Abstract
The Pseudomonas aeruginosa exotoxin A (PEA) protein requires furin-mediated cleavage for manifestation of toxicity. We show here that the small stable furin inhibitor hexa-d-arginine amide effectively blocks PEA-induced cell lysis and is itself noncytotoxic. Administration of hexa-d-arginine to PEA-treated mice significantly improves their survival rate and also decreases circulating levels of tumor necrosis factor alpha.
Furin, a member of the family of proprotein convertases, which activate precursor proteins by cleavage following basic residue consensus sequences, has been implicated in many physiological and pathological processes (for reviews see references 11 and 12). The finding that furin is required for the activation of a large number of bacterial toxins has led to the idea that inhibition of this enzyme may represent a viable approach to controlling bacterial infection. Our laboratory has developed a stable, small-molecule furin inhibitor, hexa-d-arginine amide (1) (D6R), which represents a potentially therapeutically useful molecule in this regard.
Pseudomonas aeruginosa produces a large number of toxic extracellular products; one such product is Pseudomonas exotoxin A (PEA). PEA requires intracellular proteolytic cleavage to generate a 37-kDa C-terminal fragment that translocates to the cytosol and ADP-ribosylates elongation factor 2, thereby causing cell death. Furin is involved in two separate protein processing pathways that each potentially contribute to the sensitivity of cells to PEA—the quantity of the toxin receptor expressed on target cells and the activation of PEA (2-7, 10). Inhibition of PEA cytotoxicity in A7 melanoma cells could be effected by exogenous application of the furin inhibitor alpha-1-PDX, an engineered derivative of alpha-1 antitrypsin, with 50% inhibition between 2 and 5 μM alpha-1-PDX (8), showing that furin inhibition represents a useful avenue for attenuation of toxicity.
In the study presented here, we have tested the therapeutic potential of D6R against P. aeruginosa exotoxin, both in cell culture and in live animals.
D6R inhibits the cytotoxicity of PEA in CHO cells.
CHO cells were treated with various concentrations (1 to 100 μM) of D6R (synthesized by Louisiana State University Health Sciences Center Core Laboratories) dissolved in phosphate-buffered saline and were observed under the microscope for normal morphology and growth. Even at concentrations up to 100 μM, D6R did not exhibit any apparent toxic effects (data not shown). While the amide form of D6R was used for most of these studies, the free hydroxyl form (D6R-OH) was also tested in vitro and in vivo without detectable differences in potency.
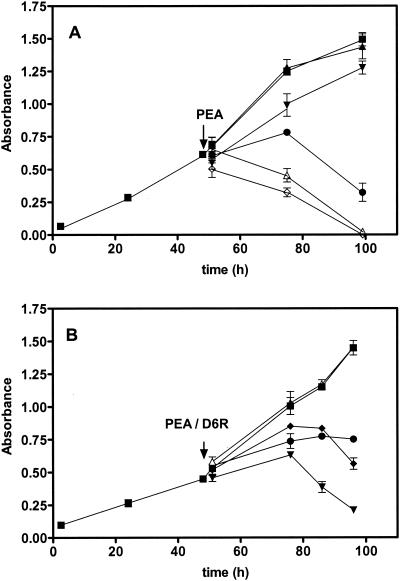
The determination of the 50% inhibitory concentration for PEA was performed with CHO cells at a concentration of 5 × 102 cells/well in 96-well plates (Fig. 1A). The cells were treated with the doses of PEA shown in Fig. 1, and cell growth was monitored with the compound WST-1 (Roche Diagnostics). This dye will reflect the activity of mitochondrial dehydrogenase present in living cells; the difference in the absorbances at 450 and 630 nm was measured 1 h after addition to cells. The 50% inhibitory concentration for PEA-mediated cell death, as assessed 24 h after application of PEA, was 10 ng/ml, and this concentration was employed in subsequent experiments. In Fig. 1B, the effects of inclusion of D6R with PEA at various concentrations on PEA-induced cytotoxicity are shown; significant protective effects were observed, especially at the highest dose (10 μM).
FIG. 1.
