Abstract
Previous studies have indicated that the ability to bind to fibronectin is a key feature in successful cell invasion by Campylobacter jejuni. Given the spatial distribution of fibronectin and the architecture of the epithelium, this suggests the possibility that C. jejuni cell invasion might preferentially occur at the basolateral cell surface. To test this hypothesis, we examined the interaction of C. jejuni with T84 human colonic cells. When grown under the appropriate conditions, T84 cells form a polarized cell monolayer. C. jejuni translocation of a T84 cell monolayer appeared to occur via a paracellular (extracellular) route as opposed to a transcellular (intracellular) route based on the finding that a C. jejuni noninvasive mutant translocated as efficiently as its isogenic parent. Additional studies revealed that two distinct C. jejuni wild-type isolates could compete with one another for host cell receptors, whereas a C. jejuni fibronectin-binding-deficient mutant could not compete with a wild-type isolate for host cell receptors. Further, C. jejuni adherence and internalization were significantly inhibited by antifibronectin antibodies but only when cells were first treated with EGTA to expose basolateral cell surfaces. Together, these results support the theory that C. jejuni invasion occurs preferentially at the basolateral surface of eukaryotic cells.
Campylobacter jejuni is one of the leading causes of human gastrointestinal disease in the United States (1, 2). The ability of C. jejuni to cause disease is dependent upon multiple factors including motility (6, 29, 44), chemotaxis (38, 45), host cell translocation (7, 11, 14, 24), host cell adherence (17, 20, 32), host cell invasion (11, 18, 23, 35), and toxin production (33, 43). Of particular significance to the present study is whether C. jejuni translocation, or migration across the intestinal epithelium, is an important virulence attribute, since the pathology of Campylobacter-mediated enteritis is generally confined to the intestinal epithelium.
Cell adherence by C. jejuni is multifactorial, with a number of adhesins identified. The best-characterized C. jejuni adhesins to date include CadF, JlpA, and PEB1 (17, 20, 32). With one exception, the targets of these binding proteins remain unknown. The target of the CadF adhesin is fibronectin (Fn), a component of the extracellular matrix (20). Fn appears to be a common host cell target, as numerous pathogens, including C. jejuni (20, 27), Staphylococcus aureus (26, 36), Streptococcus pyogenes (16, 30), Salmonella enterica serovar Enteritidis (5), Escherichia coli (13, 42), Neisseria gonorrhoeae (41), Mycobacterium avium (37), and Treponema species (9, 10, 40), possess Fn binding ability. To date, the in vitro studies performed to determine the role of CadF, and all other C. jejuni adhesins, have been limited to the use of nonpolarized cells. Unfortunately, the architecture of cells grown on a plastic substrate differs substantially from that of cells in vivo, where Fn is localized to the basolateral cell surface.
While the intestinal epithelium provides a primary defense against invading organisms, several pathogenic bacteria possess the ability to translocate an epithelial or endothelial cell barrier (12, 25). Such translocation is an important virulence attribute, as it allows the invader access to underlying tissues and may permit the organism to disseminate throughout the host. The Caco-2, HT29, and T84 human colonic cell lines posses the ability to form polarized cell monolayers when grown under appropriate conditions, thereby affording a model to assess the ability of bacteria to translocate across an intact epithelial cell barrier (8). Polarized cells are characterized by defined apical and basolateral cell surfaces separated by tight junctions, which limit the passage of solutes through the paracellular spaces (28). Transepithelial electrical resistance (TER) is frequently used as an index of tight junction permeability and monolayer integrity. Disruption of the intercellular tight junctions results in a decrease in TER. Previous work has revealed that C. jejuni can translocate a Caco-2 polarized cell monolayer without a concomitant loss in TER (11, 14, 24), indicating that C. jejuni can translocate across a cell monolayer whose integrity remains intact. A consensus is yet to be reached among investigators as to the mechanism of translocation. More specifically, whether C. jejuni translocates via a paracellular route (migration from the apical to the basolateral cell surface by passage between cells) or a transcellular route (migration from the apical to the basolateral cell surface by host cell uptake, followed by intracellular trafficking) remains debatable.
This study was initiated to further examine the binding, internalization, and translocation properties of C. jejuni by using a polarized cell model system. We specifically chose T84 cells for their phenotypic similarity to colonic crypt cells (31). Histological examination of C. jejuni-infected humans and animals has revealed pathology primarily in the colon (3, 6, 35). We were thus able to characterize the role of the CadF outer membrane protein in promoting the interactions of C. jejuni with cells whose architecture models that of the organism's in vivo target.
MATERIALS AND METHODS
Bacterial isolates and growth conditions.
C. jejuni F38011, 81-176 (Tetr) (4), and the F38011 isogenic cadF mutant (Kanr), ciaB mutant (Kanr), and ciaB transformant harboring the pMEK100 shuttle plasmid (Tetr and Kanr) were cultured on Mueller-Hinton (MH) agar plates supplemented with antibiotics (12.5 μg of tetracycline/ml and 200 μg of kanamycin/ml) under microaerophilic conditions at 37°C. The pMEK100 shuttle plasmid contains a 2,248-bp fragment of DNA harboring the entire ciaB gene from C. jejuni F38011 (34). A C. jejuni F38011 (Str/Nalr) isolate was also cultured on MH agar plates supplemented with 200 μg of streptomycin/ml and 50 μg of nalidixic acid/ml. All isolates were subcultured every 24 to 48 h. E. coli MRF and Salmonella enterica serovar Typhimurium SL1344 were cultured in Luria-Bertani (LB) broth (10 g of Bacto Tryptone, 5 g of yeast extract, and 10 g of sodium chloride per liter) and on LB agar plates (LB broth with 15 g of Bacto Agar per liter) in a 37°C incubator.
Tissue culture.
Stock cultures of T84 cells (human colonic cell line, ATCC CCL248) were grown in Eagle's minimal essential medium (EMEM) supplemented with 10% (vol/vol) fetal bovine serum (FBS; HyClone Laboratories Inc., Logan, Utah) and maintained at 37°C in a humidified, 5% CO2 incubator.
T84 nonpolarized adherence and internalization assays.
