Abstract
Recently, it was reported that a streptococcal Mac protein (designated Mac5005) made by serotype M1 group A Streptococcus (GAS) is a homologue of human CD11b that inhibits opsonophagocytosis and killing of GAS by human polymorphonuclear leukocytes (PMNs) (B. Lei, F. R. DeLeo, N. P. Hoe, M. R. Graham, S. M. Mackie, R. L. Cole, M. Liu, H. R. Hill, D. E. Low, M. J. Federle, J. R. Scott, and J. M. Musser, Nat. Med. 7:1298-1305, 2001). To study mac variation and expression of the Mac protein, the gene in 67 GAS strains representing 36 distinct M protein serotypes was sequenced. Two distinct genetic complexes were identified, and they were designated complex I and complex II. Mac variants in each of the two complexes were closely related, but complex I and complex II variants differed on average at 50.66 ± 5.8 amino acid residues, most of which were located in the middle one-third of the protein. Complex I Mac variants have greater homology with CD11b than complex II variants. GAS strains belonging to serotypes M1 and M3, the most abundant M protein serotypes responsible for human infections in many case series, have complex I Mac variants. The mac gene was cloned from representative strains assigned to complexes I and II, and the Mac proteins were purified to apparent homogeneity. Both Mac variants had immunoglobulin G (IgG)-endopeptidase activity. In contrast to Mac5005 (complex I), Mac8345 (complex II) underwent autooxidation of its cysteine residues, resulting in the loss of IgG-endopeptidase activity. A Mac5005 Cys94Ala site-specific mutant protein was unable to cleave IgG but retained the ability to inhibit IgG-mediated phagocytosis by human PMNs. Thus, the IgG-endopeptidase activity was not essential for the key biological function of Mac5005. Although Mac5005 and Mac8345 each have an Arg-Gly-Asp (RGD) motif, the proteins differed in their interactions with human integrins αvβ3 and αIIbβ3. Binding of Mac5005 to integrins αvβ3 and αIIbβ3 was mediated primarily by the RGD motif in Mac5005, whereas binding of Mac8345 involved the RGD motif and a region in the middle one-third of the molecule whose sequence is different in Mac8345 and Mac5005. Taken together, the data add to the emerging theme in GAS pathogenesis that allelic variation in virulence genes contributes to fundamental differences in host-pathogen interactions among strains.
Group A Streptococcus (GAS) is a gram-positive bacterial pathogen that is responsible for human morbidity and mortality globally (22). This organism causes diseases such as pharyngitis, cellulitis, bacteremia, rheumatic fever, and necrotizing fasciitis. The success of GAS as a pathogen is dependent on its ability to survive host defenses, including phagocytosis and killing by polymorphonuclear leukocytes (PMNs) and complement-mediated cell lysis. Several GAS molecules have been described that participate in these processes and act as virulence factors (reviewed in reference 8). For example, the hyaluronic acid capsule made by GAS is antiphagocytic (17). In addition, extracellular proteins made by GAS also are known to detrimentally alter host defenses, most notably the complement system. M protein, a highly polymorphic cell surface molecule that is made by all GAS strains, has been known for decades to be antiphagocytic (11, 12, 35). GAS also expresses an endopeptidase virulence factor that cleaves and inactivates complement protein C5a (13, 34). An extracellular protein referred to as streptococcal inhibitor of complement (Sic) inhibits the normal cytolytic activity of the complement membrane attack complex in vitro (1, 9). Sic expression increases persistence of GAS in the upper respiratory tract of the host and enhances pathogen durability on the human mucosal surface (18). GAS strains also express several superantigens that perturb normal host immune function and may contribute to pathogen survival (19).
M1 organisms have been the most abundant cause of human invasive infections in many population-based epidemiologic studies. Serotype M3 organisms also commonly cause invasive infections and are significantly associated with a high rate of morbidity and mortality (28). M1 and M3 organisms are also common causes of pharyngitis. Proteome analysis of serotype M1 and M3 strains identified many previously undescribed extracellular proteins, including a protein with homology to the α-subunit of human Mac-1 protein, designated streptococcal Mac protein (15). Analysis of sera obtained from mice with soft-tissue infections identified antibodies against this protein, indicating that Mac is made in the course of host-pathogen interactions.
Mac-1 (αMβ2, CD11b/CD18), a leukocyte integrin that is a member of the β2 integrin family, has been implicated in diverse biologic processes (7). Mac-1 is the primary fibrin(ogen) receptor on leukocytes and facilitates leukocyte recruitment to sites of fibrin and platelet deposition. By binding these proteins and other ligands, such as ICAM-1 and C3bi, Mac-1 regulates several critical innate leukocyte functions, including adhesion, migration, phagocytosis, and oxidative burst. Interestingly, streptococcal Mac has an Arg-Gly-Asp (RGD) motif, an amino acid sequence that commonly mediates binding of proteins to human integrins. Recently, Lei et al. (16) reported that streptococcal Mac produced by a serotype M1 strain bound to the surface of human PMNs and inhibited opsonophagocytosis and production of reactive oxygen species (ROS), which resulted in significantly decreased pathogen killing. Moreover, data were presented in the previous studies which indicate that Mac blocks the binding of antibody to the PMN Fc receptor, CD16 (16). Hence, Mac is a secreted bacterial protein that directly promotes pathogen survival. Mac was identified in culture supernatants of the majority of strains tested (including all serotype M1 and M3 strains), and patients with GAS infections made anti-Mac antibodies, indicating that the protein is made in vivo. Subsequently, von Pawel-Rammingen et al. (33) reported that Mac contains immunoglobulin G (IgG)-endopeptidase activity and proposed that the ability of Mac to block PMN opsonophagocytosis can be attributed to the proteinase activity.
The goal of the present study was to characterize the mac gene and Mac protein in natural populations of GAS. We report here that there are two major allele families of mac that differ from one another largely due to substantial divergence in the middle one-third of the mac gene and Mac protein. Mac variants (Mac5005, complex I; Mac8345, complex II) differentially bound to human integrins αvβ3 and αIIbβ3 expressed on cultured cells. In contrast to purified complex I Mac, complex II Mac was sensitive to autooxidation, which resulted in a loss of IgG-endopeptidase activity. Although IgG-endopeptidase activity has been proposed to be responsible for the ability of Mac5005 to block phagocytosis (33), we found that a complex I mutant (Cys94Ala) protein was unable to cleave IgG but significantly inhibited IgG-mediated PMN phagocytosis. Taken together, the data add to the important theme in GAS pathogenesis that allelic variation in virulence genes contributes to fundamental differences in host-pathogen interactions among strains.
MATERIALS AND METHODS
Materials.
2′,7′-Dichlorodihydrofluorescein diacetate was obtained from Molecular Probes, Inc. (Eugene, Oreg.). Ficoll-Paque Plus, DEAE-Sepharose, and phenyl-Sepharose were purchased from Amersham-Pharmacia Biotech (Piscataway, N.J.), and endotoxin-free 0.9% NaCl and water were obtained from Baxter Healthcare Corp. (Deerfield, Ill.). A QCL-1000 Limulus amebocyte lysate assay kit was obtained from BioWhittaker, Inc. (Walkersville, Md.). Rabbit polyclonal anti-Mac5005 antibody has been described previously (14). Affinity-purified rabbit polyclonal anti-Mac8345 antibody was prepared by Bethyl Laboratories (Montgomery, Tex.) by using recombinant Mac8345 as the antigen and immunoabsorbent. Anti-αvβ3 monoclonal antibody (MAb) LM609 and anti-CD11b M1/70 MAb were purchased from Chemicon (Temecula, Calif.) and Pharmingen, Inc. (San Diego, Calif.), respectively. Anti-αIIb MAb and anti-CD61 (β3) MAb were obtained from Immunotech (Westbrook, Maine). A peptide (VFTRGDQSK) corresponding to amino acid residues 211 to 219 and 213 to 221 in Mac5005 and Mac8345, respectively, and a control scrambled peptide (TVRQSDFGK) were purchased from Bethyl Laboratories. The peptides were purified by high-performance liquid chromatography and had the amide form located at the carboxy terminus.
Bacterial strains and growth.
The 67 GAS strains used are described in Table 1. These strains represent 36 M protein serotypes and a broad array of infection types and geographic sources. They represent M types responsible for 75% of human invasive infections in the United States and for a large percentage of pharyngitis cases in many western countries (2, 20).
TABLE 1.