D6R protects against the cytotoxic effects of PEA in vitro. (A) Cytotoxicity of PEA. CHO cells were treated with various concentrations of PEA (0 to 100 ng/ml) 48 h after seeding. Filled squares, untreated cells; filled triangles, 0 ng of PEA/ml; filled inverted triangles, 1 ng/ml; filled circles, 10 ng of PEA/ml; open triangles, 100 ng of PEA/ml; open diamonds, 1,000 ng of PEA/ml. The experiment was repeated four times with consistent results, and the results depicted represent the means ± standard deviations of all four experiments. (B) Protection against PEA intoxication by D6R. Cells were treated with 10 ng of PEA/ml and simultaneously with either 0, 1, or 10 μM D6R. Cytotoxic effects were measured at the incubation times shown. Filled squares, untreated cells; filled triangles, no PEA and 10 μM D6R; filled inverted triangles, PEA in the absence of D6R; filled circles, PEA in the presence of 10 μM D6R; filled diamonds, PEA in the presence of 1 μM D6R.
D6R blocks PEA intoxication in vivo.
We found that administration of D6R was also not toxic in vivo. Six-week-old FVB and 129/Sv mice, 10 animals per group, were treated with either 0.1, 1, or 10 nmol of D6R (with 100 μl of 1, 10, or 100 μM D6R dissolved in physiological saline), either intraperitoneally, intravenously, or subcutaneously. For intravenous administration of D6R, animals were first anesthetized with Avertin; intravenous administration was performed through the jugular vein. The control groups were injected with 100 μl of physiological saline by the same routes. After administration of peptide, animals were observed for 1 month for their behavior as well as for food and water intake. D6R-injected mice did not exhibit any adverse physiological signs or aberrant behaviors compared to the control groups; FVB mice given up to 1 nmol of D6R (3.8 mg/kg of body weight) did not show any apparent signs of toxicosis. D6R also did not induce an increase in the production of tumor necrosis factor alpha (TNF-α) in treated animals compared with control groups, indicating that D6R administration itself does not provoke an inflammatory response. It should, however, be noted that no tissue microhistology was performed; thus, it is possible that D6R could produce undetected organ damage.
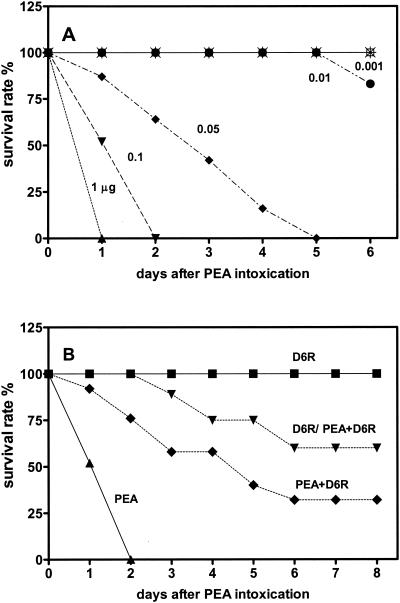
In vivo experiments showed that an intraperitoneal injection of 0.1 μg of PEA/mouse to 6-week-old FVB mice resulted in complete mortality within 2 days (Fig. 2A). D6R was then administered to groups of mice receiving this dose of PEA. One group of mice was treated with a single dose of 1 nmol of D6R intraperitoneally per day for 2 days prior to intoxication with 0.1 μg of PEA; this procedure resulted in significant protection from survival (50% survival at 7 days after PEA injection [Fig. 2B]). A second group was treated with both toxin and D6R simultaneously; this protocol resulted in 25% survival, representing a significantly better survival rate than that for the group not given D6R but worse than that for the group pretreated with D6R.
FIG. 2.
D6R protects against PEA intoxication in vivo. (A) Dose-response curve to PEA. FVB mice (10 animals per group) were given different doses of PEA by intraperitoneal injection, and mortality was assessed 2 days after administration; the 100% lethal dose for PEA this time was found to be 0.1 μg/mouse. Filled triangles, 1 μg of PEA/mouse; filled inverted triangles, 0.1 μg of PEA/mouse; filled diamonds, 0.05 μg of PEA/mouse; filled circles, 0.01 μg of PEA/mouse; crossed open squares, 0.001 μg of PEA/mouse. Results are presented as the survival rate (percentage of animals surviving 6 days after PEA administration). (B) Effect of D6R administration on PEA-induced mortality. Groups of FVB mice (10 animals per group) were given 0.1 μg of PEA/mouse (filled triangles). One group of mice was administered 1 nmol of D6R (approximately 3.8 mg/kg) intraperitoneally immediately after intoxication (filled diamonds). A second group of mice was treated similarly but was also administered 1 nmol of D6R 48 and 24 h prior to intoxication with PEA (filled inverted triangles). As a control, one group of mice was given only 1 nmol of D6R (no exposure to PEA; filled squares). Results are presented as the survival rate (percentage of animals surviving at 8 days).
