Abstract
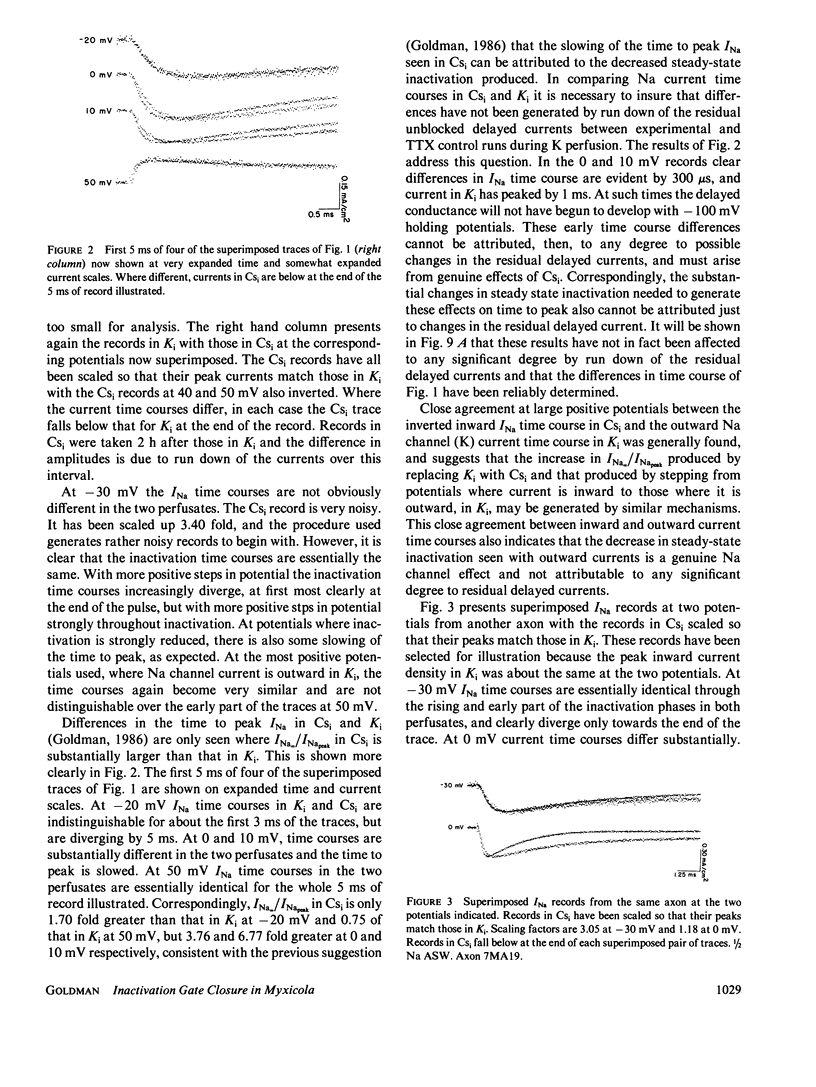
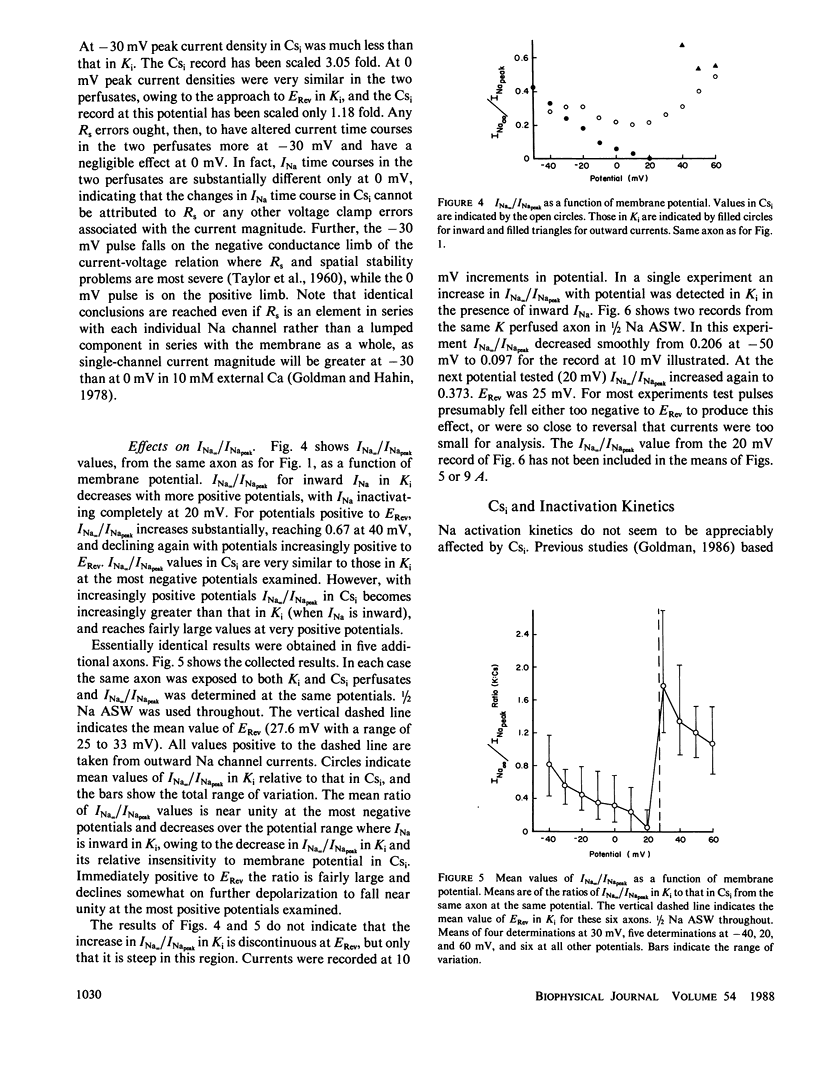
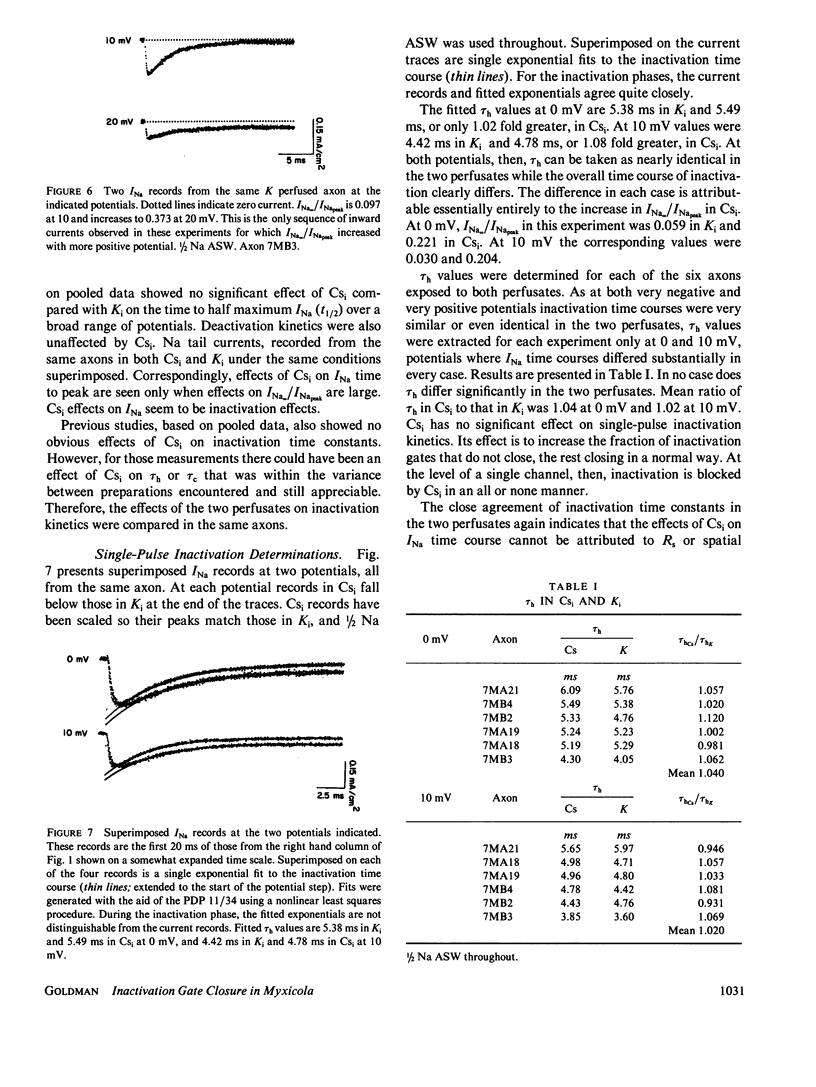
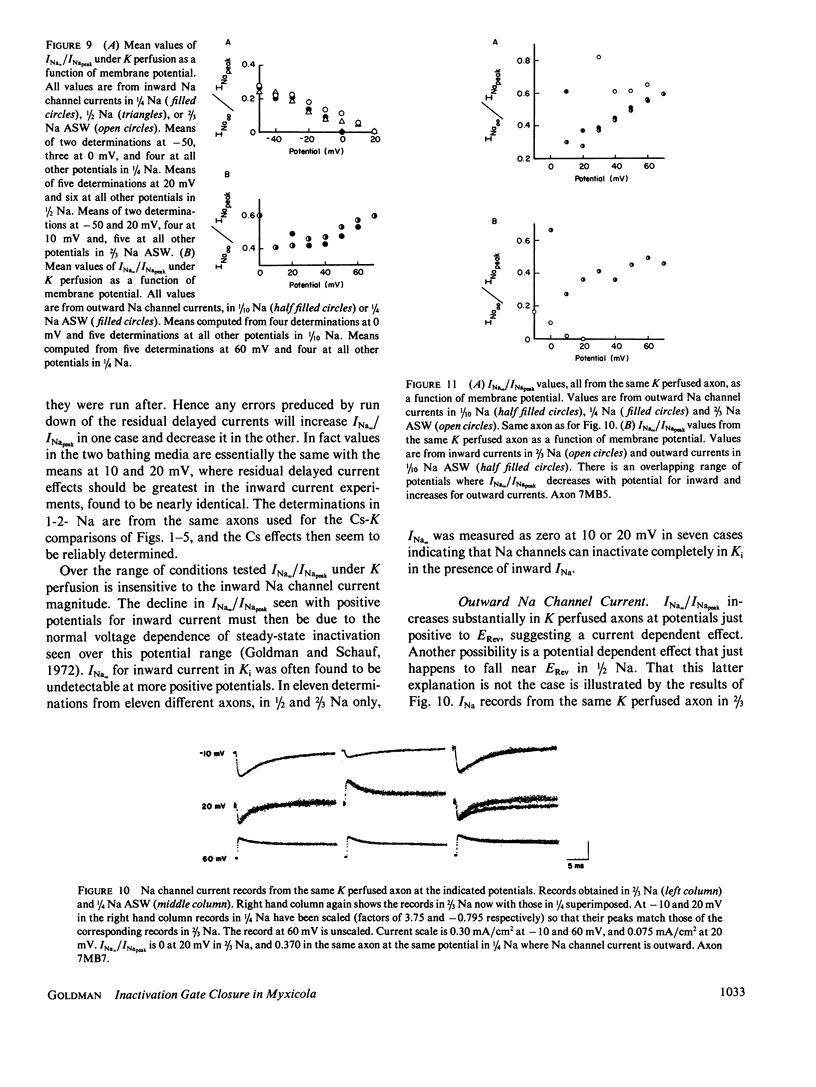
Steady state to peak Na current ratio (INa,/INapeak) in Myxicola is greater, under some conditions, in internal Cs than in K, indicating less steady state inactivation in Csi. Csi effects are selective for steady state inactivation, with negligible effects on single-pulse inactivation time constants (Th). Mean Th ratios (Csi to Ki) were 1.04 and 1.02 at 0 and 10 mV. Two pulse inactivation time constants were also little affected. Inactivation is blocked in an all or none manner. Ki has little effect on steady state inactivation in the presence of inward INa, with INa/INapeak often declining to zero at positive potentials and independent of external Na concentration from 1/4 to 2/3 artificial sea water (ASW). Cs also has little effect at more negative potentials, but more with either more positive potentials or Na reduction, both reducing inward INa. K effects are evident when Na channel current is outward. A site in the current pathway when occupied selectively blocks inactivation gate closure. As occupancy does not depend significantly on potential, the site must not be very deep into the membrane field. Inactivation gates may associate with these sites on closure. The inactivated state may consist of a positively-charged structure occluding the inner channel mouth.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman W. J., Jr, Senft J. P. Voltage clamp studies on the effect of internal cesium ion on sodium and potassium currents in the squid giant axon. J Gen Physiol. 1966 Nov;50(2):279–293. doi: 10.1085/jgp.50.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldrich R. W., Stevens C. F. Inactivation of open and closed sodium channels determined separately. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 1):147–153. doi: 10.1101/sqb.1983.048.01.017. [DOI] [PubMed] [Google Scholar]