Adherence and internalization assays were performed with T84 cells in 24-well tissue culture trays (Costar, Cambridge, Mass.) as outlined elsewhere (19). To determine the number of adherent bacteria, T84 cell monolayer cultures were inoculated with 5 × 107 CFU of a bacterial suspension in prewarmed EMEM plus 1% FBS (EMEM-1% FBS), centrifuged at 600 × g for 5 min to promote bacterium-host cell contact, and incubated for 30 min in a humidified, 5% CO2 incubator at 37°C. Monolayers were rinsed three times with phosphate-buffered saline (PBS), epithelial cells were lysed with a solution of 0.25% (wt/vol) sodium deoxycholate, and serial dilutions of the lysates were plated on MH-blood plates. The number of adherent bacteria was determined by counting the resulting colonies. Internalization assays were performed similarly. Infected cell monolayers were incubated for 3 h, rinsed three times with EMEM-1% FBS, and incubated for an additional 3 h with EMEM-1% FBS and a bactericidal concentration of gentamicin (Invitrogen, Carlsbad, Calif.). After incubation, cell monolayers were rinsed three times with PBS, T84 cells were lysed with 0.25% (wt/vol) sodium deoxycholate, and dilutions of the lysates were plated on MH-blood agar plates. The number of internalized bacteria was determined by counting the resultant colonies. Where indicated, a final concentration of 10 μM EGTA (Sigma, St. Louis, Mo.) was added to the cells 15 min prior to inoculation with C. jejuni to induce cellular retraction. EGTA was removed prior to inoculation of T84 cells with C. jejuni. T84 cell viability, following EGTA treatment, was assessed by rinsing the T84 cells twice with PBS and staining the cells for 5 min with 0.5% trypan blue. The cells were then rinsed twice with PBS, counterstained for 5 min with 0.5 ml of 0.5% phenol red, and visualized with an inverted microscope to assess viability (15). Anti-human Fn antibodies (Telios Pharmaceuticals, Inc., San Diego, Calif.) were used at a 1:50 dilution, and assays were conducted in EMEM without FBS. For assays in which serovar Typhimurium was used, the bacteria were preincubated anaerobically at 37°C for 3 h prior to infection. Results are presented as the means ± standard deviations of the numbers of viable adherent or internalized bacteria.
T84 polarized translocation, adherence, and internalization assays.
The BIOCOAT intestinal epithelium differentiation environment was used to prepare polarized T84 cells according to the manufacturer's specifications (Becton Dickinson, Bedford, Mass.). Translocation experiments were performed by adding 5 × 107 CFU (multiplicity of infection [MOI] of approximately 200) in prewarmed Entero-STIM to the apical chamber of a transwell unit and incubating bacteria in a humidified, 5% CO2 incubator at 37°C. At time points indicated, the transwells were transferred to a 24-well plate containing fresh prewarmed Entero-STIM medium. Bacteria which translocated across the cell monolayer into the basolateral medium were enumerated by plating 10-fold serial dilutions of the basolateral medium on MH-blood agar plates. Cell-associated bacteria were quantified by washing the apical and basolateral surfaces of a transwell membrane three times with PBS. Following the washes, the transwell membranes were excised with a sterile scalpel and placed in a solution of 0.25% (wt/vol) sodium deoxycholate to lyse the T84 cells. Internalized bacteria were quantified in a similar manner with modifications. Following apical and basolateral washes, Entero-STIM medium containing a bactericidal concentration of gentamicin was placed in both the apical and basolateral chambers to kill all extracellular bacteria. Following incubation, the transwell membranes were rinsed with PBS and excised, and internalized bacteria were enumerated.
To determine if C. jejuni preferentially remains cell associated rather than translocating into the basolateral medium, two identical sets of C. jejuni-inoculated transwell units were used. The first set was used to determine the number of cell-associated C. jejuni bacteria at the 4-h time point as outlined above. The second set of samples were identically inoculated, with the apical and basolateral surface of each cell monolayer being washed three times with Entero-STIM at the 4-h time point to remove nonadherent bacteria. The transwell units were then placed in a 24-well plate containing fresh Entero-STIM medium. The number of bacteria present in the basolateral chamber medium was determined after each additional hour of incubation, at which time the transwell units were transferred to a 24-well plate containing fresh Entero-STIM medium. The number of remaining cell-associated C. jejuni bacteria was determined at the 7-h time point. Where indicated, EGTA (10 μM) was added 15 min prior to infection to induce cellular retraction of T84 cells. EGTA was removed prior to inoculation of T84 cells with C. jejuni. Anti-human Fn antibodies (Telios Pharmaceuticals) were used at a 1:50 dilution with Entero-STIM in the absence of manufacturer-provided serum supplement. Translocation kinetic studies with C. jejuni F38011 and the isogenic cadF mutant were performed at MOIs of approximately 3, 30, and 300. E. coli MRF served as a control for the integrity of the T84 polarized cell monolayers over the course of most assays. E. coli MRF, inoculated in an identical manner as C. jejuni, was not recovered from the basolateral medium. TER measurements were made with a Millicell-ERS volt-ohmmeter (Millipore, Bedford, Mass.).
Competitive binding assays.
Polarized cell monolayers were prepared as outlined above. C. jejuni 81-176 (Tetr) was added to the apical chamber of the transwell unit alone or in combination with a 25- to 90-fold excess of C. jejuni F38011 (Str/Nalr) and the C. jejuni F38011 isogenic cadF mutant (Kanr). Following a 4-h incubation, transwells were rinsed and membranes were excised as indicated above. The number of each cell-associated isolate was determined by diluting lysates and plating bacteria on MH-blood plates containing the appropriate selective antibiotic.
Statistical analysis.
Significance between samples was determined by Student's t test following logarithmic [log × (base 10)] transformation of the data. Two-tailed P values were determined for each sample, and a P value of <0.01 was considered significant.
RESULTS
Translocation across T84 polarized cells is independent of Cia protein secretion.
