Abstract
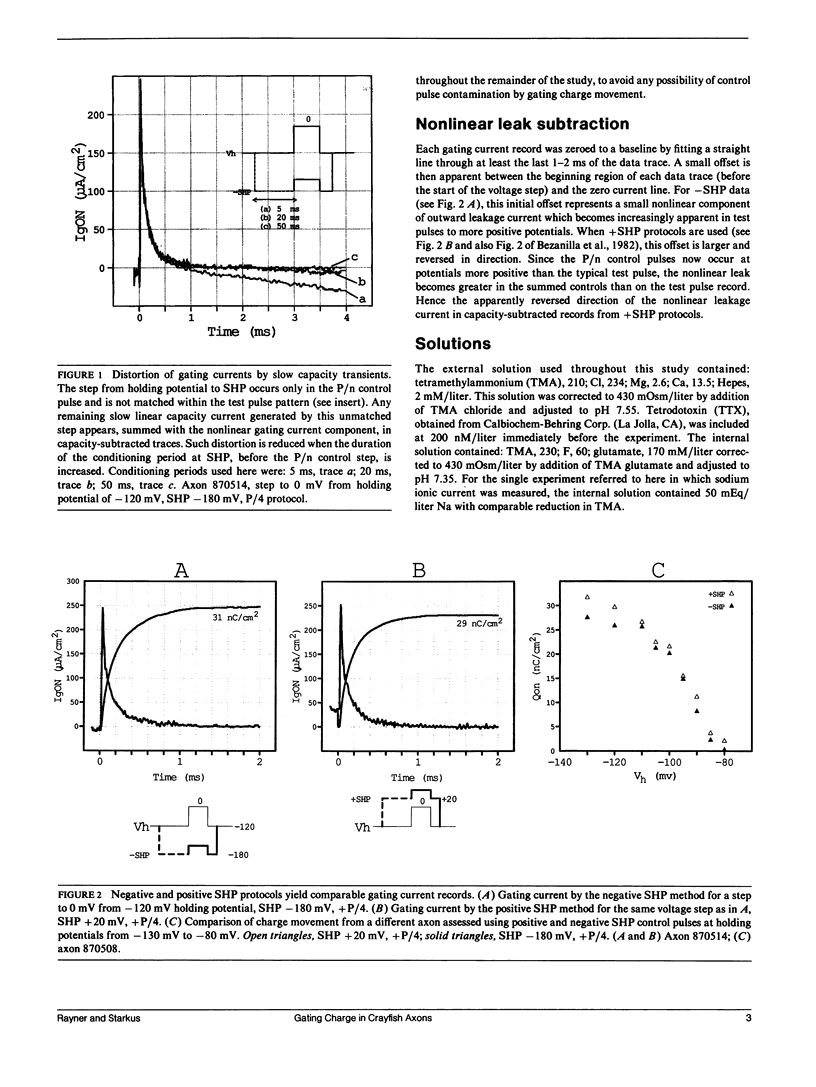
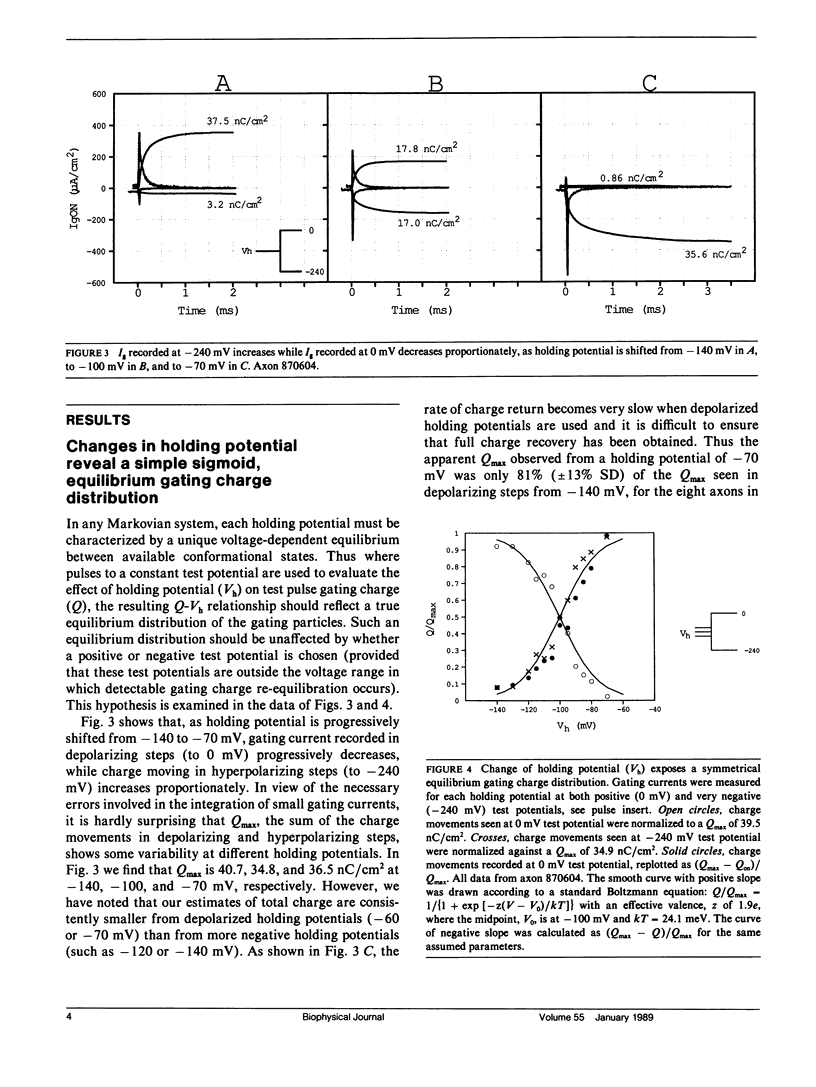
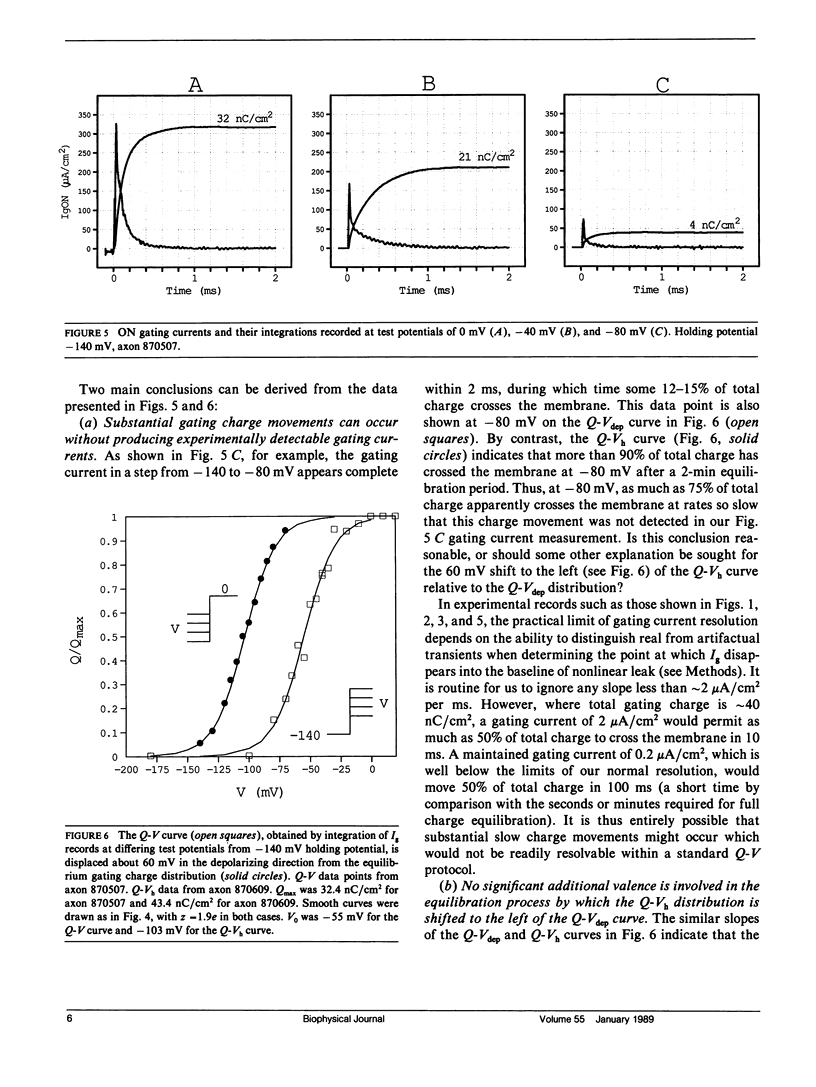
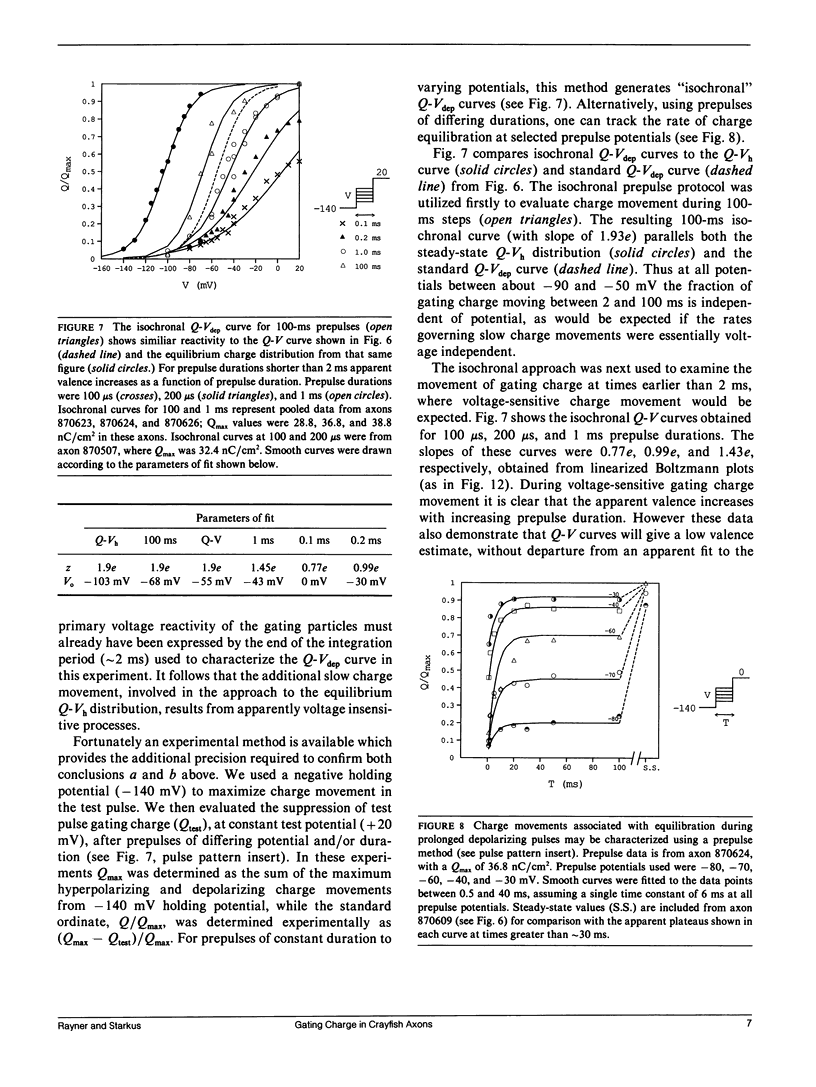
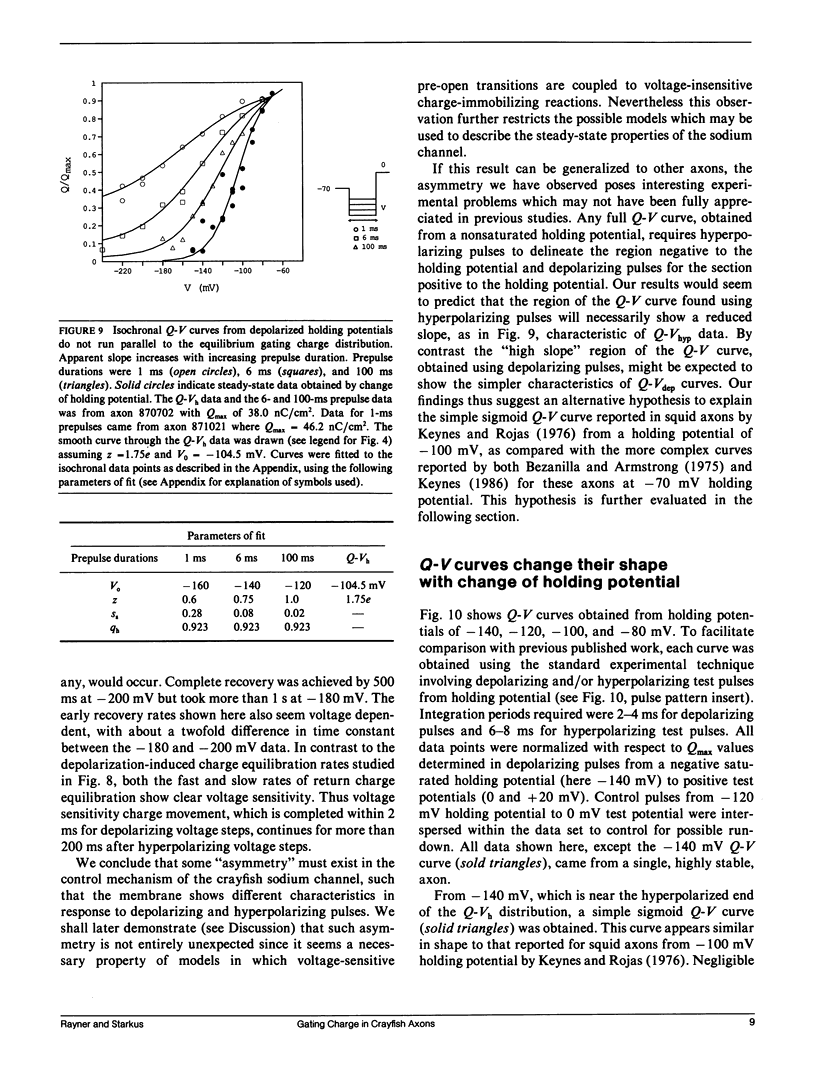
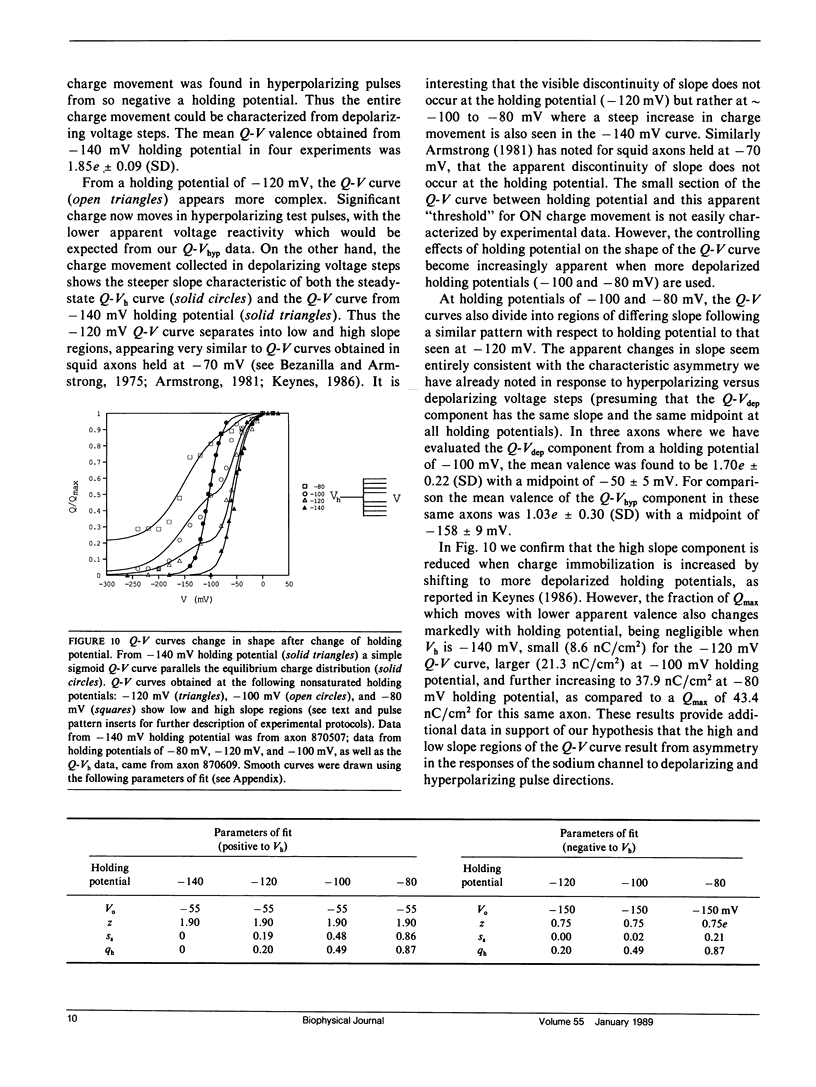
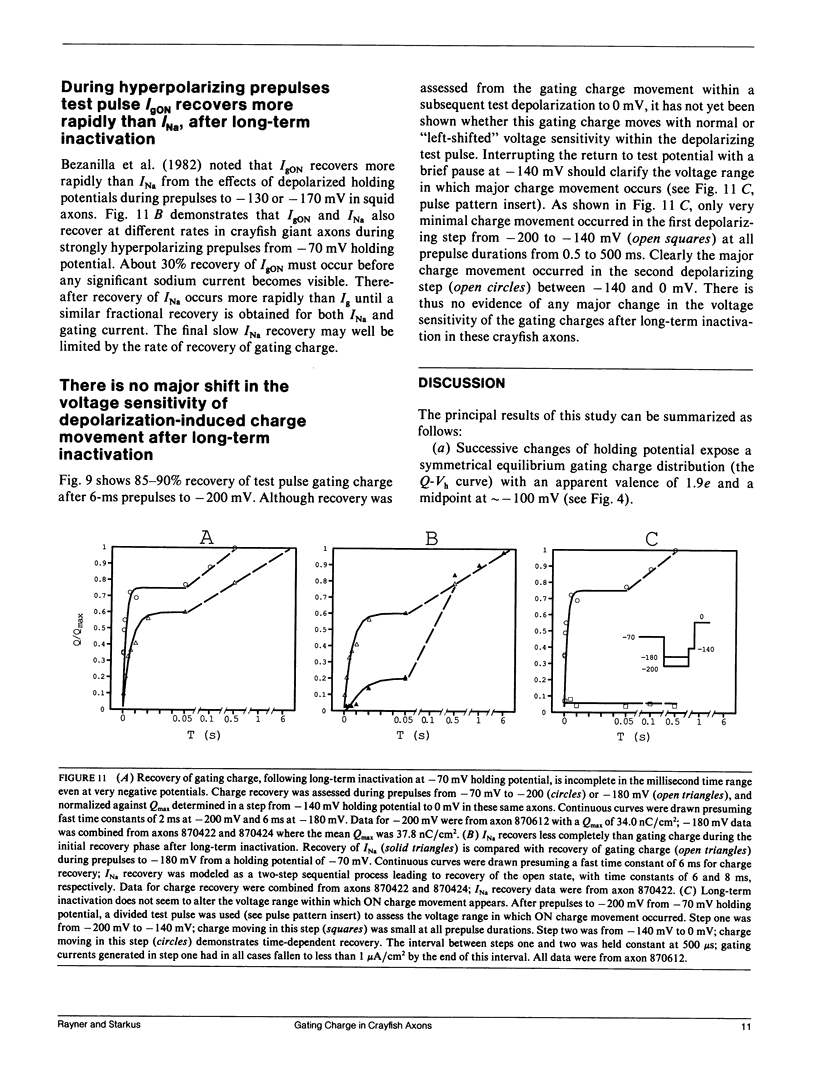
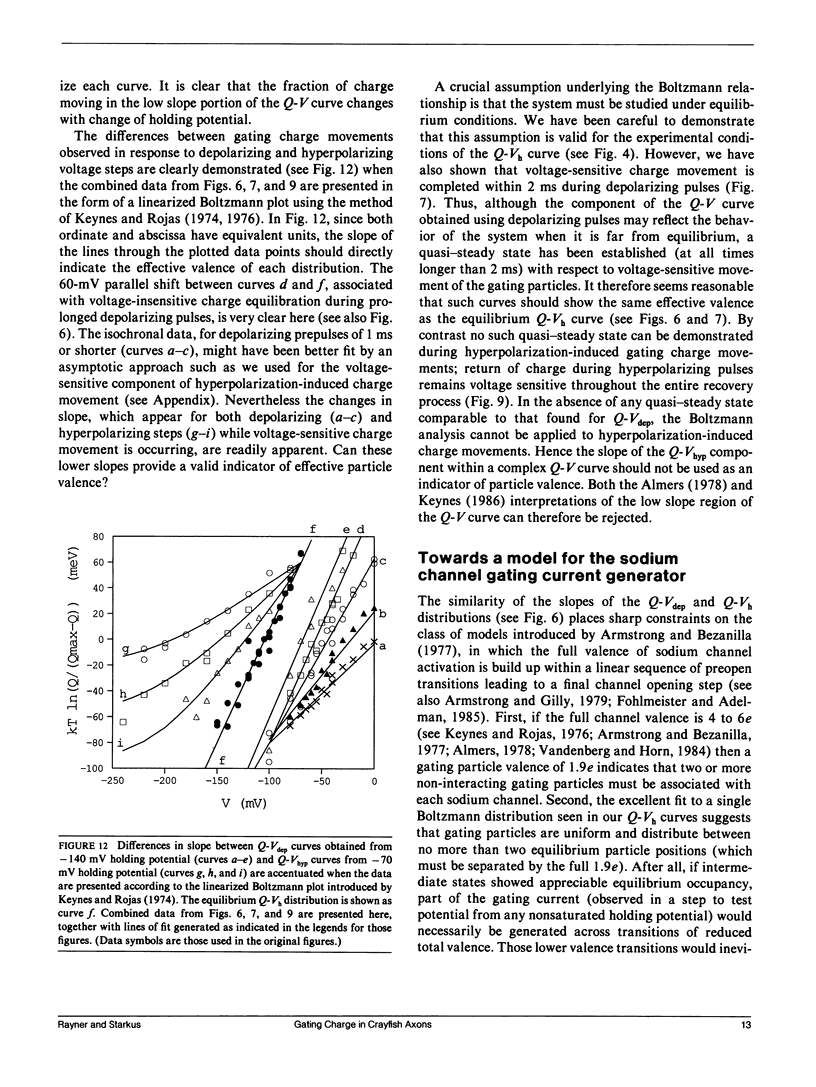
Progressive shifts of holding potential (Vh) in crayfish giant axons, from -140 to -70 mV, reduce gating currents seen in depolarizing steps (to 0 mV test