- Aldrich R. W., Stevens C. F. Voltage-dependent gating of single sodium channels from mammalian neuroblastoma cells. J Neurosci. 1987 Feb;7(2):418–431. doi: 10.1523/JNEUROSCI.07-02-00418.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M., Bezanilla F. Inactivation of the sodium channel. II. Gating current experiments. J Gen Physiol. 1977 Nov;70(5):567–590. doi: 10.1085/jgp.70.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M., Bezanilla F., Rojas E. Destruction of sodium conductance inactivation in squid axons perfused with pronase. J Gen Physiol. 1973 Oct;62(4):375–391. doi: 10.1085/jgp.62.4.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P. Sodium channel inactivation in the crayfish giant axon. Must channels open before inactivating? Biophys J. 1981 Sep;35(3):595–614. doi: 10.1016/S0006-3495(81)84815-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binstock L., Goldman L. Current- and voltage-clamped studies on Myxicola giant axons. Effect of tetrodotoxin. J Gen Physiol. 1969 Dec;54(6):730–740. doi: 10.1085/jgp.54.6.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binstock L., Goldman L. Rectification in instantaneous potassium current-voltage relations in Myxicola giant axons. J Physiol. 1971 Sep;217(3):517–531. doi: 10.1113/jphysiol.1971.sp009583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Meves H. Evidence for two types of sodium conductance in axons perfused with sodium fluoride solution. J Physiol. 1970 Dec;211(3):653–678. doi: 10.1113/jphysiol.1970.sp009298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Meves H. Rate constants associated with changes in sodium conductance in axons perfused with sodium fluoride. J Physiol. 