While some investigators have proposed that C. jejuni translocates across polarized cells via a transcellular route, others have proposed a paracellular route of translocation. To address the route of translocation, a binding and internalization experiment was initially performed with a C. jejuni ciaB mutant and T84 nonpolarized cells, as the phenotypic properties of the C. jejuni ciaB mutant with these cells had not been assessed previously. Consistent with previous work with INT 407 cells (22), adherence assays revealed no significant differences in the numbers of the C. jejuni F38011 wild-type isolate, ciaB mutant, and ciaB mutant transformed with pMEK100 bound to the T84 cells (Table 1). However, a significant difference was noted for the invasiveness of the ciaB mutant when it was compared to either the complemented ciaB mutant or the F38011 isolate (Table 1). Previous work has revealed that CiaB is a protein synthesized and secreted by C. jejuni upon cocultivation with eukaryotic cells and that a C. jejuni ciaB mutant does not secrete any proteins (22, 34). The present findings suggest that one or more of the Cia proteins are required for maximal invasion of T84 nonpolarized cells by C. jejuni.
TABLE 1.
Cia proteins are required for maximal invasion of T84 nonpolarized cells by C. jejuni
| Isolate | No. of bacteria/wella
|
|
|---|---|---|
| Adherence | Internalization | |
| C. jejuni F38011 | (2.5 ± 0.3) × 106 | (3.6 ± 0.2) × 104 |
| C. jejuni ciaB mutant | (2.4 ± 0.4) × 106 | (2.6 ± 1.0) × 103b |
| C. jejuni ciaB mutant + pMEK100 | (2.5 ± 0.5) × 106 | (1.8 ± 0.1) × 104 |
| C. jejuni 81-176 | (2.2 ± 0.5) × 106 | (2.7 ± 0.4) × 104 |
Adherence and internalization assays were performed as outlined in Materials and Methods. Results are presented as the means ± standard deviations of triplicate determinations.
The value was significantly different (P < 0.01) from that obtained with the C. jejuni F38011 wild-type isolate and C. jejuni ciaB mutant transformant harboring pMEK100.
Assays were then performed with the C. jejuni F38011 wild-type isolate and isogenic ciaB mutant to determine if C. jejuni translocates a T84 cell monolayer via a paracellular versus a transcellular route. The C. jejuni 81-176 wild-type isolate and C. jejuni ciaB transformant harboring pMEK100 were included as controls. All C. jejuni isolates displayed similar kinetics of translocation (Table 2), suggesting a paracellular route of translocation. Given this finding, the adherence and invasive potential of each isolate were assessed. The results obtained with the T84 polarized cells mirrored those obtained with the T84 nonpolarized cells with respect to the numbers of cell-associated and internalized bacteria. No difference was noted in the binding of the C. jejuni ciaB mutant compared to that of the C. jejuni F38011 wild-type isolate (Table 2). To assess cell invasion, gentamicin was added to both the apical and the basolateral chambers and the C. jejuni-inoculated cell monolayers were incubated for an additional 3 h to ensure killing of all extracellular bacteria. In contrast to adherence, differences were observed in the invasiveness of the C. jejuni isolates tested (Table 2). The invasive potential of the C. jejuni 81-176 and F38011 clinical isolates was found to be the greatest, followed by the C. jejuni ciaB-pMEK100 transformant and then the C. jejuni ciaB mutant. These results suggest that Cia proteins are required for the maximal invasion of polarized cells and yet play no role in C. jejuni translocation.
TABLE 2.
Ability of C. jejuni to translocate across T84 polarized cells is independent of invasive potential
| C. jejuni strain | No. of bacteria
|
|||||
|---|---|---|---|---|---|---|
| In basolateral medium
|
Cell associateda (4 h) | Internalizedb (7 h) | ||||
| 0-1 h | 1-2 h | 2-3 h | 3-4 h | |||
| 81-176 | (1.1 ± 0.1) × 106 | (1.4 ± 0.1) × 106 | (1.4 ± 0.2) × 106 | (1.1 ± 0.2) × 106 | (2.2 ± 0.7) × 106 | (4.5 ± 0.5) × 104 |
| F38011 | (1.3 ± 0.2) × 106 | (1.1 ± 0.1) × 106 | (9.0 ± 0.8) × 105 | (8.8 ± 0.5) × 105 | (1.1 ± 0.3) × 106 | (3.7 ± 0.4) × 104 |
| ciaB mutant | (1.5 ± 0.3) × 106 | (1.3 ± 0.8) × 106 | (2.0 ± 0.9) × 106 | (1.9 ± 0.5) × 106 | (1.2 ± 0.3) × 106 | (5.1 ± 0.2) × 103c |
| ciaB mutant plus pMEK100 | (2.3 ± 0.3) × 106 | (1.9 ± 0.3) × 106 | (2.3 ± 0.3) × 106 | (2.5 ± 0.1) × 106 | (1.2 ± 0.2) × 106 | (1.2 ± 0.1) × 104 |
C. jejuni-inoculated T84 cells were rinsed three times in PBS after a 4-h incubation period. The number of cell-associated (adherent) bacteria was determined as outlined in Materials and Methods.
C. jejuni-inoculated T84 cells were rinsed three times in Entero-STIM after 4 h of incubation, followed by 3 h of incubation with gentamicin to kill extracellular bacteria as outlined in Materials and Methods.
Mean value is significantly different (P < 0.01) from that obtained with the C. jejuni wild-type isolate and C. jejuni ciaB mutant harboring pMEK100.
Prior to the experiment outlined above, the relationship between C. jejuni invasion and translocation of T84 polarized cells was investigated by determining how many bacteria remain associated with a polarized cell monolayer over the course of a 3-h incubation period. This assay was necessary to assess the invasive potential of C. jejuni with polarized cells, as an extended period of incubation with gentamicin is required to kill extracellular bacteria. Moreover, if the intracellular bacteria transcytosed the cells within a 3-h period, an accurate assessment of the number of intracellular bacteria could not have been made, as they would have been exposed to gentamicin-containing medium. For this assay, one set of T84 polarized cells was inoculated with the C. jejuni clinical isolates F38011 and 81-176 and incubated for 4 h, after which time the monolayers were rinsed and cell association was quantified (Table 3). A second set of the C. jejuni-inoculated polarized cells was rinsed after the 4-h incubation period and placed in a 24-well plate containing fresh medium. C. jejuni bacteria present in the basolateral medium were quantified hourly for an additional 3-h period (Table 3). Following this incubation, the numbers of cell-associated C. jejuni bacteria were determined (Table 3). The results of this assay revealed that the majority of the cell-associated C. jejuni bacteria remained cell associated rather than translocating to the medium in the basolateral chamber (Table 3). Indeed, 96.9% of C. jejuni F38011 bacteria and 85.2% of C. jejuni 81-176 bacteria that were associated with the polarized cell monolayers after 4 h of incubation remained cell associated after the additional 3-h incubation period.