potential) while proportionately increasing gating currents in hyperpolarizing steps (to -240 mV). The resulting sigmoid equilibrium charge distribution (Q-Vh curve) shows an effective valence of 1.9e and a midpoint of -100 mV. By contrast, Q-V curves obtained using hyperpolarizing and/or depolarizing steps from a single holding potential, change their "shape" depending on the chosen holding potential. For holding potentials at the negative end of the Q-Vh distribution (e.g., -140 mV), negligible charge moves in hyperpolarizing pulses and the Q-V curve can be characterized entirely from depolarizing voltage steps. The slope of the resulting simple sigmoid Q-V curve also indicates an effective valence of 1.9e. When the axon is held at less negative potentials significant charge moves in hyperpolarizing voltage steps. The component of the Q-V curve collected using hyperpolarizing pulses shows a significantly reduced slope (approximately 0.75e) by comparison with the 1.9e slope found using depolarizing pulses or from the Q-Vh curve. As holding potential is shifted in the depolarizing direction along the Q-Vh curve, an increasing fraction of total charge movement must be assessed in hyperpolarizing voltage steps. Thus charge moving in the low slope component of the Q-V curve increases as holding potential is depolarized, while charge moving with high apparent valence decreases proportionately. Additional results, together with simulations based on a simple kinetic model, suggest that the reduced apparent valence of the low slope component of the Q-V curve results from gating charge immobilization occurring at holding potential. Immobilization selectively retards that fraction of total charge moving in hyperpolarizing pulses. Misleading conclusions, as to the number and valence of the gating particles, may therefore be derived from Q-V curves obtained by other than depolarizing pulses from negative saturated holding potentials.