1970 Dec;211(3):679–705. doi: 10.1113/jphysiol.1970.sp009299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Meves H. Sodium and potassium currents in squid axons perfused with fluoride solutions. J Physiol. 1970 Dec;211(3):623–652. doi: 10.1113/jphysiol.1970.sp009297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danko M., Smith-Maxwell C., McKinney L., Begenisich T. Block of sodium channels by internal mono- and divalent guanidinium analogues. Modulation by sodium ion concentration. Biophys J. 1986 Feb;49(2):509–519. doi: 10.1016/S0006-3495(86)83661-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert G. A., Goldman L. Internal perfusion of the myxicola giant axon. Biophys J. 2009 Jan 1;15(5):495–499. doi: 10.1016/S0006-3495(75)85833-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert G. A., Goldman L. The permeability of the sodium channel in Myxicola to the alkali cations. J Gen Physiol. 1976 Sep;68(3):327–340. doi: 10.1085/jgp.68.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The after-effects of impulses in the giant nerve fibres of Loligo. J Physiol. 1956 Feb 28;131(2):341–376. doi: 10.1113/jphysiol.1956.sp005467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. I., Meves H. The time course of sodium inactivation in squid giant axons. J Physiol. 1980 Feb;299:289–307. doi: 10.1113/jphysiol.1980.sp013125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman L., Chandler R. E. Geographical distribution and inactivation kinetics in internally perfused Myxicola giant axons. Biophys J. 1986 Mar;49(3):761–766. doi: 10.1016/S0006-3495(86)83702-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman L., Hahin R. Initial conditions and the kinetics of the sodium conductance in Myxicola giant axons. II. Relaxation experiments. J Gen Physiol. 