Abstract
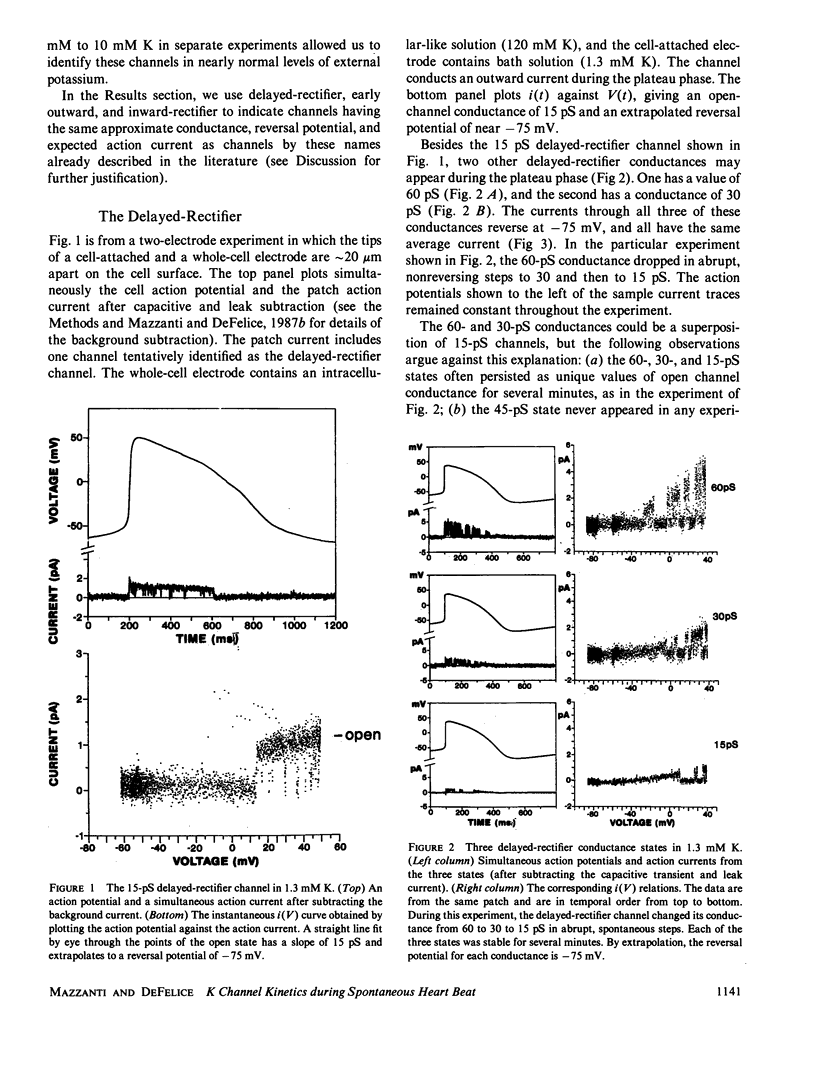
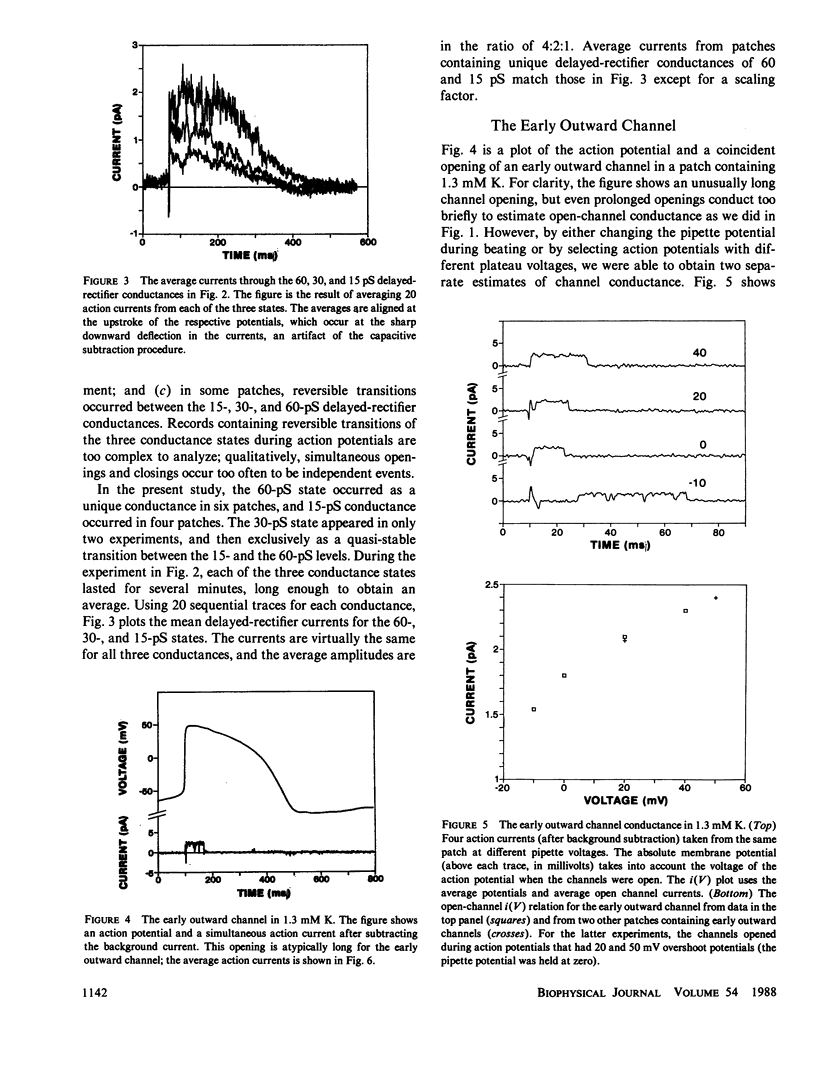
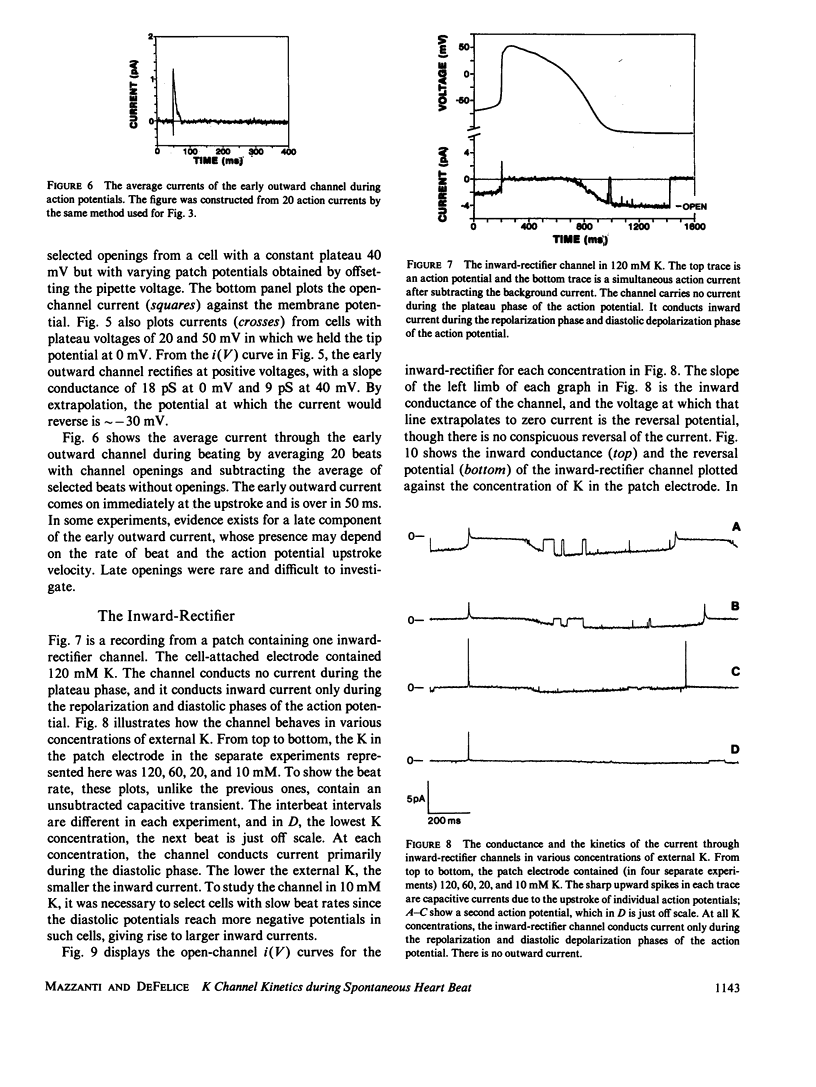
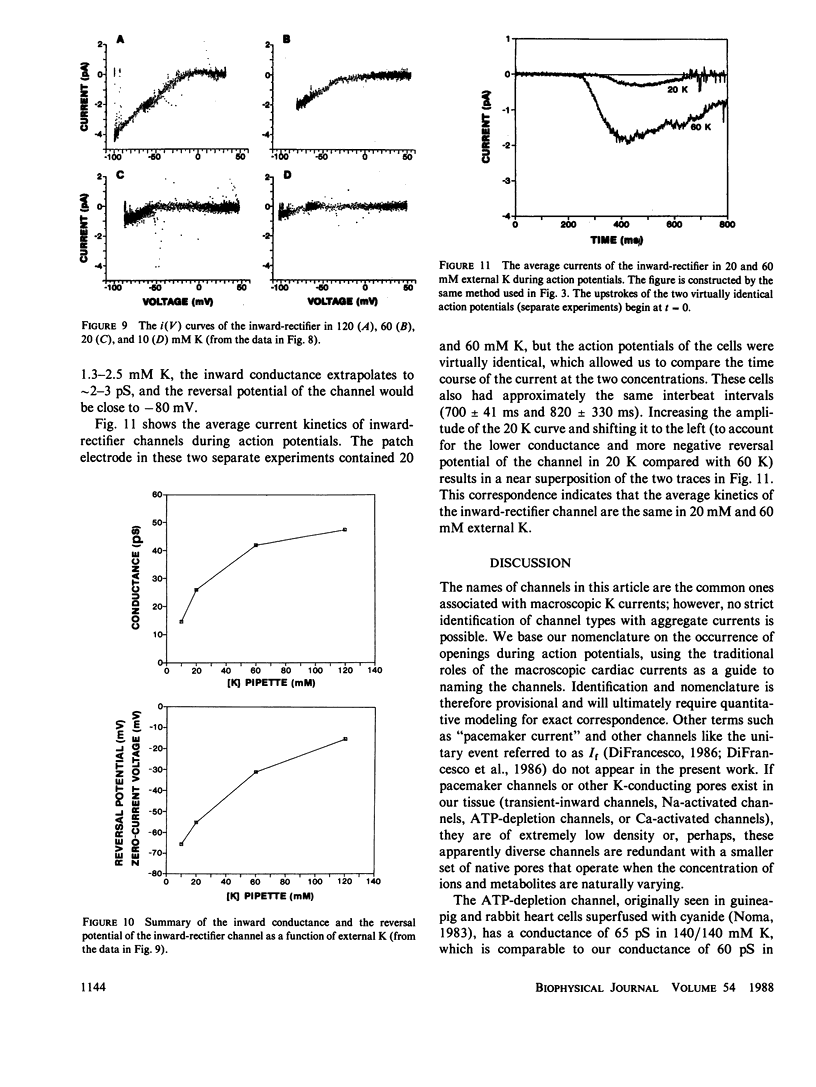
By averaging the current that passes through cell-attached patches on beating heart cells, while measuring action potentials with a whole-cell electrode, we were able to study K channels during beating. In 7-d chick ventricle in 1.3 mM K physiological solutions at room temperature, delayed-rectifier channels have three linear conductance states: 60, 30, and 15 pS. The 60 and 15 pS conductances can exist alone, but all three states may appear in the same patch as interconverting conductance levels. The delayed-rectifier conductance states have low densities (less than 10 channels per 10-microns diam cell), and all have a reversal potential near -75 mV and the same average kinetics. Outward K current through delayed-rectifier channels follows the upstroke without appreciable delay and lasts throughout the action potential. No inward current flows through delayed-rectifier channels during beating. The early outward channel has a nonlinear conductance of 18-9 pS depending on the potential. It also turns on immediately after the upstroke of the action potential and lasts on average only 50 ms. The early outward channel has an extrapolated reversal potential near -30 mV; no inward current flows during beating. The inward-rectifier has an extrapolated conductance and reversal potential of 2-3 pS and -80 mV in 1.3 mM K. Channel kinetics are independent of external K between 10 and 120 mM, and the channel conducts current only during the late repolarization and diastolic phases of the action potential. No outward current flows through inward-rectifier channels during beating. This work parallels a previous study of Na channels using similar techniques (Mazzanti, M., and L. J. DeFelice. 1987, Biophys. J. 52:95-100).