TABLE 3.
C. jejuni bacteria that become cell associated remain associated with T84 polarized cells
| C. jejuni strain | No. of bacteria
|
% of cell-associated bacteria
|
|||||
|---|---|---|---|---|---|---|---|
| Cell associated at 4 h | In basolateral medium
|
Cell associated at 7 h | Translocated to basolateral mediuma | Remaining cell associatedb | |||
| 4-5 h | 5-6 h | 6-7 h | |||||
| F38011 | (9.8 ± 1.3) × 105 | (1.7 ± 0.4) × 104 | (8.0 ± 1.4) × 103 | (6.8 ± 0.5) × 103 | (9.5 ± 1.3) × 105 | 3.2 | 96.9 |
| 81-176 | (8.8 ± 1.0) × 105 | (4.1 ± 0.7) × 104 | (3.8 ± 1.0) × 104 | (3.3 ± 1.0) × 104 | (7.5 ± 0.6) × 105 | 12.7 | 85.2 |
(Total number of translocated bacteria/number of cell-associated bacteria) × 100.
(Number of cell-associated bacteria at 7 h/number of cell-associated bacteria at 4 h) × 100.
Collectively, these data indicate that (i) C. jejuni translocates across a cell monolayer via a paracellular route, (ii) the Cia proteins play no role in C. jejuni cellular translocation, (iii) the Cia proteins are required for the maximal invasion of polarized cells, and (iv) the bacteria that become associated with the polarized cells remain cell associated.
Translocation results from saturation of host cell receptors.
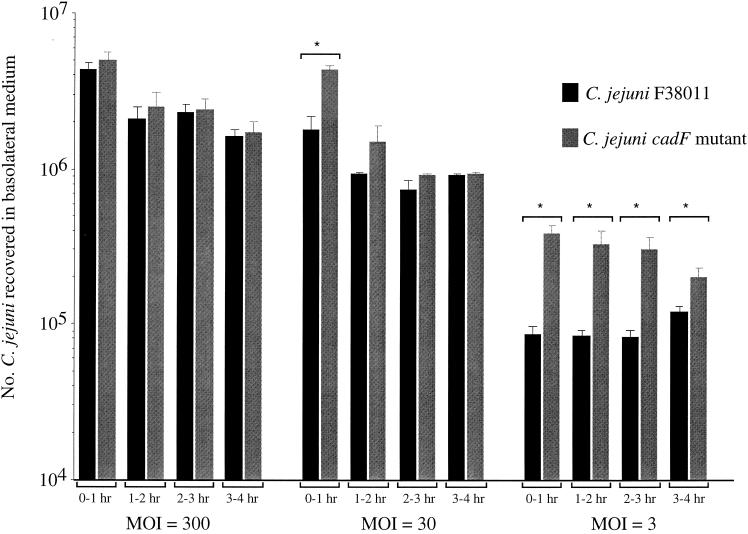
Based on the finding that C. jejuni preferentially remains cell associated (apically or basolaterally) rather than translocating to the medium in the basolateral chamber (Table 3), assays were conducted to compare the translocation kinetics of the C. jejuni F38011 wild-type isolate and the C. jejuni isogenic cadF mutant (20). Experiments were simultaneously performed to determine host cell association at 4 h at different MOIs. At an MOI of 300, both C. jejuni isolates translocated across the T84 polarized cell monolayers, into the basolateral medium, with approximately the same efficiency. As the MOI was decreased from 300 to 3, a significant decrease was noted in the ability of C. jejuni F38011 to translocate into the basolateral medium compared to that for the cadF mutant (Fig. 1). We propose that, at the lowest MOI, the C. jejuni wild-type isolate does not translocate to the basolateral medium as efficiently as does the C. jejuni cadF mutant because the former binds to Fn. The difference in translocation is masked at higher MOIs because the accessible host cell receptors to which the C. jejuni wild-type isolate binds are occupied. Also noteworthy is that, at an MOI of 30, a significant difference was observed between the translocation of the C. jejuni wild-type isolate and that of the cadF mutant at the 1-h interval. However, after the first hour of incubation, no significant difference was observed in the translocation kinetics of the two isolates. This finding suggests that Fn is available for C. jejuni to bind throughout the first hour of incubation, after which time all available host receptor molecules, including Fn, are bound. Consistent with previous work with INT 407 cells (28a), a significant decrease in adherence to T84 polarized cells was observed for the C. jejuni cadF mutant compared to the C. jejuni wild-type isolate, regardless of MOI (Table 4).
FIG. 1.
Translocation kinetics of C. jejuni F38011 and the isogenic cadF mutant across polarized T84 cells at various MOIs. Each bar represents the number of C. jejuni bacteria recovered from the basolateral chamber over the course of each 1-h interval. *, value significantly different (P < 0.01) from the C. jejuni F38011 wild-type isolate.
TABLE 4.
CadF protein is required for maximal T84 cell association regardless of MOI
| C. jejuni strain | No. of cell-associated bacteria at increasing MOIa:
|
||
|---|---|---|---|
| 3 | 30 | 300 | |
| F38011 | (4.3 ± 0.7) × 104 | (4.3 ± 0.8) × 105 | (2.1 ± 0.2) × 106 |
| cadF mutant | (1.5 ± 0.2) × 104b | (1.3 ± 0.4) × 105b | (1.1 ± 0.2) × 106b |
Assays were performed as outlined in Materials and Methods. C. jejuni cell association was measured at 4 h after infection of T84 polarized cells.
Value significantly different (P < 0.01) from that obtained with the C. jejuni F38011 wild-type isolate.
