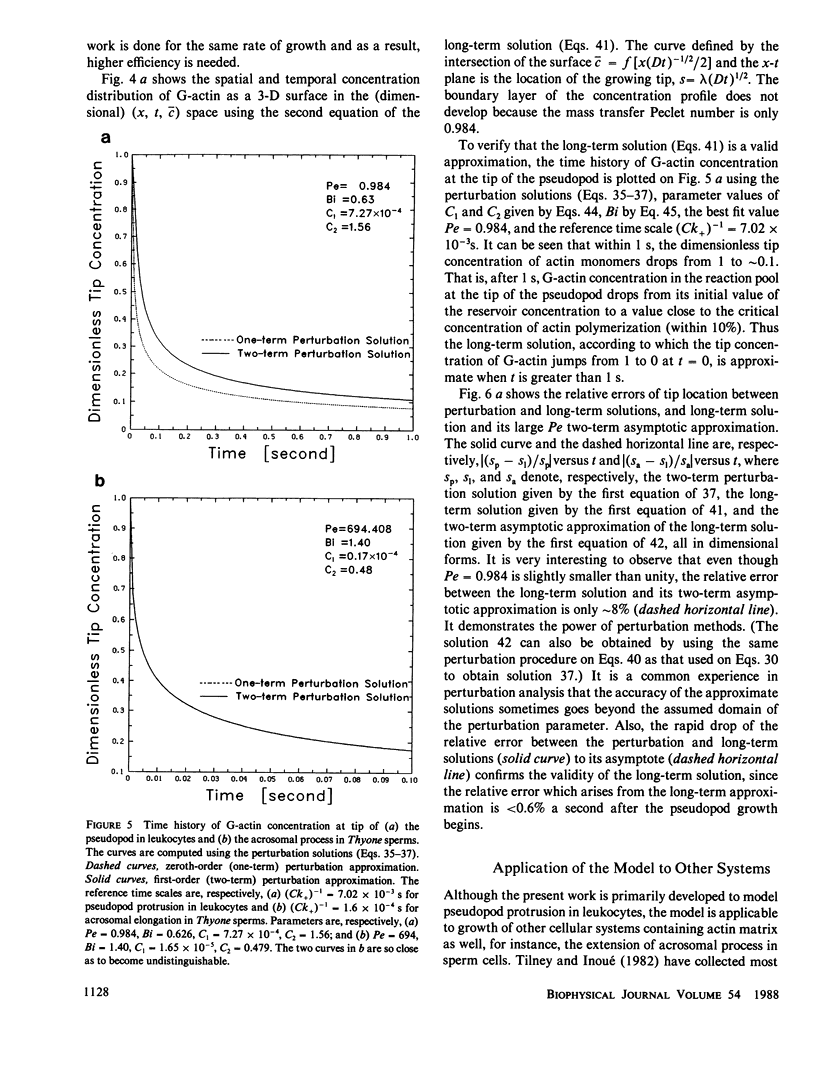
Abstract
The morphology of human leukocytes, the biochemistry of actin polymerization, and the theory of continuum mechanics are used to model the pseudopod protrusion process of leukocytes. In the proposed model, the pseudopod is considered as a porous solid of F-actin network, the pores of which are full of aqueous solution. G-actin is considered as a "solute" transported by convection and diffusion in the fluid phase. The pseudopod grows as actin filaments elongate at their barbed ends at the tip of the pseudopod. The driving force of extension is hypothesized as being provided by the actin polymerization. It is assumed that elongation of actin filaments, powered by chemical energy liberated from the polymerization reaction, does mechanical work against opposing pressure on the membrane. This also gives rise to a pressure drop in the fluid phase at the tip of the pseudopod, which is formulated by an equation relating the work done by actin polymerization to the local state of pressure. The pressure gradient along the pseudopod drives the fluid filtration through the porous pseudopod according to Darcy's Law, which in turn brings more actin monomers to the growing tip. The main cell body serves as a reservoir of G-actin. A modified first-order equation is used to describe the kinetics of polymerization. The rate of pseudopod growth is modulated by regulatory proteins. A one-dimensional moving boundary problem based on the proposed mechanism has been constructed and approximate solutions have been obtained. Comparison of the solutions with experimental data shows that the model is compatible with available observations. The model is also applicable to growth of other cellular systems such as elongation of acrosomal process in sperm cells.