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bechem M., Glitsch H. G., Pott L. Properties of an inward rectifying K channel in the membrane of guinea-pig atrial cardioballs. Pflugers Arch. 1983 Nov;399(3):186–193. doi: 10.1007/BF00656713. [DOI] [PubMed] [Google Scholar]
- Beeler G. W., Reuter H. Reconstruction of the action potential of ventricular myocardial fibres. J Physiol. 1977 Jun;268(1):177–210. doi: 10.1113/jphysiol.1977.sp011853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begenisich T. Magnitude and location of surface charges on Myxicola giant axons. J Gen Physiol. 1975 Jul;66(1):47–65. doi: 10.1085/jgp.66.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J. E., Miller C. Effects of phospholipid surface charge on ion conduction in the K+ channel of sarcoplasmic reticulum. Biophys J. 1984 Jan;45(1):279–287. doi: 10.1016/S0006-3495(84)84154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benndorf K., Markwardt F., Nilius B. Two types of transient outward currents in cardiac ventricular cells of mice. Pflugers Arch. 1987 Aug;409(6):641–643. doi: 10.1007/BF00584667. [DOI] [PubMed] [Google Scholar]
- Bennett P. B., McKinney L. C., Kass R. S., Begenisich T. Delayed rectification in the calf cardiac Purkinje fiber. Evidence for multiple state kinetics. Biophys J. 1985 Oct;48(4):553–567. doi: 10.1016/S0006-3495(85)83813-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callewaert G., Vereecke J., Carmeliet E. Existence of a calcium-dependent potassium channel in the membrane of cow cardiac Purkinje cells. Pflugers Arch. 1986 Apr;406(4):424–426. doi: 10.1007/BF00590947. [DOI] [PubMed] [Google Scholar]
- Clapham D. E., Defelice L. J. Voltage-activated k channels in embryonic chick heart. Biophys J. 1984 Jan;45(1):40–42. doi: 10.1016/S0006-3495(84)84099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham D. E., Logothetis D. E. Delayed rectifier K+ current in embryonic chick heart ventricle. Am J Physiol. 1988 Jan;254(1 Pt 2):H192–H197. doi: 10.1152/ajpheart.1988.254.1.H192. [DOI] [PubMed] [Google Scholar]
- Clay J. R., Hill C. E., Roitman D., Shrier A. Repolarization current in embryonic chick atrial heart cells. J Physiol. 1988 Sep;403:525–537. doi: 10.1113/jphysiol.1988.sp017262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay J. R., Shrier A. Analysis of subthreshold pace-maker currents in chick embryonic heart cells. J Physiol. 1981 Mar;312:471–490. doi: 10.1113/jphysiol.1981.sp013639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J Physiol. 1971 Feb;213(1):21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti F., De Felice L. J., Wanke E. Potassium and sodium ion current noise in the membrane of the squid giant axon. J Physiol. 1975 Jun;248(1):45–82. doi: 10.1113/jphysiol.1975.sp010962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti F., Hille B., Nonner W. Non-stationary fluctuations of the potassium conductance at the node of ranvier of the frog. J Physiol. 1984 Aug;353:199–230. doi: 10.1113/jphysiol.1984.sp015332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti F., Neher E. Single channel recordings of K+ currents in squid axons. Nature. 1980 May 15;285(5761):140–143. doi: 10.1038/285140a0. [DOI] [PubMed] [Google Scholar]
- Coraboeuf E., Carmeliet E. Existence of two transient outward currents in sheep cardiac Purkinje fibers. Pflugers Arch. 1982 Feb;392(4):352–359. doi: 10.1007/BF00581631. [DOI] [PubMed] [Google Scholar]
- DeHann R. L. Regulation of spontaneous activity and growth of embryonic chick heart cells in tissue culture. Dev Biol. 1967 Sep;16(3):216–249. doi: 10.1016/0012-1606(67)90025-5. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D. Characterization of single pacemaker channels in cardiac sino-atrial node cells. Nature. 1986 Dec 4;324(6096):470–473. doi: 10.1038/324470a0. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D., Ferroni A., Mazzanti M., Tromba C. Properties of the hyperpolarizing-activated current (if) in cells isolated from the rabbit sino-atrial node. J Physiol. 1986 Aug;377:61–88. doi: 10.1113/jphysiol.1986.sp016177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D., Noble D. A model of cardiac electrical activity incorporating ionic pumps and concentration changes. Philos Trans R Soc Lond B Biol Sci. 1985 Jan 10;307(1133):353–398. doi: 10.1098/rstb.1985.0001. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D., Noma A., Trautwein W. Kinetics and magnitude of the time-dependent potassium current in the rabbit sinoatrial node: effect of external potassium. Pflugers Arch. 1979 Sep;381(3):271–279. doi: 10.1007/BF00583259. [DOI] [PubMed] [Google Scholar]