Competition assays with polarized cells were conducted to further confirm that Fn serves as a host cell receptor for C. jejuni clinical isolates F38011 and 81-176. It was possible to specifically determine the number of each bacterial isolate associated with the epithelial cells based on their antibiotic resistances. C. jejuni 81-176, along with a 25- to 90-fold excess of either C. jejuni F38011 or the isogenic cadF mutant, was used to inoculate the apical surface of the T84 polarized cells. The number of each C. jejuni isolate associated with the monolayer was then determined as outlined in Materials and Methods. C. jejuni 81-176 was not significantly prevented from associating with the polarized monolayer in the presence of a 50- and a 90-fold excess of the cadF mutant (Table 5). In contrast, C. jejuni F38011 significantly reduced the association of C. jejuni 81-176 with the T84 polarized cells in a dose-dependent manner. Notably, the total number of adherent C. jejuni 81-176 bacteria, at an MOI of approximately 3, was comparable to that previously observed for C. jejuni F38011 at the same MOI (Table 4). These data further support the notion that the binding of C. jejuni to Fn promotes bacterium-host cell association.
TABLE 5.
Functional role of CadF as an adhesin is shared between the C. jejuni clinical isolates 81-176 and F38011
| Competitor and fold excess |
C. jejuni 81-176 wild-type isolate bacteria bounda
|
|
|---|---|---|
| No. | % | |
| No competitor | (3.7 ± 0.3) × 104 | 100 |
| C. jejuni cadF mutant | ||
| 50 | (3.5 ± 0.4) × 104 | 95 ± 10 |
| 90 | (2.9 ± 0.4) × 104 | 78 ± 11 |
| C. jejuni F38011 | ||
| 25 | (2.0 ± 0.2) × 104 | 54 ± 5b |
| 50 | (1.4 ± 0.3) × 104 | 38 ± 8b |
Values represent the numbers of C. jejuni 81-176 bacteria bound in the presence of the competing organism divided by the numbers of C. jejuni 81-176 bacteria bound in the absence of the competitor, multiplied by 100.
Value significantly different (P < 0.01) from that obtained with the C. jejuni 81-176 wild-type isolate in the absence of the competitor.
Invasion of T84 cells occurs primarily via the basolateral surface.
Because the two wild-type isolates competed for cell association (adherence and internalization), additional experiments were performed to determine whether adherence and internalization could be retarded by blocking receptors with anti-Fn antibodies. Initial experiments to test this hypothesis were conducted with T84 nonpolarized cells. However, the adherence to and invasion of T84 nonpolarized cells by C. jejuni were reduced to only 81 and 76%, respectively (Table 6), in the presence of the anti-Fn antibodies compared to the control. Because Fn would be expected to predominate at cell-substrate contacts, monolayers were pretreated with EGTA prior to infection to expose the extracellular matrix. Upon treatment of T84 cells with EGTA, C. jejuni F38011 adherence and invasion increased 144 and 288%, respectively, compared to untreated monolayers (Table 6). When anti-Fn antibodies were included in the medium following EGTA treatment, the numbers of adherent and internalized bacteria were significantly reduced from 144 to 50% and 288 to 65%, respectively. These data suggest that increased exposure of C. jejuni to Fn promotes maximal host cell interaction, which in turn leads to increased invasion into T84 nonpolarized cells. Serovar Typhimurium was included as a control to ensure that the increase in C. jejuni adherence and invasion did not result directly from an increase in exposed cell surface area following treatment with EGTA. No significant difference in serovar Typhimurium adherence or invasion was noted following EGTA treatment of host cells or in the presence of anti-Fn antibodies. Furthermore, transient chelation of extracellular divalent cations did not appear to affect host cell endocytic activity as judged by efficient uptake of serovar Typhimurium over the course of the assay or T84 cell viability as judged by staining with trypan blue.
TABLE 6.
C. jejuni association with Fn maximizes bacterial adherence and invasion of T84 nonpolarized cells
| Bacterial strain and type | % of adherent and internalized bacteriaa
|
||
|---|---|---|---|
| Anti-Fn | EGTA | EGTA + anti-Fn | |
| C. jejuni F38011 | |||
| Adherent | 81 ± 5 | 144 ± 12b | 50 ± 8c |
| Internalized | 76 ± 12 | 288 ± 36b | 65 ± 17c |
| Serovar Typhimurium SL1344 | |||
| Adherent | 95 ± 20 | 113 ± 15 | 115 ± 25 |
| Internalized | 92 ± 4 | 96 ± 8 | 84 ± 8 |
Assays were performed as outlined in Materials and Methods. Results are presented as the percent adherent and internalized bacteria relative to the values (100%) obtained for the wild-type isolates alone.
Value significantly different (P < 0.01) from that obtained from the inoculation of T84 cells with the C. jejuni F38011 wild-type isolate.
Value significantly different (P < 0.01) from that obtained from inoculation of the EGTA-treated T84 cells with the C. jejuni F38011 wild-type isolate.
Identical experiments were also performed with T84 polarized cells to determine the effects of EGTA treatment on C. jejuni adherence and invasion. Anti-Fn antibodies added to the non-EGTA-treated polarized cells had no effect on C. jejuni adherence and invasion, presumably due to the lack of antibody access to Fn localized to the basolateral surface. As observed with nonpolarized cells, pretreatment of the cell monolayers with EGTA resulted in a significant increase in the number of adherent and internalized bacteria to 185 and 367%, respectively (Table 7). Thus, a 1.8-fold increase in binding was observed and a disproportional increase of 3.6-fold in internalization was noted, suggesting that a greater proportion of the bacteria are bound to receptors involved in C. jejuni uptake. This observation was also noted when nonpolarized cells were used (Table 6). Inclusion of anti-Fn antibodies with the infection inoculum, following EGTA treatment of T84 polarized cells, significantly decreased adherence and invasion from 185 to 35% and 367 to 67%, respectively. Noteworthy is that the addition of anti-Fn antibodies following EGTA treatment of the polarized cells resulted in a proportional decrease in adherence (5.3-fold decrease) and internalization (5.5-fold decrease).
TABLE 7.