Full text
PDF





















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebi U., Fowler W. E., Isenberg G., Pollard T. D., Smith P. R. Crystalline actin sheets: their structure and polymorphism. J Cell Biol. 1981 Nov;91(2 Pt 1):340–351. doi: 10.1083/jcb.91.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson M., Weber A., Zigmond S. H. An actin-nucleating activity in polymorphonuclear leukocytes is modulated by chemotactic peptides. J Cell Biol. 1986 Dec;103(6 Pt 2):2707–2714. doi: 10.1083/jcb.103.6.2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Blum J. D., Pollard T. D. Acanthamoeba castellanii capping protein: properties, mechanism of action, immunologic cross-reactivity, and localization. J Cell Biol. 1984 Jul;99(1 Pt 1):217–225. doi: 10.1083/jcb.99.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry F. E. Determinants of capillary permeability: a review of mechanisms based on single capillary studies in the frog. Circ Res. 1986 Oct;59(4):367–380. doi: 10.1161/01.res.59.4.367. [DOI] [PubMed] [Google Scholar]
- Dembo M., Harlow F. Cell motion, contractile networks, and the physics of interpenetrating reactive flow. Biophys J. 1986 Jul;50(1):109–121. doi: 10.1016/S0006-3495(86)83444-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dembo M. The mechanics of motility in dissociated cytoplasm. Biophys J. 1986 Dec;50(6):1165–1183. doi: 10.1016/S0006-3495(86)83560-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detmers P. A., Goodenough U. W., Condeelis J. Elongation of the fertilization tubule in Chlamydomonas: new observations on the core microfilaments and the effect of transient intracellular signals on their structural integrity. J Cell Biol. 1983 Aug;97(2):522–532. doi: 10.1083/jcb.97.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C., Skalak R., Sung K. L., Schmid-Schönbein G. W., Chien S. Passive deformation analysis of human leukocytes. J Biomech Eng. 1988 Feb;110(1):27–36. doi: 10.1115/1.3108402. [DOI] [PubMed] [Google Scholar]
- Frieden C. Actin and tubulin polymerization: the use of kinetic methods to determine mechanism. Annu Rev Biophys Biophys Chem. 1985;14:189–210. doi: 10.1146/annurev.bb.14.060185.001201. [DOI] [PubMed] [Google Scholar]
- Gershon N. D., Porter K. R., Trus B. L. The cytoplasmic matrix: its volume and surface area and the diffusion of molecules through it. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5030–5034. doi: 10.1073/pnas.82.15.5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Kaulfus P., Weber K. F actin assembly modulated by villin: Ca++-dependent nucleation and capping of the barbed end. Cell. 1981 May;24(2):471–480. doi: 10.1016/0092-8674(81)90338-x. [DOI] [PubMed] [Google Scholar]
- Gordon D. J., Yang Y. Z., Korn E. D. Polymerization of Acanthamoeba actin. Kinetics, thermodynamics, and co-polymerization with muscle actin. J Biol Chem. 1976 Dec 10;251(23):7474–7479. [PubMed] [Google Scholar]
- Hartwig J. H., Shevlin P. The architecture of actin filaments and the ultrastructural location of actin-binding protein in the periphery of lung macrophages. J Cell Biol. 1986 Sep;103(3):1007–1020. doi: 10.1083/jcb.103.3.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. L. Microfilament or microtubule assembly or disassembly against a force. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5613–5617. doi: 10.1073/pnas.78.9.5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson K., Wojcieszyn J. The translational mobility of substances within the cytoplasmic matrix. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6747–6751. doi: 10.1073/pnas.81.21.6747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janmey P. A., Chaponnier C., Lind S. E., Zaner K. S., Stossel T. P., Yin H. L. Interactions of gelsolin and gelsolin-actin complexes with actin. Effects of calcium on actin nucleation, filament severing, and end blocking. Biochemistry. 1985 Jul 2;24(14):3714–3723. doi: 10.1021/bi00335a046. [DOI] [PubMed] [Google Scholar]
- Korn E. D. Actin polymerization and its regulation by proteins from nonmuscle cells. Physiol Rev. 1982 Apr;62(2):672–737. doi: 10.1152/physrev.1982.62.2.672. [DOI] [PubMed] [Google Scholar]