- Fischmeister R., DeFelice L. J., Ayer R. K., Jr, Levi R., DeHaan R. L. Channel currents during spontaneous action potentials in embryonic chick heart cells. The action potential patch clamp. Biophys J. 1984 Aug;46(2):267–271. doi: 10.1016/S0006-3495(84)84020-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatman P. W. Magnesium transport across cell membranes. J Membr Biol. 1984;80(1):1–14. doi: 10.1007/BF01868686. [DOI] [PubMed] [Google Scholar]
- Fozzard H. A., Sheu S. S. Intracellular potassium and sodium activities of chick ventricular muscle during embryonic development. J Physiol. 1980 Sep;306:579–586. doi: 10.1113/jphysiol.1980.sp013416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S., Ayer R. K., Jr, DeHaan R. L. Development of the fast sodium current in early embryonic chick heart cells. J Membr Biol. 1988 Mar;101(3):209–223. doi: 10.1007/BF01872836. [DOI] [PubMed] [Google Scholar]
- Fukushima Y. Single channel potassium currents of the anomalous rectifier. Nature. 1981 Nov 26;294(5839):368–371. doi: 10.1038/294368a0. [DOI] [PubMed] [Google Scholar]
- Giles W. R., Shibata E. F. Voltage clamp of bull-frog cardiac pace-maker cells: a quantitative analysis of potassium currents. J Physiol. 1985 Nov;368:265–292. doi: 10.1113/jphysiol.1985.sp015857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles W. R., van Ginneken A. C. A transient outward current in isolated cells from the crista terminalis of rabbit heart. J Physiol. 1985 Nov;368:243–264. doi: 10.1113/jphysiol.1985.sp015856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume J. R., Giles W. Ionic currents in single isolated bullfrog atrial cells. J Gen Physiol. 1983 Feb;81(2):153–194. doi: 10.1085/jgp.81.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume J. R., Uehara A. Ionic basis of the different action potential configurations of single guinea-pig atrial and ventricular myocytes. J Physiol. 1985 Nov;368:525–544. doi: 10.1113/jphysiol.1985.sp015874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephson I. R., Sanchez-Chapula J., Brown A. M. Early outward current in rat single ventricular cells. Circ Res. 1984 Feb;54(2):157–162. doi: 10.1161/01.res.54.2.157. [DOI] [PubMed] [Google Scholar]
- Kameyama M., Kiyosue T., Soejima M. Single channel analysis of the inward rectifier K current in the rabbit ventricular cells. Jpn J Physiol. 1983;33(6):1039–1056. doi: 10.2170/jjphysiol.33.1039. [DOI] [PubMed] [Google Scholar]
- Kass R. S. Delayed rectification in the cardiac Purkinje fiber is not activated by intracellular calcium. Biophys J. 1984 Apr;45(4):837–839. doi: 10.1016/S0006-3495(84)84227-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kell M. J., DeFelice L. J. Surface charge near the cardiac inward-rectifier channel measured from single-channel conductance. J Membr Biol. 1988 Apr;102(1):1–10. doi: 10.1007/BF01875348. [DOI] [PubMed] [Google Scholar]
- Kukushkin N. I., Gainullin R. Z., Sosunov E. A. Transient outward current and rate dependence of action potential duration in rabbit cardiac ventricular muscle. Pflugers Arch. 1983 Oct;399(2):87–92. doi: 10.1007/BF00663902. [DOI] [PubMed] [Google Scholar]
- Kurachi Y. Voltage-dependent activation of the inward-rectifier potassium channel in the ventricular cell membrane of guinea-pig heart. J Physiol. 1985 Sep;366:365–385. doi: 10.1113/jphysiol.1985.sp015803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H., Saigusa A., Irisawa H. Ohmic conductance through the inwardly rectifying K channel and blocking by internal Mg2+. Nature. 1987 Jan 8;325(7000):156–159. doi: 10.1038/325156a0. [DOI] [PubMed] [Google Scholar]