C. jejuni association with Fn localized to the basolateral surface of T84 polarized cells maximizes bacterial adherence and invasion
| Type of C. jejuni F38011 cells | % of adherent and internalized bacteriaa
|
||
|---|---|---|---|
| Anti-Fn | EGTA | EGTA + anti-Fn | |
| Adherent | 97 ± 9 | 185 ± 15b | 35 ± 3c |
| Internalized | 108 ± 16 | 367 ± 21b | 67 ± 13c |
Assays were performed as outlined in Materials and Methods. Results are presented as the percent adherent and internalized bacteria relative to the values (100%) obtained for the wild-type isolate alone.
Value significantly different (P < 0.01) from that obtained from the inoculation of T84 cells with the C. jejuni F38011 wild-type isolate.
Value significantly different (P < 0.01) from that obtained from inoculation of the EGTA-treated T84 cells with the C. jejuni F38011 wild-type isolate.
DISCUSSION
The goal of this study was to further characterize the interaction of C. jejuni with cells by using a model that mimics the in vivo environment that the organism encounters. Because histological examination of C. jejuni-infected humans and animals has revealed pathology in the colon (3, 6, 35), we chose to examine the interactions of C. jejuni with T84 cells. Phenotypically, T84 cells are thought to be similar to colonic crypt cells (31). The primary purpose of this study was to characterize the binding, entry, and translocation of polarized cells by C. jejuni.
In agreement with the work of Harvey et al. (14) and Everest et al. (11) but in contrast to the work of Brás and Ketley (7), we found no correlation in an isolate's invasive and translocation potential. Harvey et al. (14) compared the abilities of four clinical C. jejuni isolates to translocate across polarized Caco-2 cells with their abilities to invade nonpolarized cells. They also noted that the integrity of the cell monolayer was maintained over the course of the assay (14). We also noted that the final TER values were comparable to starting baseline values (data not shown). Taken together, these data suggest that C. jejuni can translocate across an intact cell monolayer. Work by others has revealed that cellular tight junctions can reseal following bacterial penetration (39), thus providing a basis for C. jejuni to utilize a paracellular route of passage without the long-term disruption of the integrity of the cell monolayer. Previous studies have also demonstrated that tight junctions can temporarily relax to allow regulated passage of both solutes and neutrophils (28).
Investigators have utilized polarized cell culture systems to address the virulence properties of a wide variety of pathogens (12). For example, Kops et al. (25) found that while both Salmonella enterica serovar Typhi and serovar Typhimurium translocate across polarized cell monolayers, serovar Typhi translocates much earlier and in greater numbers than does serovar Typhimurium. The translocation of serovar Typhi across the cell monolayers was accompanied by cell death and extrusion, which was reflected in a rapid decrease in TER values. The authors proposed that their findings were reflective of in vivo disease presentations, as serovar Typhi is commonly septic in nature and serovar Typhimurium is a localized superficial invader of the intestinal mucosa. Finlay and Falkow (12) also noted that the morphological effects of serovar Typhimurium on Caco-2 polarized cells were similar to those obtained with animal infection models. While the results presented herein indicate that C. jejuni possesses the ability to translocate across a cell monolayer, they also suggest that the migration of the organism from the apical (e.g., lumen) to the basolateral (e.g., lamina propria) chamber may not, per se, be reflective of the organism's preference. More specifically, when the inoculum was removed from the apical chamber and the monolayers were rinsed, the majority of C. jejuni bacteria were found to remain associated with the polarized cells rather than to translocate into the basolateral chamber medium. Based on this observation, we believe that the ability of C. jejuni to translocate across a cell barrier is relevant with respect to the organism gaining access to receptor molecules located on the basolateral surface of a host cell. Our data suggest that the C. jejuni detected in the basolateral chamber represents those organisms that continue to migrate beyond the cell monolayer as the available host cell receptor molecules to which they bind are saturated with bound bacteria. This finding is further supported by the fact that translocation of C. jejuni F38011 and the cadF mutant differs. At a high MOI (300 bacteria per cell), Fn appears to become saturated, as suggested by similar translocation kinetics for the C. jejuni F38011 parental isolate and the cadF mutant. However, at the lowest MOI (three bacteria per cell), C. jejuni F38011 translocation is retarded, which may be due to delayed saturation of available Fn binding sites. A similar phenomenon may occur in vivo where, early in an infection, most bacteria colonize the intestinal mucosa and begin to multiply. Thereafter, a significant number of C. jejuni bacteria may reach the lamina propria due to an increase in the bacterial load at the site of infection.
While the functions of the Cia proteins are not known, the results presented herein support a link between Cia secretion and C. jejuni host cell invasion. The relationship between Cia protein secretion and C. jejuni invasion was evident regardless of whether the T84 host cells were cultured by conventional methods or with conditions that allowed the establishment of a polarized cell monolayer. The results are consistent with those previously obtained with INT 407 (22, 34) and Caco-2 (unpublished data) cells.
In summary, C. jejuni appears to preferentially invade the basolateral surface of polarized cells based on competition assays whereby two C. jejuni wild-type isolates, in contrast to the C. jejuni cadF mutant, competed with one another for host cell receptors. Pretreatment of polarized cells with EGTA to increase exposure of cell-bound Fn resulted in a significant increase in both C. jejuni adherence and invasion. The increase in C. jejuni invasion of EGTA-treated cells was not due to an increase in cell surface area, as serovar Typhimurium adherence and invasion frequencies were not affected when host cells were pretreated with EGTA. The specificity of the binding of C. jejuni to Fn was further demonstrated with antibodies that specifically reacted against Fn. Based on these data, we propose a model whereby C. jejuni translocates the intestinal epithelia, binds to Fn, and then invades the target cell. Interestingly, studies with N. gonorrhoeae have suggested that the OpaA adhesin serves as a molecular bridge between Fn and β1-integrins, which lead to bacterial uptake (41). Work in our laboratory has indicated that the binding of C. jejuni to Fn via CadF promotes the phosphorylation of paxillin, a focal adhesion protein (28a). Based on the data presented herein, CadF also appears to play a role in promoting the binding of C. jejuni to polarized cells. Because cell binding and the stimulation of cell signaling events are a prerequisite for C. jejuni uptake by nonprofessional phagocytic cells, CadF may be an essential virulence determinant. In support of this notion, CadF has been found previously to be conserved among C. jejuni isolates (21). Further studies will be conducted to determine whether C. jejuni environmental isolates display the same virulence attributes as the C. jejuni clinical isolates F38011 and 81-176.