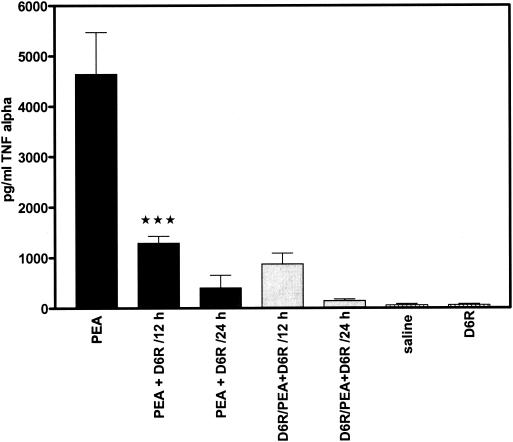
Sepsis is often a fatal condition; excessive production of host inflammatory mediators including cytokines is considered to contribute to its lethality. For example, in murine sepsis due to P. aeruginosa circulating TNF-α, interleukin-1 (IL-1), IL-6, and IL-10 are significantly increased (9). Schumann et al. (13) demonstrated that PEA is responsible for enhancing the production of TNF-α in P. aeruginosa bacteremia in mice. The same protocol for PEA administration as described above for toxicity studies was used to measure levels of TNF-α in plasma, a measure of physiological response to toxin. Trunk blood was collected from all groups at 12 and 24 h after intoxication with PEA, and TNF-α (CytElisa; ALPCO Diagnostics) levels in plasma were measured by a sensitive immunoassay. Treatment of mice with D6R alone did not result in elevated production of TNF-α in plasma (59 ± 22 pg/ml [Fig. 3 ]). As expected, mice given 0.1 μg of PEA exhibited a significant increase in TNF-α levels in plasma (4,640 ± 830 pg/ml) compared with the control group injected with physiological saline. However, in the PEA-treated group given D6R, decreased TNF-α levels were observed 12 h after intoxication (1,290 ± 140 pg/ml; P < 0.0001). Twenty-four hours after intoxication there was a further decrease in circulating TNF-α compared with the group given toxin but no D6R (403 ± 250 pg/ml). Pretreatment of mice with D6R 48 h prior to intoxication resulted in a slight but significant decrease in TNF-α production at both 12 and 24 h following intoxication (Fig. 3), indicating a lesser degree of cytokine response to the same dose of toxin.
FIG. 3.
Production of TNF-α increases during PEA-induced toxicosis but is reduced by treatment with D6R. Levels of TNF-α in plasma were measured 12 and 24 h after PEA-induced toxicosis; separate groups of mice were treated with either 0.9% NaCl or 1 nmol of D6R. PEA, mice treated with PEA alone, with plasma TNF-α levels measured 12 h after PEA administration; PEA + D6R/12 h, animals treated with PEA and D6R at the same time, with plasma TNF-α levels measured 12 h after PEA administration; PEA + D6R/24 h, animals treated with PEA and D6R, with plasma TNF-α levels measured 24 h after PEA administration; D6R/PEA+D6R/12 h and D6R/PEA+D6R/24 h, animals pretreated with D6R 48 h prior to administration of PEA. Triple star, P < 0.0001.
D6R as a potentially useful therapeutic.
The data presented above show that D6R is able to attenuate PEA toxicity both in cell culture and in live animals without itself evoking a cytokine response. D6R appears to exhibit other potentially promising attributes of a therapeutically useful furin inhibitor in being able to cross cell membranes (data not shown), being small enough to achieve useful therapeutic concentrations, and exhibiting no apparent cytotoxicity.