Full text
PDF





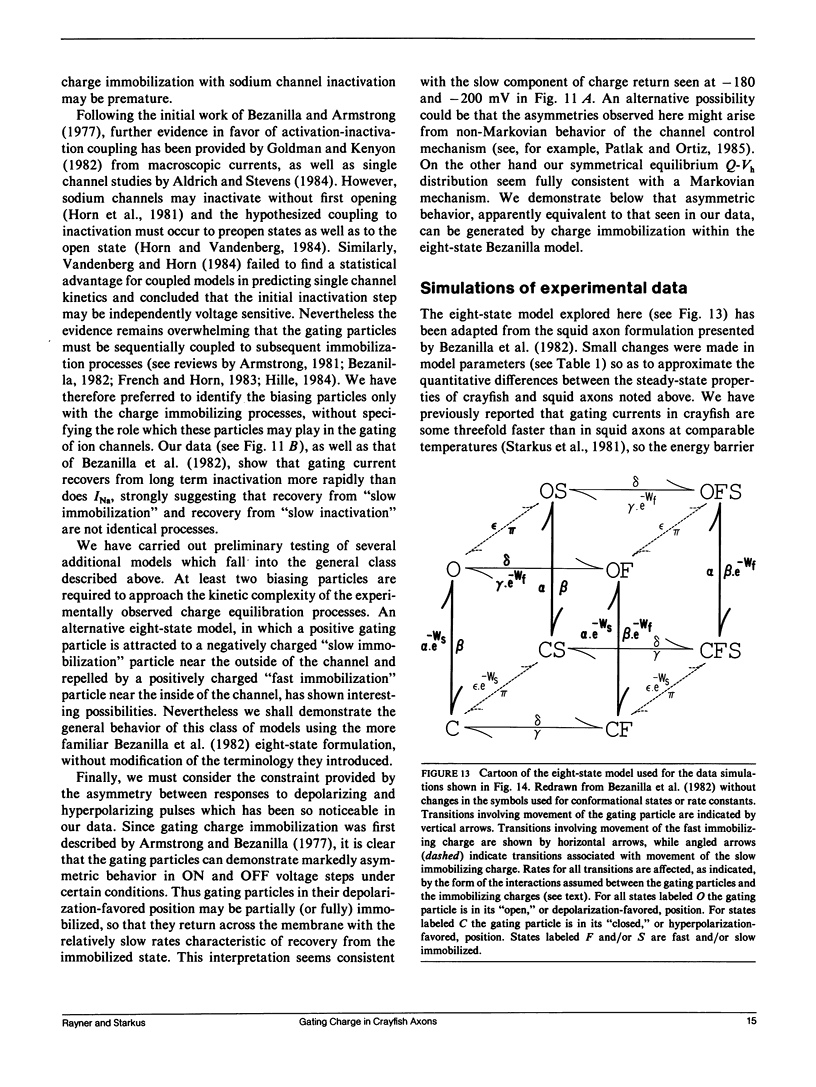
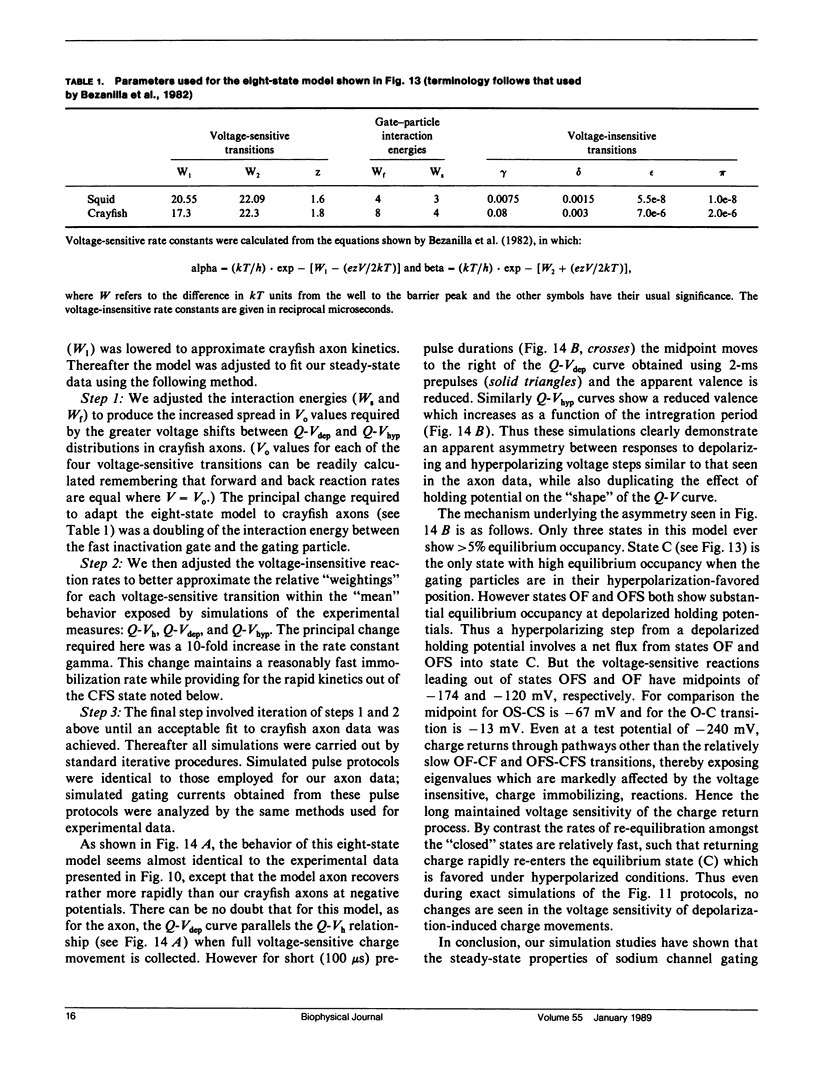
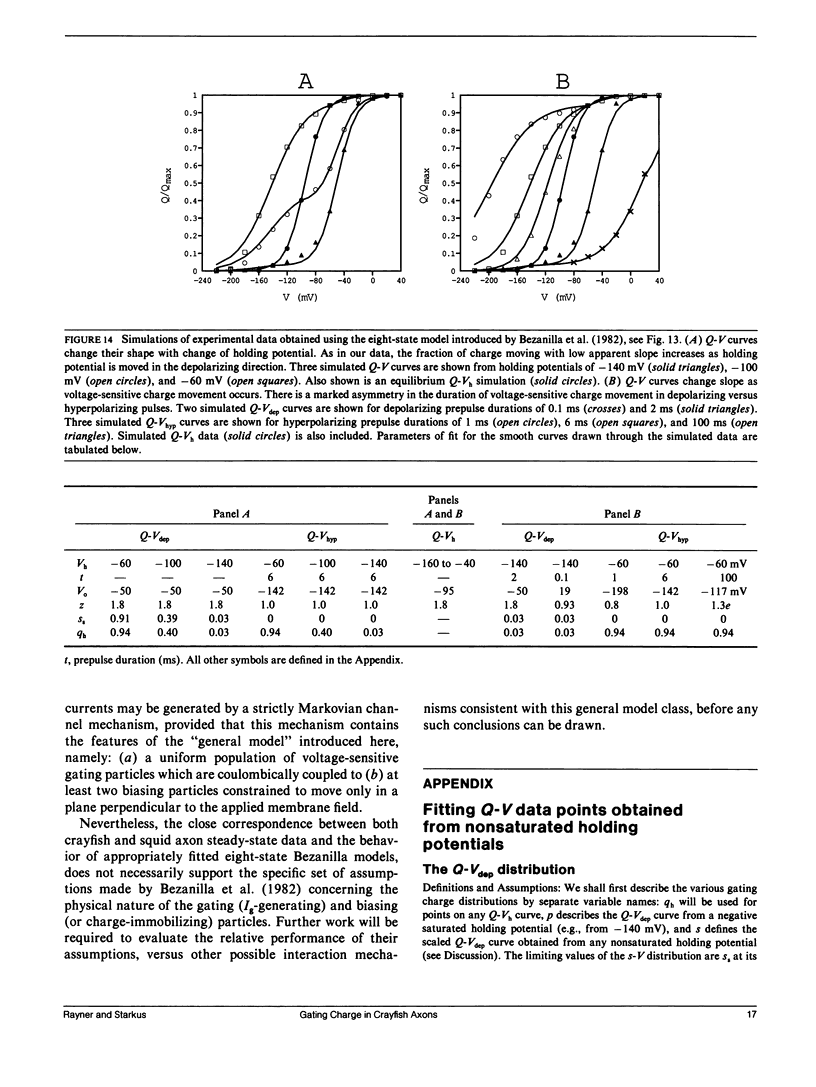


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldrich R. W., Stevens C. F. Inactivation of open and closed sodium channels determined separately. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 1):147–153. doi: 10.1101/sqb.1983.048.01.017. [DOI] [PubMed] [Google Scholar]
- Almers W. Gating currents and charge movements in excitable membranes. Rev Physiol Biochem Pharmacol. 1978;82:96–190. doi: 10.1007/BFb0030498. [DOI] [PubMed] [Google Scholar]
- Armstrong C. M., Bezanilla F. Charge movement associated with the opening and closing of the activation gates of the Na channels. J Gen Physiol. 1974 May;63(5):533–552. doi: 10.1085/jgp.63.5.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M., Bezanilla F. Inactivation of the sodium channel. II. Gating current experiments. J Gen Physiol. 1977 Nov;70(5):567–590. doi: 10.1085/jgp.70.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M., Gilly W. F. Fast and slow steps in the activation of sodium channels. J Gen Physiol. 1979 Dec;74(6):691–711. doi: 10.1085/jgp.74.6.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M. Sodium channels and gating currents. Physiol Rev. 1981 Jul;61(3):644–683. doi: 10.1152/physrev.1981.61.3.644. [DOI] [PubMed] [Google Scholar]
- Bezanilla F., Armstrong C. M. Inactivation of the sodium channel. I. Sodium current experiments. J Gen Physiol. 1977 Nov;70(5):549–566. doi: 10.1085/jgp.70.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F., Armstrong C. M. Properties of the sodium channel gating current. Cold Spring Harb Symp Quant Biol. 1976;40:297–304. doi: 10.1101/sqb.1976.040.01.030. [DOI] [PubMed] [Google Scholar]
- Bezanilla F. Gating charge movements and kinetics of excitable membrane proteins. Prog Clin Biol Res. 1982;79:3–16. [PubMed] [Google Scholar]