- Kreis T. E., Geiger B., Schlessinger J. Mobility of microinjected rhodamine actin within living chicken gizzard cells determined by fluorescence photobleaching recovery. Cell. 1982 Jul;29(3):835–845. doi: 10.1016/0092-8674(82)90445-7. [DOI] [PubMed] [Google Scholar]
- Lal A. A., Korn E. D. Reinvestigation of the inhibition of actin polymerization by profilin. J Biol Chem. 1985 Aug 25;260(18):10132–10138. [PubMed] [Google Scholar]
- Lanir Y. Biorheology and fluid flux in swelling tissues, II. Analysis of unconfined compressive response of transversely isotropic cartilage disc. Biorheology. 1987;24(2):189–205. doi: 10.3233/bir-1987-24211. [DOI] [PubMed] [Google Scholar]
- Lanir Y. Biorheology and fluid flux in swelling tissues. I. Bicomponent theory for small deformations, including concentration effects. Biorheology. 1987;24(2):173–187. doi: 10.3233/bir-1987-24210. [DOI] [PubMed] [Google Scholar]
- Lanni F., Taylor D. L., Ware B. R. Fluorescence photobleaching recovery in solutions of labeled actin. Biophys J. 1981 Aug;35(2):351–364. doi: 10.1016/S0006-3495(81)84794-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanni F., Ware B. R. Detection and characterization of actin monomers, oligomers, and filaments in solution by measurement of fluorescence photobleaching recovery. Biophys J. 1984 Jul;46(1):97–110. doi: 10.1016/S0006-3495(84)84002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassing I., Lindberg U. Specific interaction between phosphatidylinositol 4,5-bisphosphate and profilactin. Nature. 1985 Apr 4;314(6010):472–474. doi: 10.1038/314472a0. [DOI] [PubMed] [Google Scholar]
- Luby-Phelps K., Taylor D. L., Lanni F. Probing the structure of cytoplasm. J Cell Biol. 1986 Jun;102(6):2015–2022. doi: 10.1083/jcb.102.6.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIHASHI K. MOLECULAR CHARACTERISTICS OF G-ADP ACTIN. Arch Biochem Biophys. 1964 Sep;107:441–448. doi: 10.1016/0003-9861(64)90300-5. [DOI] [PubMed] [Google Scholar]
- Montague C., Rhee K. W., Carlson F. D. Measurement of the translational diffusion constant of G-actin by photon correlation spectroscopy. J Muscle Res Cell Motil. 1983 Feb;4(1):95–101. doi: 10.1007/BF00711960. [DOI] [PubMed] [Google Scholar]
- Nossal R. On the elasticity of cytoskeletal networks. Biophys J. 1988 Mar;53(3):349–359. doi: 10.1016/S0006-3495(88)83112-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosawa F. The flexibility of F-actin. Biophys Chem. 1980 Jun;11(3-4):443–446. doi: 10.1016/0301-4622(80)87021-9. [DOI] [PubMed] [Google Scholar]
- Orenstein J. M., Shelton E. Membrane phenomena accompanying erythrophagocytosis. A scanning electron microscope study. Lab Invest. 1977 Apr;36(4):363–374. [PubMed] [Google Scholar]
- Oster G. F. On the crawling of cells. J Embryol Exp Morphol. 1984 Nov;83 (Suppl):329–364. [PubMed] [Google Scholar]
- Oster G. F., Perelson A. S. The physics of cell motility. J Cell Sci Suppl. 1987;8:35–54. doi: 10.1242/jcs.1987.supplement_8.3. [DOI] [PubMed] [Google Scholar]
- Pollard T. D., Cooper J. A. Actin and actin-binding proteins. A critical evaluation of mechanisms and functions. Annu Rev Biochem. 1986;55:987–1035. doi: 10.1146/annurev.bi.55.070186.005011. [DOI] [PubMed] [Google Scholar]
- Pollard T. D., Cooper J. A. Quantitative analysis of the effect of Acanthamoeba profilin on actin filament nucleation and elongation. Biochemistry. 1984 Dec 18;23(26):6631–6641. doi: 10.1021/bi00321a054. [DOI] [PubMed] [Google Scholar]
- Pollard T. D., Mooseker M. S. Direct measurement of actin polymerization rate constants by electron microscopy of actin filaments nucleated by isolated microvillus cores. J Cell Biol. 1981 Mar;88(3):654–659. doi: 10.1083/jcb.88.3.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard T. D. Rate constants for the reactions of ATP- and ADP-actin with the ends of actin filaments. J Cell Biol. 1986 Dec;103(6 Pt 2):2747–2754. doi: 10.1083/jcb.103.6.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryzwansky K. B., Schliwa M., Porter K. R. Comparison of the three-dimensional organization of unextracted and Triton-extracted human neutrophilic polymorphonuclear leukocytes. Eur J Cell Biol. 1983 Mar;30(1):112–125. [PubMed] [Google Scholar]