1978 Dec;72(6):879–898. doi: 10.1085/jgp.72.6.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman L. Internal cesium and the sodium inactivation gate in Myxicola giant axons. Biophys J. 1986 Aug;50(2):231–238. doi: 10.1016/S0006-3495(86)83457-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman L., Kenyon J. L. Delays in inactivation development and activation kinetics in myxicola giant axons. J Gen Physiol. 1982 Jul;80(1):83–102. doi: 10.1085/jgp.80.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman L., Kenyon J. L. Internally perfused Myxicola giant axons showing long-term survival. Biophys J. 1979 Nov;28(2):357–361. doi: 10.1016/S0006-3495(79)85182-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman L., Schauf C. L. Inactivation of the sodium current in Myxicola giant axons. Evidence for coupling to the activation process. J Gen Physiol. 1972 Jun;59(6):659–675. doi: 10.1085/jgp.59.6.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ionic selectivity, saturation, and block in sodium channels. A four-barrier model. J Gen Physiol. 1975 Nov;66(5):535–560. doi: 10.1085/jgp.66.5.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R., Patlak J., Stevens C. F. Sodium channels need not open before they inactivate. Nature. 1981 Jun 4;291(5814):426–427. doi: 10.1038/291426a0. [DOI] [PubMed] [Google Scholar]
- Horn R., Vandenberg C. A. Statistical properties of single sodium channels. J Gen Physiol. 1984 Oct;84(4):505–534. doi: 10.1085/jgp.84.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxford G. S., Wu C. H., Narahashi T. Removal of sodium channel inactivation in squid giant axons by n-bromoacetamide. J Gen Physiol. 1978 Mar;71(3):227–247. doi: 10.1085/jgp.71.