Acknowledgments
We thank John Klena, Anthony Garza, and Ray Larsen for reviewing the manuscript.
This work was supported by a grant from the NIH (DK58911) and the USDA National Research Initiative Competitive Grants Program (USDA/NRICGP, 99-35201-8579) awarded to M.E.K.
Editor: B. B. Finlay
REFERENCES
- 1.Allos, B. M. 2001. Campylobacter jejuni infections: update on emerging issues and trends. Clin. Infect. Dis. 32:1201-1206. [DOI] [PubMed] [Google Scholar]
- 2.Altekruse, S. F., N. J. Stern, P. I. Fields, and D. L. Swerdlow. 1999. Campylobacter jejuni—an emerging foodborne pathogen. Emerg. Infect. Dis. 5:28-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babakhani, F. K., G. A. Bradley, and L. A. Joens. 1993. Newborn piglet model for campylobacteriosis. Infect. Immun. 61:3466-3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bacon, D. J., R. A. Alm, D. H. Burr, L. Hu, D. J. Kopecko, C. P. Ewing, T. J. Trust, and P. Guerry. 2000. Involvement of a plasmid in virulence of Campylobacter jejuni 81-176. Infect. Immun. 68:4384-4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baloda, S. B., A. Faris, G. Fröman, and T. Waldström. 1985. Fibronectin binding to Salmonella strains. FEMS Microbiol. Lett. 28:1-5. [Google Scholar]
- 6.Black, R. E., M. M. Levine, M. L. Clements, T. P. Hughes, and M. J. Blaser. 1988. Experimental Campylobacter jejuni infection in humans. J. Infect. Dis. 157:472-479. [DOI] [PubMed] [Google Scholar]
- 7.Brás, A. M., and J. M. Ketley. 1999. Transcellular translocation of Campylobacter jejuni across human polarised epithelial monolayers. FEMS Microbiol. Lett. 179:209-215. [DOI] [PubMed] [Google Scholar]
- 8.Cruz, N., Q. Lu, X. Alvarez, and E. A. Deitch. 1994. Bacterial translocation is bacterial species dependent: results using the human Caco-2 intestinal cell line. J. Trauma 36:612-616. [PubMed] [Google Scholar]
- 9.Dawson, J. R., and R. P. Ellen. 1994. Clustering of fibronectin adhesins toward Treponema denticola tips upon contact with immobilized fibronectin. Infect. Immun. 62:2214-2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dawson, J. R., and R. P. Ellen. 1990. Tip-oriented adherence of Treponema denticola to fibronectin. Infect. Immun. 58:3924-3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Everest, P. H., H. Goossens, J. P. Butzler, D. Lloyd, S. Knutton, J. M. Ketley, and P. H. Williams. 1992. Differentiated Caco-2 cells as a model for enteric invasion by Campylobacter jejuni and C. coli. J. Med. Microbiol. 37:319-325. [DOI] [PubMed] [Google Scholar]
- 12.Finlay, B. B., and S. Falkow. 1990. Salmonella interactions with polarized human intestinal Caco-2 epithelial cells. J. Infect. Dis. 162:1096-1106. [DOI] [PubMed] [Google Scholar]
- 13.Fröman, G., L. M. Switalski, A. Faris, T. Wadström, and M. Höök. 1984. Binding of Escherichia coli to fibronectin. J. Biol. Chem. 259:14899-14905. [PubMed] [Google Scholar]
- 14.Harvey, P., T. Battle, and S. Leach. 1999. Different invasion phenotypes of Campylobacter isolates in Caco-2 cell monolayers. J. Med. Microbiol. 48:461-469. [DOI] [PubMed] [Google Scholar]
- 15.Humason, G. L. 1979. Animal tissue techniques, 4th ed. W. H. Freeman and Company, San Francisco, Calif.
- 16.Jaffe, J., S. Natanson-Yaron, M. G. Caparon, and E. Hanski. 1996. Protein F2, a novel fibronectin-binding protein from Streptococcus pyogenes, possesses two binding domains. Mol. Microbiol. 21:373-384. [DOI] [PubMed] [Google Scholar]
- 17.Jin, S., A. Joe, J. Lynett, E. K. Hani, P. Sherman, and V. L. Chan. 2001. JlpA, a novel surface-exposed lipoprotein specific to Campylobacter jejuni, mediates adherence to host epithelial cells. Mol. Microbiol. 39:1225-1236. [DOI] [PubMed] [Google Scholar]
- 18.Konkel, M. E., and W. Cieplak, Jr. 1992. Altered synthetic response of Campylobacter jejuni to cocultivation with human epithelial cells is associated with enhanced internalization. Infect. Immun. 60:4945-4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Konkel, M. E., M. D. Corwin, L. A. Joens, and W. Cieplak, Jr. 1992. Factors that influence the interaction of Campylobacter jejuni with cultured mammalian cells. J. Med. Microbiol. 37:30-37. [DOI] [PubMed] [Google Scholar]
- 20.Konkel, M. E., S. G. Garvis, S. L. Tipton, D. E. Anderson, Jr., and W. Cieplak, Jr. 1997. Identification and molecular cloning of a gene encoding a fibronectin-binding protein (CadF) from Campylobacter jejuni. Mol. Microbiol. 24:953-963. [DOI] [PubMed] [Google Scholar]
- 21.Konkel, M. E., S. A. Gray, B. J. Kim, S. G. Garvis, and J. Yoon. 1999. Identification of the enteropathogens Campylobacter jejuni and Campylobacter coli based on the cadF virulence gene and its product. J. Clin. Microbiol. 37:510-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Konkel, M. E., B. J. Kim, V. Rivera-Amill, and S. G. Garvis. 1999. Bacterial secreted proteins are required for the internalization of Campylobacter jejuni into cultured mammalian cells. Mol. Microbiol. 32:691-701. [DOI] [PubMed] [Google Scholar]
- 23.Konkel, M. E., D. J. Mead, and W. Cieplak, Jr. 1993. Kinetic and antigenic characterization of altered protein synthesis by Campylobacter jejuni during cultivation with human epithelial cells. J. Infect. Dis. 168:948-954. [DOI] [PubMed] [Google Scholar]