- Bezanilla F., Taylor R. E., Fernández J. M. Distribution and kinetics of membrane dielectric polarization. 1. Long-term inactivation of gating currents. J Gen Physiol. 1982 Jan;79(1):21–40. doi: 10.1085/jgp.79.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández J. M., Bezanilla F., Taylor R. E. Distribution and kinetics of membrane dielectric polarization. II. Frequency domain studies of gating currents. J Gen Physiol. 1982 Jan;79(1):41–67. doi: 10.1085/jgp.79.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fohlmeister J. F., Adelman W. J., Jr Gating current harmonics. II. Model simulations of axonal gating currents. Biophys J. 1985 Sep;48(3):391–400. doi: 10.1016/S0006-3495(85)83795-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French R. J., Horn R. Sodium channel gating: models, mimics, and modifiers. Annu Rev Biophys Bioeng. 1983;12:319–356. doi: 10.1146/annurev.bb.12.060183.001535. [DOI] [PubMed] [Google Scholar]
- Goldman L., Kenyon J. L. Delays in inactivation development and activation kinetics in myxicola giant axons. J Gen Physiol. 1982 Jul;80(1):83–102. doi: 10.1085/jgp.80.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heggeness S. T., Starkus J. G. Saxitoxin and tetrodotoxin. Electrostatic effects on sodium channel gating current in crayfish axons. Biophys J. 1986 Mar;49(3):629–643. doi: 10.1016/S0006-3495(86)83690-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R., Patlak J., Stevens C. F. Sodium channels need not open before they inactivate. Nature. 1981 Jun 4;291(5814):426–427. doi: 10.1038/291426a0. [DOI] [PubMed] [Google Scholar]
- Horn R., Vandenberg C. A. Statistical properties of single sodium channels. J Gen Physiol. 1984 Oct;84(4):505–534. doi: 10.1085/jgp.84.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynes R. D. Properties of the sodium gating current in the squid giant axon. Ann N Y Acad Sci. 1986;479:431–438. doi: 10.1111/j.1749-6632.1986.tb15586.x. [DOI] [PubMed] [Google Scholar]