GAS strains and mac complex assignments
| MGAS no.a | Location of isolation | Serotype | mac complex |
|---|---|---|---|
| 5005 | Canada | M1 | I |
| 7320 | Finland | M1 | I |
| 7638 | Illinois | M1 | I |
| 8520 | Utah | M1 | I |
| 6270 | Texas | M2 | II |
| 7563 | Illinois | M2 | II |
| 7635 | Illinois | M2 | II |
| 315 | Texas | M3 | I |
| 6256 | Texas | M3 | I |
| 7361 | Texas | M3 | I |
| 7595 | Illinois | M3 | I |
| 8580 | Utah | M3 | I |
| 8730 | Texas | M3 | I |
| 8514 | Texas | M4 | I |
| 8718 | Texas | M4 | I |
| 6007 | Texas | M5 | II |
| 6179 | Texas | M6 | II |
| 7572 | Illinois | M6 | II |
| 8651 | Utah | M6 | II |
| 61522 | Texas | M9 | I |
| 6166 | Texas | M12 | II |
| 6866 | Finland | M12 | II |
| 8481 | Utah | M12 | II |
| 1836 | United Kingdom | M13 | I |
| 6157 | Texas | M18 | II |
| 7558 | Illinois | M18 | II |
| 7629 | Illinois | M18 | II |
| 7788 | Utah | M18 | II |
| 8512 | Utah | M18 | II |
| 8738 | Texas | M18 | II |
| 378 | New York | M19 | II |
| 381 | New York | M19 | II |
| 8577 | Utah | M22 | I |
| 347 | New York | M24 | II |
| 1220 | New York | M24 | II |
| 1902 | New York | M24 | II |
| 1838 | United Kingdom | M27 | I |
| 6141 | Texas | M28 | II |
| 7498 | Montana | M28 | II |
| 7577 | Illinois | M28 | II |
| 8345 | Finland | M28 | II |
| 8535 | Utah | M28 | II |
| 451 | New York | M29 | II |
| 349 | New York | M30 | II |
| 1839 | New York | M30 | II |
| 427 | New York | M31 | II |
| 6183 | Texas | M41 | II |
| 6255 | Texas | M43 | II |
| 438 | New York | M46 | II |
| 1222 | New York | M46 | II |
| 8557 | Utah | M49 | I |
| 1862 | United Kingdom | M53 | II |
| 8716 | Texas | M56 | I |
| 4825 | New Zealand | M58 | I |
| 3427 | Canada | M61 | I |
| 1884 | United Kingdom | M63 | I |
| 7343 | Texas | M75 | II |
| 8071 | Utah | M75 | II |
| 58804 | Texas | M77 | II |
| 1868 | United Kingdom | M80 | I |
| 5423 | Texas | M82 | II |
| 13111 | Texas | M87 | I |
| 8517 | Utah | M89 | II |
| 56814 | Texas | M89 | II |
| 6610 | Texas | M89 | II |
| 6253 | Texas | M92 | I |
| 2105 | Texas | M114 | II |
MGAS, Musser group A Streptoccoccus culture collection.
Escherichia coli NovaBlue and BL21(DE3) (Novagen, Madison, Wis.) were used for gene cloning and protein expression, respectively. GAS strains were grown routinely in Todd-Hewitt broth (Difco Laboratories, Detroit, Mich.) supplemented with 0.2% yeast extract in the presence of 5% CO2. Brain heart infusion agar (Difco Laboratories) or tryptose agar with 5% sheep blood (Becton Dickinson, Cockeysville, Md.) was used as the solid medium.
DNA sequencing and molecular evolutionary genetic analyses.
Chromosomal DNA was isolated with a Puregene DNA isolation kit (Gentra Systems, Minneapolis, Minn.). Sequence data were obtained from both DNA strands with an Applied Biosystems 3700 automated sequencer (Applied Biosystems, Inc., Foster City, Calif.). Multiple-sequence alignment of the inferred amino acid sequences of the Mac variants was conducted with Clustal W (version 1.8) (31), and a gene tree was constructed with MEGA, version 2.1 (http://www.megasoftware.net/). The proportions of polymorphic synonymous sites (pS) and nonsynonymous sites (pN) were calculated by the method of Nei and Gojobori (23). To examine variation across the molecule, pS and pN were calculated by a sliding window analysis of 30 codons along the mac gene with the program PSWIN (25). The level of differences between pS and pN among strains belonging to the same mac complex was compared to the level of divergence between the two allele complexes by use of the method of Whittam and Nei (36). Estimates of the sampling variance of these statistics were obtained by Monte Carlo simulation or by bootstrapping.
Gene cloning and mutagenesis.
Cloning of the mac gene from serotype M1 strain MGAS5005 has been described previously (15). A gene representing the second major mac variant complex identified by comparative sequencing of 67 GAS strains also was cloned (see below). The source strain for the second mac gene was MGAS8345 (serotype M28). The mac gene was amplified by PCR with primers 5′-GTGTTCATATGGATAGTTTTTCTGCTAATCAAG-3′ and 5′-AGGATCCTTAATTGGTCTGATTCCAAC-3′. The sequence of the underlined nucleotides of the former primer was altered to introduce an NdeI restriction enzyme site and start codon. The underlined bases of the latter primer were changed to introduce a BamHI site. The PCR products were digested with NdeI and BamHI and ligated into pET21b at the same sites to obtain recombinant plasmid pSP22-2. The recombinant genes were sequenced to rule out the possibility that spurious mutations were introduced.
Residue 94 of Mac5005 was changed from cysteine to alanine by using a QuickChange XL site-directed mutagenesis kit (Stratagene, La Jolla, Calif.) and primers 5′-GAAAAGACGATCTTCTTGCAGGGGCTGCCACAGCAGG-3′ and 5′-CCTGCTGTGGCAGCCCCTGCAAGAAGATCGTCTTTTC-3′ according to the manufacturer's protocol. The entire mutant gene was sequenced to confirm the presence of the desired mutation and to rule out the possibility that spurious mutations were introduced.
Expression and purification of recombinant Mac.
Recombinant Mac5005 and Mac8345 were purified from E. coli BL21(DE3) containing plasmids pSP22 (15) and pSP22-2, respectively. Bacteria were grown for 10 h at 37°C in 6 liters of Luria-Bertani broth supplemented with 100 mg of ampicillin per liter. Cell paste (30 g) was sonicated for 20 min at 4°C in 60 ml of 5 mM Tris-HCl buffer (pH 8.0). The cell lysate was loaded onto a DEAE-Sepharose column (2.5 by 30 cm) equilibrated with the same buffer. The column was sequentially treated with 200 ml of 2.5 mM sodium phosphate and 200 ml of 7.5 mM sodium phosphate buffer (pH 7.5). Mac was identified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and peak fractions were pooled. Ammonium sulfate was added to the pooled fractions to a concentration of 1.5 M, and the resulting solution was applied to a phenyl-Sepharose column (1.5 by 10 cm), which was washed with a linear gradient of 1.5 to 0 M (NH4)2SO4 in 50 mM sodium phosphate. The recombinant protein was concentrated by precipitation with (NH4)2SO4 and dialyzed against 3 liters of 2.5 mM sodium phosphate (pH 7.4) for 20 h at 4°C. The DEAE-Sepharose chromatography and protein concentration procedures were repeated. Mac obtained by using this purification procedure was more than 98% pure as assessed by Coomassie brilliant blue staining after SDS-PAGE. The identity of the recombinant proteins was confirmed by amino-terminal amino acid sequencing and Western immunoblot analysis with specific rabbit polyclonal antiserum raised against Mac5005. The Mac5005 Cys94Ala mutant was purified by using conditions identical to those used for Mac5005 purification. Contaminating endotoxin was removed with Detoxi-gel endotoxin-removing affinity gel purchased from Pierce Chemical Co. (Rockford, Ill.).
PMN isolation and assays for ROS production, phagocytosis, and bactericidal activity.
Human PMNs were isolated from heparinized venous blood by using dextran sedimentation and Hypaque-Ficoll density gradient separation followed by hypotonic lysis of erythrocytes (4). Purified PMNs were resuspended in Dulbecco's phosphate-buffered saline containing 10 mM d-glucose (DPBS/g) or RPMI 1640 (Invitrogen) buffered with 10 mM HEPES (for phagocytosis experiments [see below]). All reagents used for preparation of PMNs contained <10.0 pg of endotoxin per ml as determined by the Limulus amebocyte lysate assay (Biowhittaker, Inc.)
