensitive sodium channels of excitable membranes. Prog Biophys Mol Biol. 1985;45(2):57–148. doi: 10.1016/0079-6107(85)90005-7. [DOI] [PubMed] [Google Scholar]
- Meeder T., Ulbricht W. Action of benzocaine on sodium channels of frog nodes of Ranvier treated with chloramine-T. Pflugers Arch. 1987 Jul;409(3):265–273. doi: 10.1007/BF00583475. [DOI] [PubMed] [Google Scholar]
- Meves H., Rubly N., Watt D. D. Gating current experiments on frog nodes of Ranvier treated with Centruroides sculpturatus toxins or aconitine. Pflugers Arch. 1987 Aug;409(4-5):381–393. doi: 10.1007/BF00583792. [DOI] [PubMed] [Google Scholar]
- Meves H., Vogel W. Inactivation of the asymmetrical displacement current in giant axons of Loligo forbesi. J Physiol. 1977 May;267(2):377–393. doi: 10.1113/jphysiol.1977.sp011818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumcke B., Schwarz J. R., Stämpfli R. A comparison of sodium currents in rat and frog myelinated nerve: normal and modified sodium inactivation. J Physiol. 1987 Jan;382:175–191. doi: 10.1113/jphysiol.1987.sp016362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonner W. Relations between the inactivation of sodium channels and the immobilization of gating charge in frog myelinated nerve. J Physiol. 1980 Feb;299:573–603. doi: 10.1113/jphysiol.1980.sp013143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonner W., Rojas E., Stämpfli H. Displacement currents in the node of Ranvier. Voltage and time dependence. Pflugers Arch. 1975;354(1):1–18. doi: 10.1007/BF00584499. [DOI] [PubMed] [Google Scholar]
- Oakley B., 2nd, Flaming D. G., Brown K. T. Effects of the rod receptor potential upon retinal extracellular potassium concentration. J Gen Physiol. 1979 Dec;74(6):713–737. doi: 10.1085/jgp.74.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxford G. S. Some kinetic and steady-state properties of sodium channels after removal of inactivation. J Gen Physiol. 1981 Jan;77(1):1–22. doi: 10.1085/jgp.77.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxford G. S., Wu C. H., Narahashi T. Removal of sodium channel inactivation in squid giant axons by n-bromoacetamide. J Gen Physiol. 1978 Mar;71(3):227–247. doi: 10.1085/jgp.71.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rack M., Rubly N., Waschow C. Effects of some chemical reagents on sodium current inactivation in myelinated nerve fibers of the frog. Biophys J. 1986 Oct;50(4):557–564. doi: 10.1016/S0006-3495(86)83495-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidtmayer J. Behaviour of chemically modified sodium channels in frog nerve supports a three-state model of inactivation. Pflugers Arch. 1985 May;404(1):21–28. doi: 10.1007/BF00581486. [DOI] [PubMed] [Google Scholar]
- Stimers J. R., Bezanilla F., Taylor R. E. Sodium channel activation in the squid giant axon. Steady state properties. J Gen Physiol. 1985 Jan;85(1):65–82. doi: 10.1085/jgp.85.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G. K., Brodwick M. S., Eaton D. C. Removal of sodium channel inactivation in squid axon by the oxidant chloramine-T. J Gen Physiol. 1985 Aug;86(2):289–302. doi: 10.1085/jgp.86.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G. K. Irreversible modification of sodium channel inactivation in toad myelinated nerve fibres by the oxidant chloramine-T. J Physiol. 1984 Jan;346:127–141. doi: 10.1113/jphysiol.1984.sp015011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G. K. Modification of sodium channel inactivation in single myelinated nerve fibers by methionine-reactive chemicals. Biophys J. 1984 Jul;46(1):121–124. doi: 10.1016/S0006-3495(84)84005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh J. Z., Armstrong C. M. Immobilisation of gating charge by a substance that simulates inactivation. Nature. 1978 Jun 1;273(5661):387–389. doi: 10.1038/273387a0. [DOI] [PubMed] [Google Scholar]
- Yeh J. Z., Narahashi T. Kinetic analysis of pancuronium interaction with sodium channels in squid axon membranes. J Gen Physiol. 1977 Mar;69(3):293–323. doi: 10.1085/jgp.69.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]