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxford G. S., Yeh J. Z. Interactions of monovalent cations with sodium channels in squid axon. I. Modification of physiological inactivation gating. J Gen Physiol. 1985 Apr;85(4):583–602. doi: 10.1085/jgp.85.4.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B. Inactivation in Myxicola giant axons responsible for slow and accumulative adaptation phenomena. J Physiol. 1981 Mar;312:531–549. doi: 10.1113/jphysiol.1981.sp013642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauf C. L., Bullock J. O. Internal cesium alters sodium inactivation in Myxicola. Biophys J. 1978 Sep;23(3):473–477. doi: 10.1016/S0006-3495(78)85463-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauf C. L., Bullock J. O. Modifications of sodium channel gating in Myxicola giant axons by deuterium oxide, temperature, and internal cations. Biophys J. 1979 Aug;27(2):193–208. doi: 10.1016/S0006-3495(79)85211-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauf C. L. Tetramethylammonium ions alter sodium-channel gating in Myxicola. Biophys J. 1983 Mar;41(3):269–274. doi: 10.1016/S0006-3495(83)84437-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR R. E., MOORE J. W., COLE K. S. Analysis of certain errors in squid axon voltage clamp measurements. Biophys J. 1960 Nov;1:161–202. doi: 10.1016/s0006-3495(60)86882-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg C. A., Horn R. Inactivation viewed through single sodium channels. J Gen Physiol. 1984 Oct;84(4):535–564. doi: 10.1085/jgp.84.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto D., Yeh J. Z., Narahashi T. Interactions of permeant cations with sodium channels of squid axon membranes. Biophys J. 1985 Sep;48(3):361–368. doi: 10.1016/S0006-3495(85)83792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