Intracellular ROS production by PMNs was measured by previously described procedures (14). Briefly, PMNs (107 cells/ml) were suspended in DPBS/g containing 25 μM 2′,7′-dichlorodihydrofluorescein diacetate, equilibrated for 45 min at room temperature with gentle agitation, and chilled on ice. IgG-coated latex beads (diameter, 2.0 μm; Polysciences, Warrington, Pa.) were prepared as described previously (14). Human PMNs (5 × 106 cells) were added to wells of a chilled 96-well microtiter plate with or without Mac5005, Mac8345, control protein Spy0453, 8 × 106 IgG-coated latex beads, or buffer in a 200-μl (final volume) assay mixture. ROS production was monitored at 1-min intervals at 37°C for 90 min with a microplate fluorometer (Molecular Devices, Sunnyvale, Calif.) by using excitation and emission wavelengths of 485 and 538 nm, respectively. Vmax was defined as the highest rate of ROS production in a 5-min period.
Phagocytosis and bactericidal activity were measured as described previously (16). Strains MGAS5005 (serotype M1) and MGAS315 (serotype M3) were grown to the late exponential phase, washed with pyrogen-free phosphate-buffered saline, and suspended in DPBS/g to a concentration of 109 CFU/ml. GAS cells (25 μl) were opsonized with immune serum obtained from an individual with recent GAS-induced pharyngitis for 30 min at 37°C and chilled on ice. PMNs (2.5 × 106 cells) were combined on ice with ∼107 preopsonized GAS cells in the presence of various concentrations of Mac5005 or Mac8345, 2 μg of anti-CD11b M1/70 per ml, or 50 μg of control protein (purified streptococcal phosphoglycerate kinase) per ml as indicated below. The mixtures were rotated for 30 min at 37°C, and phagocytosis was terminated by returning the samples to ice at the end of the incubation period. Smears on microscope slides were prepared from each assay tube, and the percentage of bound or ingested GAS cells was determined by examining 250 to 350 PMNs per slide as described previously (16). PMN bactericidal activity was determined in the same experiments by plating aliquots from each assay tube on brain heart infusion agar to determine the number of viable GAS cells (16). The relative bactericidal activity, expressed as a percentage, was calculated by comparing the ability of each treatment mixture (e.g., Mac) to inhibit PMN killing of GAS with the ability of assay mixtures containing anti-CD11b to inhibit PMN killing of GAS by using the following equation, as described previously (16): [(CFU for anti-CD11b treatment − CFU for Mac treatment)/(CFU for anti-CD11b treatment − CFU for no treatment)] × 100.
The effect of the Cys94Ala mutation of Mac5005 on PMN phagocytosis was assessed by flow cytometry by using a previously described method (32), with several modifications. IgG latex beads were labeled with 0.75 μg of fluorescein isothiocyanate (FITC) (Sigma) per ml for 20 min at 37°C, and unbound label was removed with three washes in RPMI 1640 buffered with 10 mM HEPES. FITC-labeled IgG latex beads (107 beads) were mixed with 106 PMNs and Mac5005, Mac8345, the Cys94Ala Mac5005 mutant, or control protein Spy0453 at the concentrations indicated below. The samples were rotated at 37°C for 30 min. Samples were analyzed before and immediately after quenching with an equal volume of trypan blue (2 mg/ml in 0.15 M NaCl in 0.02 M citrate buffer, pH 4.4) by using a FACSCalibur flow cytometer (BD Biosciences, San Jose, Calif.). Data were analyzed with Cell Quest Pro software (BD Biosciences), and PMN phagocytosis was expressed as the percentage of PMNs containing ingested IgG latex beads.
Other assays and treatments.
Binding of Mac to human PMNs was assessed by flow cytometric analysis as described previously (16). The ability of Mac to cleave IgG was studied by incubating 20 μg of human or rabbit IgG (Sigma) with 1 μg of Mac5005 or Mac8345 in phosphate-buffered saline at 37°C for 90 min and analyzing the reaction mixture by SDS-PAGE. The presence of free sulfhydryl groups in Mac5005 and Mac8345 was measured with 5,5′-dithiobis(2-nitrobenzoic acid) in 6 M guanidine hydrochloride by using previously described procedures (27). Activation of Mac8345 was achieved by incubation with 1 mM dithiothreitol (DTT) at 4°C for 20 h. The DTT was removed by changing the buffer three times with a Nanosep centrifugal device (Pall Life Sciences, Ann Arbor, Mich.) immediately before the ROS assay. Western immunoblot analysis performed as described previously (16) was used to assess in vitro Mac production and the presence of Mac-specific antibody in patient sera. Statistical analyses were performed by using a one-way analysis of variance with Dunnett's correction for multiple comparisons (GraphPad Instat, version 3.01; GraphPad Software, San Diego, Calif.).
Mammalian cell culture and cell binding to Mac.
Previously described procedures were used to culture human kidney 293 cells, its transfected derivative cell line expressing αvβ3 (835 cells) (5, 6), Chinese hamster ovary-K1 (CHO-K1) cells, and transfected CHO-K1 cells expressing αIIbαLΔβ3 (CHO-αIIbαLΔβ3) (24). CHO-αIIbαLΔβ3 cells constitutively expressing the active heterodimeric integrin of the wild-type β3-chain and an αIIb/αLΔ chimera consisting of the extracellular and transmembrane domains of αIIb fused to an internal-deletion derivative of the cytoplasmic domain of the αL subunit have been described previously (24). Binding of the cells expressing human integrins αvβ3 and αIIbβ3 to immobilized streptococcal Mac and detection by crystal violet staining were performed as described previously (30).
Linear B-cell epitope mapping.
A total of 136 15-mer synthetic peptides representing the entire mature Mac5005 protein and the fragment of Mac8345 containing amino acid residues 99 to 218 were purchased from Chiron Technologies (San Diego, Calif.). Each sequential peptide overlapped the preceding peptide by 12 amino acids except for the last two peptides, which had an overlap of 14 amino acid residues with Mac5005. Each peptide was covalently linked at the amino terminus to biotin by a serine-glycine-serine-glycine spacer. The peptides were received dried and were reconstituted with 200 μl of 99.9% dimethyl sulfoxide (Sigma, St. Louis, Mo.). The reactivity of the peptides with rabbit anti-Mac5005 or rabbit anti-Mac8345 diluted 1:20,000 was analyzed as described previously (10).
RESULTS
mac gene and Mac protein variation and chromosomal context.
The mac gene present in strain MGAS5005 (serotype M1) consists of a 1,020-bp open reading frame that encodes a 339-amino-acid protein with an inferred molecular mass of 38,020 Da. Sequences compatible with −10, −35, and ribosomal binding sites are located immediately upstream of the ATG start codon. The inferred protein has a 29-amino-acid amino-terminal sequence with features typical of a secretion signal sequence, including a short amino-terminal hydrophilic region followed by a hydrophobic transmembrane segment and a small amino acid at the cleavage site. Cleavage of the secretion signal is predicted to occur between amino acid residues 29 (Ala) and 30 (Asp), and amino-terminal sequence data reported for GAS culture supernatant proteins indicated that this is the case (15). The predicted molecular mass of the mature protein is 34,939 Da, which is consistent with experimental results obtained by proteome analysis (15).
Molecular population genetic analysis and comparative genomics have shown that substantial allelic variation can exist in many genes in GAS (3, 26, 30). To determine the nature and extent of mac allelic variation, the gene and ∼150 bp of 5′ and 3′ flanking DNA were sequenced from 67 strains that together represent the breadth of GAS chromosomal diversity (Table 1). A total of 31 mac alleles that should encode 31 distinct Mac proteins were identified. Strains with the same M protein serotype usually had the same mac allele.
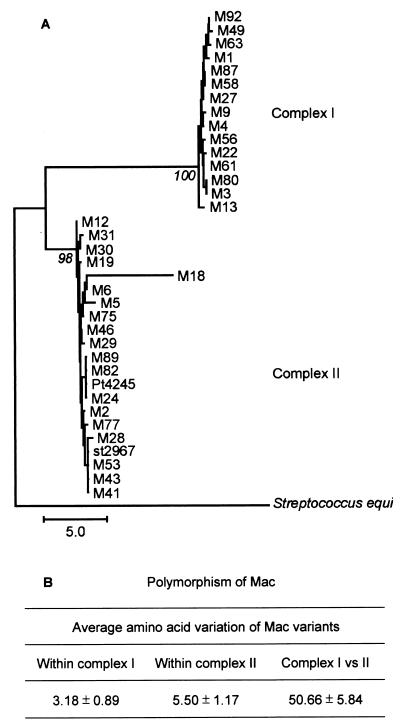
Two primary families of protein variants, designated complex I and complex II, were identified by phylogenetic analysis (21) (Fig. 1). Complex I included strains of serotypes M1, M3, M4, M9, M13, M22, M27, M49, M56, M58, M61, M63, M80, M87, and M92. Complex II included serotype M2, M5, M6, M12, M18, M19, M24, M27, M28, M29, M30, M31, M41, M43, M46, M53, M75, M77, M82, M89, and M114 GAS strains. Mac variants of each of the two complexes were closely related, differing on average by 3.18 ± 0.89 (complex I) and 5.50 ± 1.17 (complex II) amino acid residues (Fig. 1B). In contrast, complex I and complex II variants were very divergent from each other, differing at approximately one-seventh of the 340 amino acid sites (50.66 ± 5.84 amino acid differences, on average) (Fig. 1B).