- 24.Konkel, M. E., D. J. Mead, S. F. Hayes, and W. Cieplak, Jr. 1992. Translocation of Campylobacter jejuni across human polarized epithelial cell monolayer cultures. J. Infect. Dis. 166:308-315. [DOI] [PubMed] [Google Scholar]
- 25.Kops, S. K., D. K. Lowe, W. M. Bement, and A. B. West. 1996. Migration of Salmonella typhi through intestinal epithelial monolayers: an in vitro study. Microbiol. Immunol. 40:799-811. [DOI] [PubMed] [Google Scholar]
- 26.Kuusela, P. 1978. Fibronectin binds to Staphylococcus aureus. Nature (London) 276:718-720. [DOI] [PubMed] [Google Scholar]
- 27.Kuusela, P., A. P. Moran, T. Vartio, and T. U. Kosunen. 1989. Interaction of Campylobacter jejuni with extracellular matrix components. Biochim. Biophys. Acta 993:297-300. [DOI] [PubMed] [Google Scholar]
- 28.Madara, J. L. 1998. Regulation of the movement of solutes across tight junctions. Annu. Rev. Physiol. 60:143-159. [DOI] [PubMed] [Google Scholar]
- 28a.Monteville, M. R., J. E. Yoon, and M. E. Konkel. Maximal adherence and invasion of INT 407 cells by Campylobacter jejuni require the CadF outer membrane protein and microfilament reorganization. Microbiology, in press. [DOI] [PubMed]
- 29.Morooka, T., A. Umeda, and K. Amako. 1985. Motility as an intestinal colonization factor for Campylobacter jejuni. J. Gen. Microbiol. 131:1973-1980. [DOI] [PubMed] [Google Scholar]
- 30.Myhre, E. B., and P. Kuusela. 1983. Binding of human fibronectin to group A, C, and G streptococci. Infect. Immun. 40:29-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nataro, J. P., S. Hicks, A. D. Phillips, P. A. Vial, and C. L. Sears. 1996. T84 cells in culture as a model for enteroaggregative Escherichia coli pathogenesis. Infect. Immun. 64:4761-4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pei, Z., C. Burucoa, B. Grignon, S. Baqar, X.-Z. Huang, D. J. Kopecko, A. L. Bourgeois, J.-L. Fauchere, and M. J. Blaser. 1998. Mutation in the peb1A locus of Campylobacter jejuni reduces interactions with epithelial cells and intestinal colonization of mice. Infect. Immun. 66:938-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pickett, C. L., and C. A. Whitehouse. 1999. The cytolethal distending toxin family. Trends Microbiol. 7:292-297. [DOI] [PubMed] [Google Scholar]
- 34.Rivera-Amill, V., B. J. Kim, J. Seshu, and M. E. Konkel. 2001. Secretion of the virulence associated Campylobacter invasion antigens from Campylobacter jejuni requires a stimulatory signal. J. Infect. Dis. 183:1607-1616. [DOI] [PubMed] [Google Scholar]
- 35.Russell, R. G., M. O'Donnoghue, D. C. Blake, Jr., J. Zulty, and L. J. DeTolla. 1993. Early colonic damage and invasion of Campylobacter jejuni in experimentally challenged infant Macaca mulatta. J. Infect. Dis. 168:210-215. [DOI] [PubMed] [Google Scholar]
- 36.Rydén, C., K. Rubin, P. Speziale, M. Höök, M. Lindberg, and T. Wadström. 1983. Fibronectin receptors from Staphylococcus aureus. J. Biol. Chem. 258:3396-3401. [PubMed] [Google Scholar]
- 37.Schorey, J. S., M. A. Holsti, T. L. Ratliff, P. M. Allen, and E. J. Brown. 1996. Characterization of the fibronectin-attachment protein of Mycobacterium avium reveals a fibronectin-binding motif conserved among mycobacteria. Mol. Microbiol. 21:321-329. [DOI] [PubMed] [Google Scholar]
- 38.Takata, T., S. Fujimoto, and K. Amako. 1992. Isolation of nonchemotactic mutants of Campylobacter jejuni and their colonization of the mouse intestinal tract. Infect. Immun. 60:3596-3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takeuchi, A. 1967. Electron microscopic studies of experimental Salmonella infection. Penetration into the intestinal epithelium by Salmonella typhimurium. Am. J. Pathol. 50:109-136. [PMC free article] [PubMed] [Google Scholar]
- 40.Thomas, D. D., J. B. Baseman, and J. F. Alderete. 1985. Fibronectin mediates Treponema pallidum cytadherence through recognition of fibronectin cell-binding domain. J. Exp. Med. 161:514-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Putten, J. P., T. D. Duensing, and R. L. Cole. 1998. Entry of OpaA+ gonococci into HEp-2 cells requires concerted action of glycosaminoglycans, fibronectin and integrin receptors. Mol. Microbiol. 29:369-379. [DOI] [PubMed] [Google Scholar]
- 42.Visai, L., S. Bozzini, T. E. Petersen, L. Speciale, and P. Speziale. 1991. Binding sites in fibronectin for an enterotoxigenic strain of E. coli B342289c. FEBS Lett. 290:111-114. [DOI] [PubMed] [Google Scholar]
- 43.Wassenaar, T. M. 1997. Toxin production by Campylobacter spp. Clin. Microbiol. Rev. 10:466-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wassenaar, T. M., B. A. van der Zeijst, R. Ayling, and D. G. Newell. 1993. Colonization of chicks by motility mutants of Campylobacter jejuni demonstrates the importance of flagellin A expression. J. Gen. Microbiol. 139:1171-1175. [DOI] [PubMed] [Google Scholar]
- 45.Yao, R., D. H. Burr, and P. Guerry. 1997. CheY-mediated modulation of Campylobacter jejuni virulence. Mol. Microbiol. 23:1021-1031. [DOI] [PubMed] [Google Scholar]