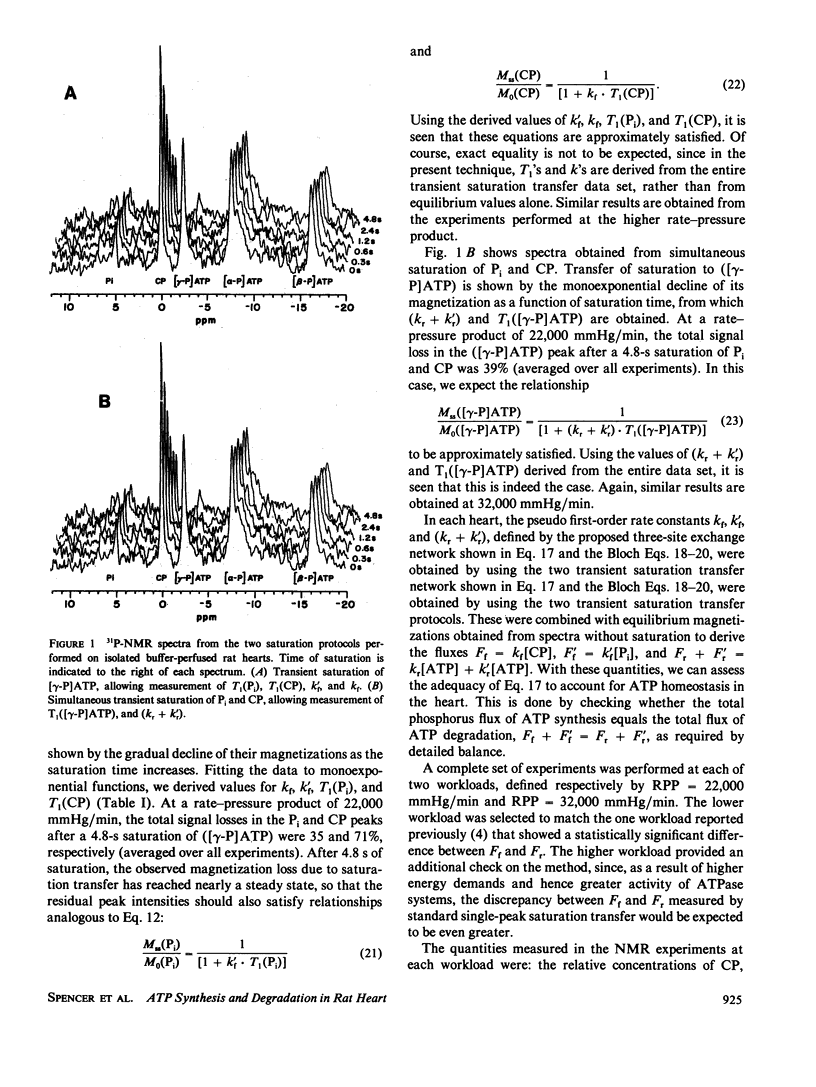
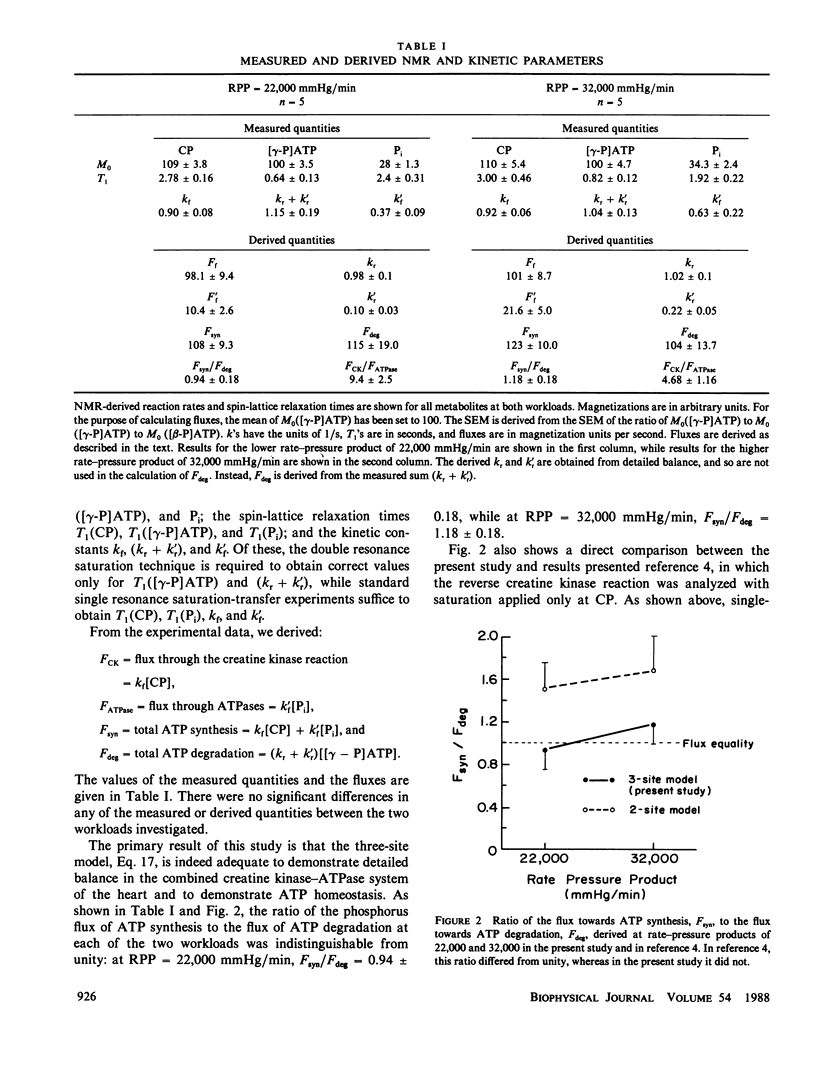
Abstract
A limitation of magnetization transfer techniques for studying enzyme kinetics in vivo has been the difficulty of treating systems with more than two exchanging species. This problem was addressed in the original papers describing saturation transfer. Since then, a number of approaches have been devised to study these complex situations. Here, we present a method based on the transient saturation transfer experiment in which spin-lattice relaxation time constants and reaction rates are obtained from the same magnetization transfer data. This technique is particularly suitable for biological samples. We apply the method to evaluate flux balance in the three-site linear exchange network composed of ATP, creatine phosphate, and inorganic phosphate in the isolated, perfused rat heart and show that the method yields reasonable values for the reaction velocities of ATP synthesis and degradation.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bittl J. A., Ingwall J. S. Reaction rates of creatine kinase and ATP synthesis in the isolated rat heart. A 31P NMR magnetization transfer study. J Biol Chem. 1985 Mar 25;260(6):3512–3517. [PubMed] [Google Scholar]