FIG. 1.
(A) Phylogeny of the GAS mac gene. The phylogenetic tree was constructed with the neighbor-joining algorithm by using the nonsynonymous rate of substitution (dN), as calculated by the Nei-Gojobori method, and was rooted with the mac homologue present in S. equi. Branch lengths are expressed as the number of nonsynonymous changes (amino acid replacements) per 100 nonsynonymous sites (dN × 100). The bootstrap confidence levels, based on 1,000 replicates, are shown for the two major Mac complexes. (B) Summary of Mac polymorphism.
The great majority of the amino acid differences occurred in the middle one-third of the protein (Fig. 2). Members of complexes I and II differed in this region, on average, by 46.85 ± 4.7 amino acid residues (>50%). With the exception of the Mac variant in M18 strains, all variants had an RGD motif at amino acid residues 214 to 216 (complex I) or 216 to 218 (complex II). The serotype M18 strains had a 7-bp deletion (nucleotides 590 to 596 of mac8345) that resulted in a 220-amino-acid truncated form of Mac which lacked the RGD motif and had a divergent 24-amino-acid carboxy terminus. Serotype M4 strains had a G-to-T nucleotide change at position 943 that resulted in a truncated protein that was 15 amino acids shorter than the Mac protein made by M1 strains. Similarly, serotype M5 strains had an A-to-T mutation at nucleotide 688 that resulted in a Mac variant that was only 230 amino acids long.
FIG. 2.
(A) Alignment of amino acid sequences of Mac proteins made by strains MGAS5005 (serotype M1; Mac5005) and MGAS8345 (serotype M28; Mac8345) and an S. equi Mac homologue (Macequi). The Macequi sequence was inferred on the basis of an open reading frame present in an S. equi genome sequence (http://www.sanger.ac.uk/Projects/S_equi/). Dots represent amino acid residues that are identical in Mac5005 and Mac8345 or Macequi; dashes represent gaps. Boldface type indicates amino acid residues in the central region of the protein that are identical in Mac8345 and Macequi but differ from the residues in Mac5005. (B) Alignment of amino acid sequences of homologous regions of CD11b, Mac5005, and Mac8345, showing a lower level of homology between CD11b and Mac8345. Boldface type indicates identical amino acid residues in CD11b and Mac5005 or Mac8345.
Evolutionary genetic analysis was performed to gain insight into the forces contributing to molecular variation in the two Mac complexes. If the divergence of the two allele complexes is due simply to the accumulation of neutral mutations, the differences (pS − pN) in the mean pS and pN for nucleotide substitutions should be similar within and between complexes (36). The values for pS − pN were found to be −0.006 ± 0.005 and −0.010 ± 0.008 for members of complex I and for members of complex II, respectively. These similar values are markedly different from the difference between complexes (pN − pS = −0.146 ± 0.038), suggesting that the diversity between Mac variants of the two complexes is not due solely to the accumulation of neutral nucleotide substitutions.
The first part (∼60 amino acid residues) of the region of homology between human CD11b and Mac5005 is located in the area of Mac that varies substantially in complex I and complex II Mac variants. Consequently, the Mac variants assigned to complex II have much less amino acid identity with human CD11b than the Mac variants in complex I (Fig. 2B).
Streptococcus equi (http://www.sanger.ac.uk/Projects/S_equi/) has a Mac homologue whose amino acid sequence is 62.4 and 67.5% identical to the amino acid sequences of Mac5005 (complex I) and Mac8345 (complex II), respectively. The S. equi Mac homologue is more closely related to Mac8345 than to Mac5005 in the central variable region (Fig. 2A).
We next examined if the chromosomal region containing the mac gene was conserved in GAS. Structural features of a 12-kb region of the genome of a serotype M1 strain containing the mac gene were compared with the structural features of an analogous region present in the genomes of M3 (4), M5 (http://www.sanger.ac.uk/Projects/S_pyogenes), and M18 (29) strains and an S. equi strain (http://www.sanger.ac.uk/Projects/S_equi/). This region of the chromosome was broadly conserved in all four GAS isolates analyzed. In contrast, the chromosomal context of the mac gene in S. equi was unrelated to that of the mac gene in the GAS isolates (data not shown).
Expression and purification of recombinant Mac.
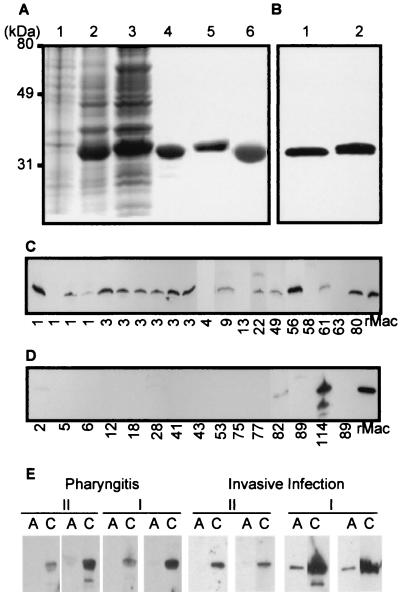
Mature Mac5005 and Mac8345 variants were overexpressed in E. coli and purified to apparent homogeneity by DEAE- and phenyl-Sepharose chromatography (Fig. 3A and B). The identity of the purified proteins was confirmed by Edman degradation of purified Mac5005 and Mac8345 (data not shown).
FIG. 3.
Purification of recombinant Mac (rMac) variants, in vitro Mac production, and anti-Mac antibody present in patient sera. (A) SDS-PAGE of a lysate of E. coli containing an empty (control) vector (lane 1), a lysate of E. coli expressing recombinant Mac5005 (lane 2), a lysate of E. coli expressing recombinant Mac8345 (lane 3), purified recombinant Mac5005 (lane 4), purified recombinant Mac8345 (lane 5), and purified Mac5005 Cys94Ala mutant (lane 6). The gel was stained with Coomassie brilliant blue. (B) Western immunoblot analysis of purified Mac5005 (lane 1) and Mac8345 (lane 2) with rabbit anti-Mac5005 antibody. (C and D) Western immunoblot analysis of Mac proteins present in culture supernatants of GAS strains possessing complex I (C) and complex II (D) mac alleles. The numbers indicate the M protein serotypes of GAS strains. The M28 isolate tested was strain MGAS8345. (E) Western immunoblot analysis of Mac5005 (I) and Mac8345 (II) proteins probed with anti-Mac5005 (I) and anti-Mac8345 (II) antibodies present in sera from patients infected with strains containing the complex I and II mac alleles, respectively. Paired acute (lanes A) and convalescent (lanes C) sera from two pharyngitis or invasive infection patients for each of two mac allele complexes were tested.
Mac production in vitro and in vivo.
To study the in vitro and in vivo production of Mac, culture supernatants of GAS strains were analyzed by Western immunoblotting with specific anti-Mac rabbit polyclonal antibody. Fifteen of twenty GAS strains containing the complex I mac alleles secreted Mac (Fig. 3C). In contrast, cultures of 13 of 15 GAS strains with the complex II mac alleles did not have detectable Mac in the culture supernatant (Fig. 3D). We previously showed that complex I Mac was made in vivo in infected mice and humans (15, 16). To test whether complex II Mac was produced in vivo during human GAS infection, the presence of Mac8345-specific antibody was assessed by Western immunoblot analysis with paired sera obtained from pharyngitis and invasive disease patients infected with GAS strains containing the complex II mac gene. Acute-phase sera had no or very low levels of Mac-specific antibody, whereas Mac-specific antibody was present in abundance in convalescent-phase sera (Fig. 3E), indicating that complex II Mac was produced in vivo during infection.
Mac8345 does not inhibit ROS production, opsonophagocytosis, and GAS killing by human PMNs.