- Sager R., Kovac P. Pre-adipocyte determination either by insulin or by 5-azacytidine. Proc Natl Acad Sci U S A. 1982 Jan;79(2):480–484. doi: 10.1073/pnas.79.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid-Schönbein G. W., Skalak R. Continuum mechanical model of leukocytes during protopod formation. J Biomech Eng. 1984 Feb;106(1):10–18. doi: 10.1115/1.3138448. [DOI] [PubMed] [Google Scholar]
- Schmid-Schönbein G. W., Sung K. L., Tözeren H., Skalak R., Chien S. Passive mechanical properties of human leukocytes. Biophys J. 1981 Oct;36(1):243–256. doi: 10.1016/S0006-3495(81)84726-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stossel T. P., Hartwig J. H., Yin H. L., Stendahl O. The motor of amoeboid leucocytes. Biochem Soc Symp. 1980;45:51–63. [PubMed] [Google Scholar]
- Sung K. L., Schmid-Schönbein G. W., Skalak R., Schuessler G. B., Usami S., Chien S. Influence of physicochemical factors on rheology of human neutrophils. Biophys J. 1982 Jul;39(1):101–106. doi: 10.1016/S0006-3495(82)84495-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait J. F., Frieden C. Polymerization and gelation of actin studied by fluorescence photobleaching recovery. Biochemistry. 1982 Jul 20;21(15):3666–3674. doi: 10.1021/bi00258a022. [DOI] [PubMed] [Google Scholar]
- Tilney L. G., Bonder E. M., DeRosier D. J. Actin filaments elongate from their membrane-associated ends. J Cell Biol. 1981 Aug;90(2):485–494. doi: 10.1083/jcb.90.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney L. G., Inoué S. Acrosomal reaction of Thyone sperm. II. The kinetics and possible mechanism of acrosomal process elongation. J Cell Biol. 1982 Jun;93(3):820–827. doi: 10.1083/jcb.93.3.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney L. G., Kallenbach N. Polymerization of actin. VI. The polarity of the actin filaments in the acrosomal process and how it might be determined. J Cell Biol. 1979 Jun;81(3):608–623. doi: 10.1083/jcb.81.3.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobacman L. S., Korn E. D. The regulation of actin polymerization and the inhibition of monomeric actin ATPase activity by Acanthamoeba profilin. J Biol Chem. 1982 Apr 25;257(8):4166–4170. [PubMed] [Google Scholar]
- Tranquillo R. T., Lauffenburger D. A. Stochastic model of leukocyte chemosensory movement. J Math Biol. 1987;25(3):229–262. doi: 10.1007/BF00276435. [DOI] [PubMed] [Google Scholar]
- Tranquillo R. T., Lauffenburger D. A., Zigmond S. H. A stochastic model for leukocyte random motility and chemotaxis based on receptor binding fluctuations. J Cell Biol. 1988 Feb;106(2):303–309. doi: 10.1083/jcb.106.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh T. P., Weber A., Higgins J., Bonder E. M., Mooseker M. S. Effect of villin on the kinetics of actin polymerization. Biochemistry. 1984 Jun 5;23(12):2613–2621. doi: 10.1021/bi00307a012. [DOI] [PubMed] [Google Scholar]
- Wang Y. L. Exchange of actin subunits at the leading edge of living fibroblasts: possible role of treadmilling. J Cell Biol. 1985 Aug;101(2):597–602. doi: 10.1083/jcb.101.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. L., Lanni F., McNeil P. L., Ware B. R., Taylor D. L. Mobility of cytoplasmic and membrane-associated actin in living cells. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4660–4664. doi: 10.1073/pnas.79.15.4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanger M., Wegner A. Equilibrium constant for binding of an actin filament capping protein to the barbed end of actin filaments. Biochemistry. 1985 Feb 12;24(4):1035–1040. doi: 10.1021/bi00325a035. [DOI] [PubMed] [Google Scholar]
- Wegner A., Savko P. Fragmentation of actin filaments. Biochemistry. 1982 Apr 13;21(8):1909–1913. doi: 10.1021/bi00537a032. [DOI] [PubMed] [Google Scholar]
- Yin H. L., Albrecht J. H., Fattoum A. Identification of gelsolin, a Ca2+-dependent regulatory protein of actin gel-sol transformation, and its intracellular distribution in a variety of cells and tissues. J Cell Biol. 1981 Dec;91(3 Pt 1):901–906. doi: 10.1083/jcb.91.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]