- Ingwall J. S. Phosphorus nuclear magnetic resonance spectroscopy of cardiac and skeletal muscles. Am J Physiol. 1982 May;242(5):H729–H744. doi: 10.1152/ajpheart.1982.242.5.H729. [DOI] [PubMed] [Google Scholar]
- Matthews P. M., Bland J. L., Gadian D. G., Radda G. K. A 31P-NMR saturation transfer study of the regulation of creatine kinase in the rat heart. Biochim Biophys Acta. 1982 Nov 17;721(3):312–320. doi: 10.1016/0167-4889(82)90084-2. [DOI] [PubMed] [Google Scholar]
- Nunnally R. L., Hollis D. P. Adenosine triphosphate compartmentation in living hearts: a phosphorus nuclear magnetic resonance saturation transfer study. Biochemistry. 1979 Aug 7;18(16):3642–3646. doi: 10.1021/bi00583a032. [DOI] [PubMed] [Google Scholar]
- Uğurbil K., Petein M., Maidan R., Michurski S., From A. H. Measurement of an individual rate constant in the presence of multiple exchanges: application to myocardial creatine kinase reaction. Biochemistry. 1986 Jan 14;25(1):100–107. doi: 10.1021/bi00349a015. [DOI] [PubMed] [Google Scholar]
- Zahler R., Bittl J. A., Ingwall J. S. Analysis of compartmentation of ATP in skeletal and cardiac muscle using 31P nuclear magnetic resonance saturation transfer. Biophys J. 1987 Jun;51(6):883–893. doi: 10.1016/S0006-3495(87)83416-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