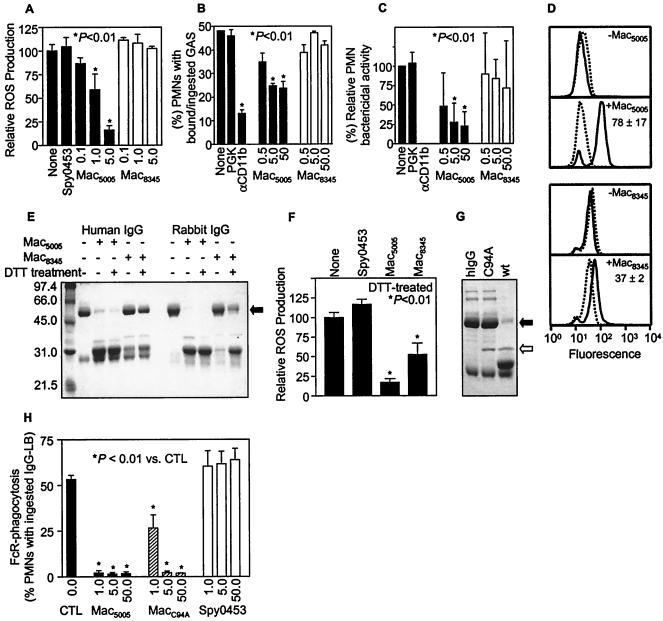
Mac5005, a representative of complex I, inhibits ROS production and opsonophagocytosis by human PMNs, resulting in significantly decreased GAS killing (16). Inasmuch as the primary structures of the Mac proteins made by members of the complexes are very different, we tested whether Mac8345, a representative of complex II, had the same biologic functions as Mac5005. In contrast to Mac5005, Mac8345 did not inhibit ROS production by human PMNs stimulated with IgG-coated latex beads (Fig. 4A). We next studied the effect of Mac8345 on opsonophagocytosis. Mac5005 significantly inhibited the association of PMNs with GAS strains opsonized with immune serum obtained from an individual with recent GAS pharyngitis, whereas Mac8345 did not have a detrimental effect on opsonophagocytosis (Fig. 4B). Consistent with the results of the ROS and opsonophagocytosis assays, GAS killing by PMNs was significantly inhibited by Mac5005 but not by Mac8345 (Fig. 4C). Taken together, the results indicate that Mac8345 does not inhibit ROS production and opsonophagocytosis and killing of GAS by human PMNs under the conditions used.
FIG. 4.
Comparison of Mac5005 and Mac8345 in terms of the ability to inhibit ROS production, phagocytosis, bacterial killing, binding to human PMNs, and IgG-endopeptidase activity. (A) Effects of Mac5005 and Mac8345 on ROS production by human PMNs. PMNs were stimulated with IgG-coated latex beads in the presence of Mac5005 or Mac8345 at different concentrations (in micrograms per milliliter) or in the presence of 5 μg of control protein Spy453 per ml. ROS production was measured for 90 min. The relative means ± standard deviations for Vmax for three triplicate experiments are shown. (B) Opsonophagocytosis of GAS. PMNs were incubated with serotype M1 or M3 GAS preopsonized with immune serum in the presence of Mac5005 or Mac8345 at different concentrations (in micrograms per milliliter), and phagocytosis was determined. Also shown are the results of assays performed without treatment or with treatment with 2 μg of antibody specific for the αM chain of human Mac-1 (αCD11b) per ml or 50 μg of recombinant GAS phosphoglycerate kinase (PGK) (purified by procedures similar to those used for Mac and used in the assays as a control protein having a similar molecular mass) per ml. (C) GAS killing. The assay conditions were identical to those used to obtain the results presented in panel B. Bactericidal activity was determined as described in the text. A statistical analysis was conducted by using a one-way analysis of variance with Dunnett's correction for multiple comparisons. The means ± standard deviations for more than five experiments are shown. (D) Flow cytometric analysis of the binding of Mac5005 and Mac8345 to human PMNs. PMNs treated with Mac (+Mac) or without Mac (−Mac) were analyzed with anti-Mac5005 or anti-Mac8345 (solid lines). The dotted lines indicate the results obtained with a control antibody. The numbers next to the lines are the means ± standard deviations for increases in fluorescence intensity in two experiments for Mac plus anti-Mac versus anti-Mac. (E) IgG-endopeptidase activity. Human or rabbit IgG was incubated with Mac5005 or Mac8345 with or without DTT treatment at 37°C for 90 min and analyzed by SDS-PAGE. For DTT treatment, Mac was incubated with 1 mM DTT at 4°C overnight and used without removal of the DTT. (F) Effect of DTT treatment of Mac on ROS production. Mac5005, Mac8345, and Spy453 were incubated with 1 mM DTT overnight, and DTT was removed immediately before the assay was conducted. The protein concentration used in the assay was 5 μg/ml. The effects on PMN ROS production of both Mac variants without DTT treatment (panel A) and with DTT treatment (panel F) were obtained in the same experiment, and DTT pretreatment did not affect the function of Mac5005. (G) Treatment of human IgG (hIgG) with purified wild-type (wt) Mac5005 or Cys94Ala Mac5005 mutant (C94A). The solid and open arrows indicate the positions of the IgG heavy chain and Mac, respectively. (H) Inhibition of IgG (FcR)-mediated PMN phagocytosis by Mac5005 and Mac5005 Cys94Ala (MacC94A) proteins. Human PMNs and FITC-labeled IgG-coated latex beads (IgG-LB) were rotated in the presence of Mac5005, Mac5005, MacC94A, or control protein Spy0453 at different concentrations (in micrograms per milliliter) at 37°C for 30 min. Phagocytosis was determined by flow cytometry as described in Materials and Methods. The results are the means ± standard deviations for three independent experiments. CTL, control.
Binding of Mac to human PMNs, Mac IgG-endopeptidase activity, and inhibition of ROS production.
It was previously reported that Mac5005 can bind to human PMNs and that binding is blocked by an anti-CD16 MAb (16). To test the ability of Mac8345 to bind to human PMNs, flow cytometry was used. Binding of Mac8345 to PMNs was detectable, but it was significantly less than the binding of Mac5005 to PMNs (Fig. 4D).
During our studies to elucidate the mechanism that Mac5005 uses to inhibit antibody binding to PMNs, ROS production, and opsonophagocytosis, Mac5005 was found to cleave the heavy chain of human IgG and rabbit IgG (Fig. 4E). This finding is consistent with the recent report that the protein has cysteine protease activity (33). We found that Mac8345 had detectable but much lower IgG-endopeptidase activity than Mac5005 under the assay conditions used (Fig. 4E). Mature Mac5005 and Mac8345 have one and two cysteine residues, respectively. To test whether there was a difference in the oxidation state of the cysteine residues in Mac5005 and Mac8345, the presence of free sulfhydryl groups in Mac proteins was measured with Ellman's agent under denaturing conditions (27). About 25% of recombinant Mac5005 had a free sulfhydryl group, whereas no free sulfhydryl group was detected in recombinant Mac8345, suggesting that Mac5005 is more resistant to oxidation than Mac8345. To test whether oxidation of the sulfhydryl group altered endopeptidase activity, the proteins were incubated with DTT overnight to reduce the oxidized cysteine side chain. DTT-treated Mac8345 had enhanced IgG-endopeptidase activity but remained less efficient than Mac5005 in terms of the ability to cleave IgG (Fig. 4E). We also noted that DTT-treated Mac8345 inhibited ROS production by human PMNs stimulated with IgG-coated latex beads, albeit not to the extent that Mac5005 inhibited ROS production (Fig. 4F).
Mac5005 Cys94Ala site-specific mutant protein lacking IgG-endopeptidase activity inhibits IgG-mediated PMN phagocytosis.
To determine if the sole cysteine residue of Mac5005 (Cys94) contributes to endopeptidase activity, this amino acid was replaced with alanine by site-directed mutagenesis. The Mac5005 Cys94Ala mutant protein was expressed in E. coli and purified to homogeneity (Fig. 3A). The purified mutant did not cleave human IgG, suggesting that the Cys94 residue is required for IgG-endopeptidase activity (Fig. 4G). To assess the effect of the Cys94Ala mutation of Mac5005 on Mac's key biological function, the mutant was tested for the ability to inhibit IgG-mediated phagocytosis by human PMNs. The mutant protein significantly inhibited phagocytosis at all concentrations tested (Fig. 4H). Importantly, there was no significant difference in the degree of blocking of phagocytosis at a concentration of either 5 or 50 μg of protein per ml between the wild-type and mutant proteins (Fig. 4H), demonstrating that the IgG-endopeptidase activity of Mac is not essential for its key biological function.