Furin has been implicated in the proteolytic activation of many bacterial toxins in addition to PEA; these include diphtheria toxin, Shiga toxin, proaerolysin, anthrax toxin, and clostridium toxins (reviewed in references 5 and 11). Excitingly, preliminary data also show that D6R is effective against the proteolytic activation of the anthrax protective antigen protein (M. S. Sarac, S. H. Leppla, and I. Lindberg, unpublished results), an event occurring at the cell surface (5).
D6R thus presents a reasonable starting molecule for the further development of stable small-molecule furin inhibitors capable of inhibiting pathophysiological processes in vivo.
Acknowledgments
We thank Joelle Finley for assistance with cell culture.
This study was supported by NIH grant NIDA grant DA05084; I. Lindberg was supported by grant DA00204.
Editor: J. T. Barbieri
REFERENCES
- 1.Cameron, A., J. Appel, R. A. Houghten, and I. Lindberg. 2000. Polyarginines are potent furin inhibitors. J. Biol. Chem. 275:36741-36749. [DOI] [PubMed] [Google Scholar]
- 2.Chiron, M., C. Fryling, and D. FitzGerald. 1997. Furin-mediated cleavage of Pseudomonas exotoxin-derived chimeric toxins. J. Biol. Chem. 272:31707-31711. [DOI] [PubMed] [Google Scholar]
- 3.Friedman, T. C., V. M. Gordon, S. H. Leppla, K. R. Klimpel, N. P. Birch, and Y. P. Loh. 1995. In vitro processing of anthrax toxin protective antigen by recombinant PC1 (SPC3) and bovine intermediate lobe secretory vesicle membranes. Arch. Biochem. Biophys. 316:5-13. [DOI] [PubMed] [Google Scholar]
- 4.Gordon, V. M., K. R. Klimpel, N. Arora, M. A. Henderson, and S. H. Leppla. 1995. Proteolytic activation of bacterial toxins by eukaryotic cells is performed by furin and by additional cellular proteases. Infect. Immun. 63:82-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gordon, V. M., and S. H. Leppla. 1994. Proteolytic activation of bacterial toxins: role of bacterial and host proteases. Infect. Immun. 62:333-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gu, M., V. Gordon, D. Fitzgerald, and S. Leppla. 1996. Furin regulates both the activation of Pseudomonas exotoxin A and the quantity of the toxin receptor expressed on target cells. Infect. Immun. 64:524-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inocencio, N. M., J. M. Moehring, and T. J. Moehring. 1994. Furin activates Pseudomonas exotoxin A by specific cleavage in vivo and in vitro. J. Biol. Chem. 269:31831-31835. [PubMed] [Google Scholar]
- 8.Jean, F., K. Stella, L. Thomas, G. Liu, Y. Xiang, A. J. Reason, and G. Thomas. 1998. α1-Antitrypsin Portland, a bioengineered serpin highly selective for furin: application as an antipathogenic agent. Proc. Natl. Acad. Sci. USA 95:7293-7298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsumoto, T., K. Tateda, S. Miyazaki, N. Furuya, A. Ohno, Y. Ishii, Y. Hirakata, and K. Yamaguchi. 1999. Paradoxical synergistic effects of tumor necrosis factor and interleukin 1 in murine gut-derived sepsis with Pseudomonas aeruginosa. Cytokine 11:366-372. [DOI] [PubMed] [Google Scholar]
- 10.Moehring, J. M., N. M. Inocencio, B. J. Robertson, and T. J. Moehring. 1993. Expression of mouse furin in a Chinese hamster cell resistant to Pseudomonas exotoxin A and viruses complements the genetic lesion. J. Biol. Chem. 268:2590-2594. [PubMed] [Google Scholar]
- 11.Molloy, S. S., E. D. Anderson, F. Jean, and G. Thomas. 1999. Bi-cycling the furin pathway: from TGN localization to pathogen activation and embryogenesis. Trends Cell Biol. 9:28-35. [DOI] [PubMed] [Google Scholar]
- 12.Nakayama, K. 1997. Furin: a mammalian subtilisin/Kex2p-like endoprotease involved in processing of a wide variety of precursor proteins. Biochem. J. 327:625-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schumann, J., S. Angermuller, R. Bang, M. Lohoff, and G. Tiegs. 1998. Acute hepatotoxicity of Pseudomonas aeruginosa exotoxin A in mice depends on T cells and TNF. J. Immunol. 161:5745-5754. [PubMed] [Google Scholar]