- Mazzanti M., DeFelice L. J. Na channel kinetics during the spontaneous heart beat in embryonic chick ventricle cells. Biophys J. 1987 Jul;52(1):95–100. doi: 10.1016/S0006-3495(87)83192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzanti M., DeFelice L. J. Regulation of the Na-conducting Ca channel during the cardiac action potential. Biophys J. 1987 Jan;51(1):115–121. doi: 10.1016/S0006-3495(87)83316-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister R. E., Noble D., Tsien R. W. Reconstruction of the electrical activity of cardiac Purkinje fibres. J Physiol. 1975 Sep;251(1):1–59. doi: 10.1113/jphysiol.1975.sp011080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald T. F., DeHaan R. L. Ion levels and membrane potential in chick heart tissue and cultured cells. J Gen Physiol. 1973 Jan;61(1):89–109. doi: 10.1085/jgp.61.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald T. F., Trautwein W. The potassium current underlying delayed rectification in cat ventricular muscle. J Physiol. 1978 Jan;274:217–246. doi: 10.1113/jphysiol.1978.sp012144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama T., Irisawa H. Transient outward current carried by potassium and sodium in quiescent atrioventricular node cells of rabbits. Circ Res. 1985 Jul;57(1):65–73. doi: 10.1161/01.res.57.1.65. [DOI] [PubMed] [Google Scholar]
- Noble D. The surprising heart: a review of recent progress in cardiac electrophysiology. J Physiol. 1984 Aug;353:1–50. doi: 10.1113/jphysiol.1984.sp015320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983 Sep 8;305(5930):147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- Payet M. D., Rousseau E., Sauvé R. Single-channel analysis of a potassium inward rectifier in myocytes of newborn rat heart. J Membr Biol. 1985;86(2):79–88. doi: 10.1007/BF01870774. [DOI] [PubMed] [Google Scholar]
- Reuter H. Ion channels in cardiac cell membranes. Annu Rev Physiol. 1984;46:473–484. doi: 10.1146/annurev.ph.46.030184.002353. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Trube G. Conductance properties of single inwardly rectifying potassium channels in ventricular cells from guinea-pig heart. J Physiol. 1984 Feb;347:641–657. doi: 10.1113/jphysiol.1984.sp015088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmann B., Trube G. Voltage-dependent inactivation of inward-rectifying single-channel currents in the guinea-pig heart cell membrane. J Physiol. 1984 Feb;347:659–683. doi: 10.1113/jphysiol.1984.sp015089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A. K., Cohen I. S., Datyner N. B. Background K+ current in isolated canine cardiac Purkinje myocytes. Biophys J. 1987 Oct;52(4):519–525. doi: 10.1016/S0006-3495(87)83241-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki T. Conductance and kinetics of delayed rectifier potassium channels in nodal cells of the rabbit heart. J Physiol. 1987 Jun;387:227–250. doi: 10.1113/jphysiol.1987.sp016571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrier A., Clay J. R. Repolarization currents in embryonic chick atrial heart cell aggregates. Biophys J. 1986 Nov;50(5):861–874. doi: 10.1016/S0006-3495(86)83527-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegelbaum S. A., Tsien R. W. Calcium-activated transient outward current in calf cardiac Purkinje fibres. J Physiol. 1980 Feb;299:485–506. doi: 10.1113/jphysiol.1980.sp013138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons M. A., Creazzo T., Hartzell H. C. A time-dependent and voltage-sensitive K+ current in single cells from frog atrium. J Gen Physiol. 1986 Dec;88(6):739–755. doi: 10.1085/jgp.88.6.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trube G., Hescheler J. Inward-rectifying channels in isolated patches of the heart cell membrane: ATP-dependence and comparison with cell-attached patches. Pflugers Arch. 1984 Jun;401(2):178–184. doi: 10.1007/BF00583879. [DOI] [PubMed] [Google Scholar]
- Vassort G., Whittembury J., Mullins L. J. Increases in internal Ca2+ and decreases in internal H+ are induced by general anesthetics in squid axons. Biophys J. 1986 Jul;50(1):11–19. doi: 10.1016/S0006-3495(86)83434-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagihara K., Noma A., Irisawa H. Reconstruction of sino-atrial node pacemaker potential based on the voltage clamp experiments. Jpn J Physiol. 1980;30(6):841–857. doi: 10.2170/jjphysiol.30.841. [DOI] [PubMed] [Google Scholar]