Binding of Mac to integrins αvβ3 and αIIbβ3 expressed on the surface of transfected cells.
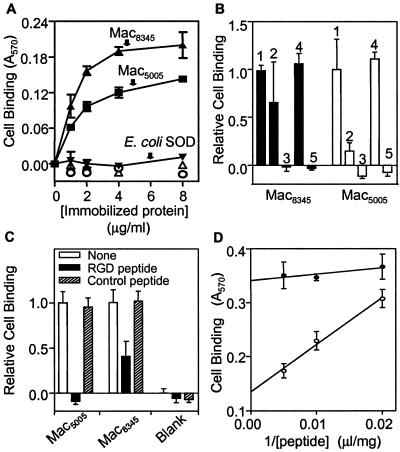
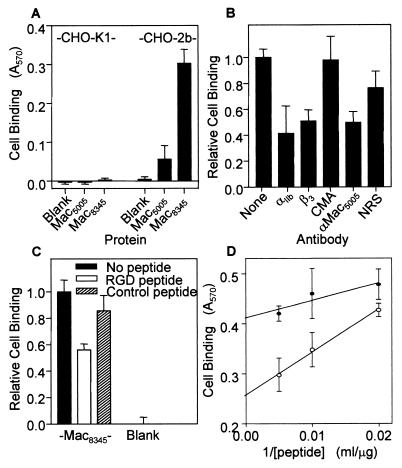
Inasmuch as virtually all Mac variants identified have an RGD motif, we tested the hypothesis that purified Mac5005 and Mac8345 bound to human integrins αvβ3 and αIIbβ3. Binding of Mac5005 and Mac8345 to these integrins was studied with transfected cells and immobilized purified Mac by using procedures described previously (30). Parent 293 cells that do not express these human integrins did not bind to Mac5005 and Mac8345. In contrast, transfected derivative 835 cells expressing integrin αvβ3 on the cell surface bound to these Mac proteins in a dose-dependent manner (Fig. 5A). Mac8345 bound significantly more transfected cells than Mac5005 bound, suggesting that Mac8345 has a higher affinity for integrin αvβ3 than Mac5005 has. The binding of the 835 cells to Mac5005 or Mac8345 was completely eliminated by anti-αvβ3 MAb but was not affected detrimentally by a control mouse ascites. Rabbit polyclonal anti-Mac5005 antibody inhibited 85% of the cell binding to Mac5005 but had significantly less inhibitory effect on the cell binding to Mac8345 (Fig. 5B).
FIG. 5.
Binding of Mac proteins to integrin αvβ3 expressed on the surface of human kidney 293 cells. Cell binding was determined as described in Materials and Methods. Mac proteins in wells were coated with 50 μl of a solution containing 2 μg of protein per ml or a different concentration. (A) Binding by transformed cells expressing integrin αvβ3 (835 cells) to immobilized Mac5005 (▴), Mac8345 (▪), or a negative control protein (E. coli superoxide dismutase [SOD]) (▾) as a function of the coating protein concentration. Insignificant binding of parent 293 cells to Mac5005 (▵) and Mac8345 (○) is also shown. The mean binding values ± standard deviations for a representative quadruplicate experiment are shown. (B) Inhibition of 835 cell binding to Mac by anti-αvβ3 MAb and polyclonal rabbit anti-Mac5005. In the inhibition assay with anti-αvβ3 MAb, 835 cells were incubated with control mouse ascites or ascites containing anti-αvβ3 MAb diluted 1:500 at 37°C for 30 min before binding to Mac. In the inhibition assay with anti-Mac5005 antibody, antibody diluted 1:500 was incubated with coated Mac at room temperature for 1 h before cell binding. Bars 1, no antibody; bars 2, anti-Mac5005; bars 3, anti-αvβ3; bars 4, control mouse ascites; bars 5, no coated protein. (C) Inhibition of 835 cell binding to Mac by a peptide encompassing the RGD motif. 835 cells were incubated with 200 μg of peptide VFTRGDQSK per ml or scrambled control peptide TVRQSDFGK at 37°C for 30 min and then added to microtiter wells coated with Mac5005 or Mac8345 for cell binding. (D) 835 cell binding to immobilized Mac8345 as a function of the reciprocal of the concentration of the RGD (○) or control (•) peptide. The means ± standard deviations for three quadruplicate experiments are shown in panels B to D.
We next used synthetic peptides to test the hypothesis that the RGD motif present in streptococcal Mac was involved in the binding of the proteins to 835 cells. A synthetic peptide (VFTRGDQSK) corresponding to residues 211 to 219 of Mac5005 completely blocked cell binding to Mac5005. In contrast, this peptide (corresponding to amino acid residues 213 to 221 of Mac8345) only partially blocked the binding of Mac8345 to 835 cells (Fig. 5C). The scrambled peptide control (TVRQSDFGK) did not detrimentally affect the binding of either Mac variant to the integrin-expressing cells. Importantly, binding of the integrin-expressing cells to Mac8345 was not blocked completely when the VFTRGDQSK peptide was present at the highest concentration used (Fig. 5D). Taken together, these results indicate that the binding of Mac to human integrin αvβ3 is primarily mediated by the RGD motif. In addition, a second region(s) of Mac8345 that is divergent from Mac5005 (either in primary amino acid sequence or in conformation) contributes to the interaction of Mac8345 with the αvβ3 integrin.
Similar experiments were performed to test the hypothesis that Mac proteins bound to integrin αIIbβ3. Parental (untransfected) CHO-K1 cells did not bind to either Mac protein, whereas transfected CHO-K1 cells expressing the αIIbαLΔβ3 integrin (CHO-αIIbαLΔβ3 cells) bound to Mac8345 very well. In contrast, the binding of integrin-expressing cells to Mac5005 was significantly less than the binding to Mac8345 (Fig. 6A). Cell binding to Mac8345 was inhibited significantly by antibodies directed against Mac5005 and integrin subunits αIIb and β3 (Fig. 6B). As observed in the αvβ3 integrin experiments, the VFTRGDQSK peptide only partially blocked binding of Mac8345 to the CHO-αIIbαLΔβ3 cells (Fig. 6C) and did not completely block cell binding when it was present at high concentrations (Fig. 6D). Taken together, these findings demonstrate that there is a difference in the binding specificities of Mac5005 and Mac8345 with integrins, suggesting that the Mac variants are structurally and topologically distinct.
FIG. 6.
Binding of Mac8345 to integrin αIIb/αLΔβ3 expressed on the surface of CHO-K1 cells. Cell binding was determined by using the conditions described in the legend to Fig. 5 except that microtiter wells were coated with 50 μl of a solution containing 8 μg of protein per ml. (A) Binding of parent CHO-K1 cells or transformed CHO-K1 cells (CHO-2b) expressing integrin αIIb/αLΔβ3 to immobilized Mac proteins. The mean binding values ± standard deviations for a representative quadruplicate experiment are shown. (B) Effects of anti-Mac5005, anti-αIIb, and anti-β3 antibodies on binding of CHO-2b cells to immobilized Mac8345. Control mouse ascites (CMA) and normal rabbit serum (NRS) were also included as controls. The means ± standard deviations for three quadruplicate experiments are shown. (C) Inhibitory effect of the RGD peptide on binding of CHO-2b cells to immobilized Mac8345. The means ± standard deviations for two quadruplicate experiments are shown. (D) Binding of CHO-2b cells to immobilized Mac8345 as a function of the reciprocal of the concentration of the RGD (○) or control (•) peptide.
Probing protein surface properties of Mac5005 and Mac8345 by linear B-cell epitope mapping.