- Keynes R. D., Rojas E. Kinetics and steady-state properties of the charged system controlling sodium conductance in the squid giant axon. J Physiol. 1974 Jun;239(2):393–434. doi: 10.1113/jphysiol.1974.sp010575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynes R. D., Rojas E. The temporal and steady-state relationships between activation of the sodium conductance and movement of the gating particles in the squid giant axon. J Physiol. 1976 Feb;255(1):157–189. doi: 10.1113/jphysiol.1976.sp011274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meves H. The effect of holding potential on the asymmetry currents in squid gaint axons. J Physiol. 1974 Dec;243(3):847–867. doi: 10.1113/jphysiol.1974.sp010780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patlak J. B., Ortiz M. Slow currents through single sodium channels of the adult rat heart. J Gen Physiol. 1985 Jul;86(1):89–104. doi: 10.1085/jgp.86.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrager P. Ionic conductance changes in voltage clamped crayfish axons at low pH. J Gen Physiol. 1974 Dec;64(6):666–690. doi: 10.1085/jgp.64.6.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkus J. G., Fellmeth B. D., Rayner M. D. Gating currents in th intact crayfish giant axon. Biophys J. 1981 Aug;35(2):521–533. doi: 10.1016/S0006-3495(81)84807-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkus J. G., Heggeness S. T., Rayner M. D. Kinetic analysis of sodium channel block by internal methylene blue in pronased crayfish giant axons. Biophys J. 1984 Aug;46(2):205–218. doi: 10.1016/S0006-3495(84)84014-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkus J. G., Shrager P. Modification of slow sodium inactivation in nerve after internal perfusion with trypsin. Am J Physiol. 1978 Nov;235(5):C238–C244. doi: 10.1152/ajpcell.1978.235.5.C238. [DOI] [PubMed] [Google Scholar]
- Vandenberg C. A., Horn R. Inactivation viewed through single sodium channels. J Gen Physiol. 1984 Oct;84(4):535–564. doi: 10.1085/jgp.84.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]