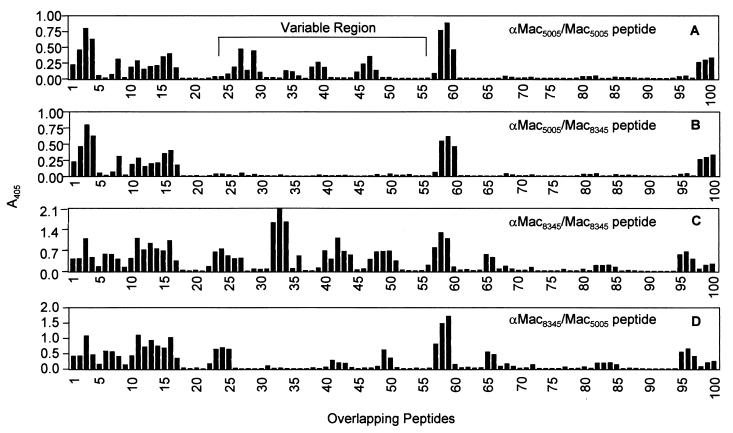
To test the hypothesis that the two Mac variants differed in their surface properties, linear B-cell epitope mapping was used. Affinity-purified rabbit antibodies specific for each Mac variant were analyzed by an enzyme-linked immunosorbent assay to determine reactivity with overlapping peptides representing the full length of mature Mac5005 and Mac8345. Antibodies recognizing four epitopes of Mac5005 present in the variable region (middle one-third of the molecule) did not react with the peptides corresponding to this region of Mac8345 (Fig. 7A and B). Similarly, anti-Mac8345 antibody also recognized four epitopes present in the central part of Mac8345. However, the positions of the epitopes were shifted such that three of them had no or diminished reactivity with the Mac5005 peptides corresponding to the analogous region of Mac8345 (Fig. 7C and D). The fourth epitope (corresponding to peptides 23 to 27) was located in a relatively conserved region of Mac (Fig. 7C and D). We inferred from these results that Mac5005 and Mac8345 differ in surface topology and structure and that the differences are attributable to amino acid divergence in the middle one-third of the molecule.
FIG. 7.
Distinct surface properties of Mac5005 and Mac8345 identified by linear B-cell epitope mapping. An enzyme-linked immunosorbent assay was used to measure the reactivities of affinity-purified rabbit polyclonal anti-Mac5005 (αMac5005) and anti-Mac8345 (αMac8345) antibodies with a library of overlapping peptides covering the entire mature Mac5005 and Mac8345 proteins. Reactivity (A405) is shown as a function of peptide. (A) Reactivity of anti-Mac5005 with Mac5005 peptides; (B) reactivity of anti-Mac5005 with Mac8345 peptides; (C) reactivity of anti-Mac8345 with Mac8345 peptides; (D) reactivity of anti-Mac8345 with Mac5005 peptides.
DISCUSSION
Mac polymorphism and recombination.
Comparative DNA sequencing analysis of the mac gene identified two complexes of Mac variants made by strains representing 37 different M protein serotypes. Mac variants assigned to the same complex are closely related, whereas Mac variants assigned to different complexes differ considerably in the middle one-third of the Mac protein. Some GAS strains with divergent genomic backgrounds, as determined by multilocus enzyme electrophoresis (21), had nearly identical mac gene sequences. Moreover, the differences between Mac variants assigned to the two distinct complexes cannot be explained merely by the accumulation of neutral nucleotide substitutions. The most likely interpretation of all the molecular population genetic data is that recombination contributed to the divergence of mac alleles, resulting in two complexes of Mac variants. We noted that the S. equi Mac homologue is more closely related to complex II Mac variants than to complex I Mac variants, especially in the central variable region, which is consistent with the idea that complex I Mac variants evolved from the complex II variants.
IgG-endopeptidase activity and Mac function.
Recently, it was reported that Mac5005 inhibited opsonophagocytosis and killing of GAS by human PMNs. The protein blocked IgG binding to the Fc receptor FcγRIII (CD16) present on the surface of PMNs and eliminated ROS production in response to stimulation with IgG- or IgG/C3bi-coated latex beads (16). In the course of the present follow-up study, we discovered that Mac had IgG-endopeptidase activity, which is consistent with data recently published by von Pawel-Rammingen et al. (33). These investigators reported that Mac (which they designated IdeS, for IgG-degrading enzyme of Streptococcus pyogenes) exhibits protease activity against IgG and proposed that the IgG-endopeptidase activity is necessary for inhibiting PMN phagocytosis (33). In contrast to this hypothesis, our results demonstrate that the ability of Mac5005 to block IgG-mediated phagocytosis is distinct from IgG-endopeptidase activity. The Mac5005 Cys94Ala mutant protein, which lacks IgG-endopeptidase activity, retained the ability to block phagocytosis at all concentrations tested (Fig. 4G), demonstrating that the IgG-endopeptidase activity of Mac is not crucial for the key biological function of Mac.
Role of Cys94 in Mac endopeptidase activity.
We found that autooxidation of a cysteine residue in Mac inactivated its endopeptidase activity. These results could be interpreted to mean either that Mac is a cysteine protease (a cysteine residue is involved in catalysis) or that a cysteine residue is present at or close to the active site but is not directly involved in catalysis. Vibrio harveyi luciferase is an example of the latter possibility; in this organism chemical modification of a cysteine residue eliminated activity, but site-specific mutation of a cysteine to an alanine residue did not (37). The Cys94Ala Mac5005 mutant lacks endopeptidase activity, which is consistent with the idea that the cysteine residue is a catalytically active residue, an idea put forth on the basis of biochemical data (33). However, given that streptococcal Mac protein lacks significant homology to known cysteine proteases, it is important to probe this issue in more detail.
In vitro and in vivo Mac expression.
The difference in in vitro Mac production associated with the two complex variants could be due either to processes such as altered transcription, translation, and/or secretion or to enhanced degradation of complex II variants compared to degradation of complex I variants. Although in vitro Mac production was not detected in most of the strains containing the complex II mac alleles, importantly Mac8345-specific antibody was present in convalescent-phase sera obtained from patients with invasive infections and pharyngitis caused by strains containing complex II mac alleles. This observation indicates that some isolates with complex II Mac alleles are capable of producing Mac in vivo during infection episodes and suggests that there are fundamental differences between GAS strains in terms of the mechanism(s) regulating Mac production. Although relatively little is known about regulation of Mac production, Lei et al. (16) showed that expression of Mac by a representative serotype M1 strain was influenced by the covR-covS two-component gene regulatory system.
Apparent surface differences between complex I and II Mac variants.
The middle one-third of the amino acid residues of complex I Mac and complex II Mac differ by approximately 50%. Linear B-cell epitope mapping suggested that the differences result in differences in the surface-exposed regions of Mac5005 and Mac8345. These differences may provide an important clue to explain the functional differences between Mac5005 and Mac8345 which we observed. For example, we found that Mac8345 has a higher affinity than Mac5005 for human integrins αvβ3 and αIIbβ3. In addition, binding of Mac5005 to integrin αvβ3 was mediated by the RGD motif, whereas the interaction of Mac8345 with integrins αvβ3 and αIIbβ3 was mediated by the RGD motif plus additional amino acid residues. The ability of Mac to interact with proteins other than IgG suggests that the endopeptidase activity of Mac may not be limited to IgG. Moreover, the functional differences between complex I and II Mac molecules which we observed emphasize the importance of understanding the contribution of allelic variation to host-pathogen interactions (26).
Editor: D. L. Burns
REFERENCES
- 1.Akesson, P., A. G. Sjoholm, and L. Bjorck. 1996. Protein SIC, a novel extracellular protein of Streptococcus pyogenes interfering with complement function. J. Biol. Chem. 271:1081-1088. [DOI] [PubMed] [Google Scholar]
- 2.Beall, B., R. Facklam, T. Hoenes, and B. Schwartz. 1997. Survey of emm gene sequences and T-antigen types from systemic Streptococcus pyogenes infection isolates collected in San Francisco, California; Atlanta, Georgia; and Connecticut in 1994 and 1995. J. Clin. Microbiol. 35:1231-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beres, S. B., G. L. Sylva, K. D. Barbian, B. Lei, J. S. Hoff, N. D. Mammarella, M. Liu, J. C. Smoot, S. F. Porcella, L. D. Parkins, J. K. McCormick, D. Y. M. Leung, P. M. Schlievert, and J. M. Musser. 2002. Genome sequence of a serotype M3 strain of group A Streptococcus: phage-encoded toxins, the high-virulence phenotype, and clone emergence. Proc. Natl. Acad. Sci. USA 99:10078-10083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyum, A. 1968. Isolation of mononuclear cells and granulocytes from human blood. J. Clin. Lab. Investig. 21:77-89. [PubMed] [Google Scholar]
- 5.Chuntharapai, A., S. Bodary, M. Horton, and K. J. Kim. 1993. Blocking monoclonal antibodies to alpha V beta 3 integrin: a unique epitope of alpha V beta 3 integrin is present on human osteoclasts. Exp. Cell Res. 205:345-352. [DOI] [PubMed] [Google Scholar]
- 6.Coburn, J., L. Magoun, S. C. Bodary, and J. M. Leong. 1998. Integrins αvβ3 and α5β1 mediate attachment of Lyme disease spirochetes to human cells. Infect. Immun. 66:1946-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corbi, A. L., T. K. Kishimoto, L. J. Miller, and T. A. Springer. 1988. The human leukocyte adhesion glycoprotein Mac-1 (complement receptor type 3, CD11b) α subunit. Cloning, primary structure, and relation to the integrins, von Willebrand factor and factor B. J. Biol. Chem. 263:12403-12411. [PubMed] [Google Scholar]
- 8.Cunningham, M. W. 2000. Pathogenesis of group A streptococcal infections. Clin. Microbiol. Rev. 13:470-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernie-King, B. A., D. J. Seilly, C. Willers, R. Wurzner, A. Davies, and P. J. Lachmann. 2001. Streptococcal inhibitor of complement (SIC) inhibits the membrane attack complex by preventing uptake of C567 onto cell membranes. Immunology 103:390-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoe, N. P., P. Kordari, R. Cole, M. Liu, T. Palzkill, W. Huang, D. McLellan, G. J. Adams, M. Hu, J. Vuopio-Varkila, T. R. Cate, M. E. Pichichero, K. M. Edwards, J. Eskola, D. E. Low, and J. M. Musser. 2000. Human immune response to streptococcal inhibitor of complement, a serotype M1 group A Streptococcus extracellular protein involved in epidemics. J. Infect. Dis. 18:1425-1436. [DOI] [PubMed] [Google Scholar]
- 11.Horstmann, R. D., H. J. Sievertsen, J. Knobloch, and V. A. Fischetti. 1988. Antiphagocytic activity of streptococcal M protein: selective binding of complement control protein factor H. Proc. Natl. Acad. Sci. USA 85:1657-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horstmann, R. D., H. J. Sievertsen, M. Leippe, and V. A. Fischetti. 1992. Role of fibrinogen in complement inhibition by streptococcal M protein. Infect. Immun. 60:5036-5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ji, Y., L. McLandsborough, A. Kondagunta, and P. P. Cleary. 1996. C5a peptidase alters clearance and trafficking of group A streptococci by infected mice. Infect. Immun. 64:503-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobayashi, S. D., J. M. Voyich, C. L. Buhl, R. M. Stahl, and F. R. DeLeo. 2002. Global changes in gene expression by human polymorphonuclear leukocytes during receptor-mediated phagocytosis: cell fate is regulated at the level of gene expression. Proc. Natl. Acad. Sci. USA 99:6901-6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lei, B., S. Mackie, S. Lukomski, and J. M. Musser. 2000. Identification and immunogenicity of group A Streptococcus culture supernatant proteins. Infect. Immun. 68:6807-6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lei, B., F. R. DeLeo, N. P. Hoe, M. R. Graham, S. M. Mackie, R. L. Cole, M. Liu, H. R. Hill, D. E. Low, M. J. Federle, J. R. Scott, and J. M. Musser. 2001. Evasion of human innate and acquired immunity by a bacterial homologue of CD11b that inhibits opsonophagocytosis. Nat. Med. 7:1298-1305. [DOI] [PubMed] [Google Scholar]
- 17.Levin, J. C., and M. R. Wessels. 1998. Identification of csrR/csrS, a genetic locus that regulates hyaluronic acid capsule synthesis in group A Streptococcus. Mol. Microbiol. 30:209-219. [DOI] [PubMed] [Google Scholar]
- 18.Lukomski, S., N. P. Hoe, I. Abdi, J. Rurangirwa, P. Kordari, M. Liu, S. J. Dou, G. G. Adams, and J. M. Musser. 2000. Nonpolar inactivation of the hypervariable streptococcal inhibitor of complement gene (sic) in serotype M1 Streptococcus pyogenes significantly decreases mouse mucosal colonization. Infect. Immun. 68:535-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCormick, J. K., J. M. Yarwood, and P. M. Schlievert. 2001. Toxic shock syndrome and bacterial superantigens: an update. Annu. Rev. Microbiol. 55:77-104. [DOI] [PubMed] [Google Scholar]
- 20.Musser, J. M., A. R. Hauser, M. H. Kim, P. M. Schlievert, K. Nelson, and R. K. Selander. 1991. Streptococcus pyogenes causing toxic-shock-like syndrome and other invasive diseases: clonal diversity and pyrogenic exotoxin expression. Proc. Natl. Acad. Sci. USA 88:2668-2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Musser, J. M., V. Kapur, J. Szeto, X. Pan, D. Swanson, and D. Martin. 1995. Genetic diversity and relationships among Streptococcus pyogenes strains expressing serotype M1 protein: recent intercontinental spread of a subclone causing episodes of invasive disease. Infect. Immun. 63:994-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Musser, J. M., and R. M. Krause. 1998. The revival of group A streptococcal diseases with a commentary on staphylococcal toxic shock syndrome, p. 185-218. In R. M. Krause and A. Fauci (ed.), Emerging infections. Academic Press, San Diego, Calif.
- 23.Nei, M., and T. Gojobori. 1986. Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol. Biol. Evol. 3:418-426. [DOI] [PubMed] [Google Scholar]
- 24.O'Toole, T. E., Y. Katagari, R. J. Faull, K., Peter, R. Tamura, V. Quaranta, J. C. Loftus, S. J. Shattil, and M. H. Ginsberg. 1994. Integrin cytoplasmic domains mediate inside-out signal transduction. J. Cell Biol. 124:1047-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reid, S. D., R. K. Selander, and T. S. Whittam. 1999. Sequence diversity of flagellin (fliC) alleles in pathogenic Escherichia coli. J. Bacteriol. 181:153-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reid, S. D., N. P. Hoe, L. M. Smoot, and J. M. Musser. 2001. Group A Streptococcus: allelic variation, population genetics, and host-pathogen interactions. J. Clin. Investig. 107:393-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riddles, P. W., R. L. Blakeley, and B. Zerner. 1983. Reassessment of Ellman's reagent. Methods Enzymol. 91:49-60. [DOI] [PubMed] [Google Scholar]
- 28.Sharkawy, A., D. E. Low, R. Saginur, D. Gregson, B. Schwartz, P. Jessamine, K. Green, and A. McGeer. 2002. Severe group A streptococcal soft-tissue infections in Ontario: 1992-1996. Clin. Infect. Dis. 34:454-460. [DOI] [PubMed] [Google Scholar]
- 29.Smoot, J. C., K. D. Barbian, J. J. Van Gompel, L. M. Smoot, M. S. Chaussee, G. L. Sylva, D. E. Sturdevant, S. M. Mackie, S. F. Porcella, L. D. Parkins, S. B. Beres, D. S. Campell, T. M. Smith, Q. Zhang, V. Kapur, J. A. Daly, L. G. Veasy, and J. M. Musser. 2002. Genome sequence and comparative microarray analysis of serotype M18 group A Streptococcus strains associated with acute rheumatic fever outbreaks. Proc. Natl. Acad. Sci. USA 99:4668-4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stockbauer, K. E., L. Magoun, M. Liu, E. H. Burns, Jr., S. Gubba, S. Renish, X. Pan, S. C. Bodary, E. Baker, J. Coburn, J. M. Leong, and J. M. Musser. 1999. A natural variant of the cysteine protease virulence factor of group A Streptococcus with an arginine-glycine-aspartic acid (RGD) motif preferentially binds human integrins αvβ3 and αIIbβ3. Proc. Natl. Acad. Sci. USA 96:242-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Eeden, S. F., M. E. Klut, B. Walker, and J. C. Hogg. 1999. The use of flow cytometry to measure neutrophil function. J. Immunol. Methods 242:23-43. [DOI] [PubMed] [Google Scholar]
- 33.von Pawel-Rammingen, U., B. P. Johansson, and L. Björck. 2002. IdeS, a novel streptococcal cysteine proteinase with unique specificity for immunoglobulin G. EMBO J. 21:1607-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wexler, D. E., D. E. Chenoweth, and P. P. Cleary. 1985. Mechanism of action of the group A streptococcal C5a inactivator. Proc. Natl. Acad. Sci. USA 82:8144-8148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whitnack, E., and E. H. Beachey. 1982. Antiopsonic activity of fibrinogen bound to M protein on the surface of group A streptococci. J. Clin. Investig. 69:1042-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whittam, T. S., and M. Nei. 1991. Neutral mutation hypothesis test. Nature 354:114-116. [DOI] [PubMed] [Google Scholar]
- 37.Xi, L., K. W. Cho, M. E. Herndon, and S.-C. Tu. 1990. Elicitation of an oxidase activity in bacterial luciferase by site-directed mutation of a noncatalytic residue. J. Biol. Chem. 265:4200-4203. [PubMed] [Google Scholar]