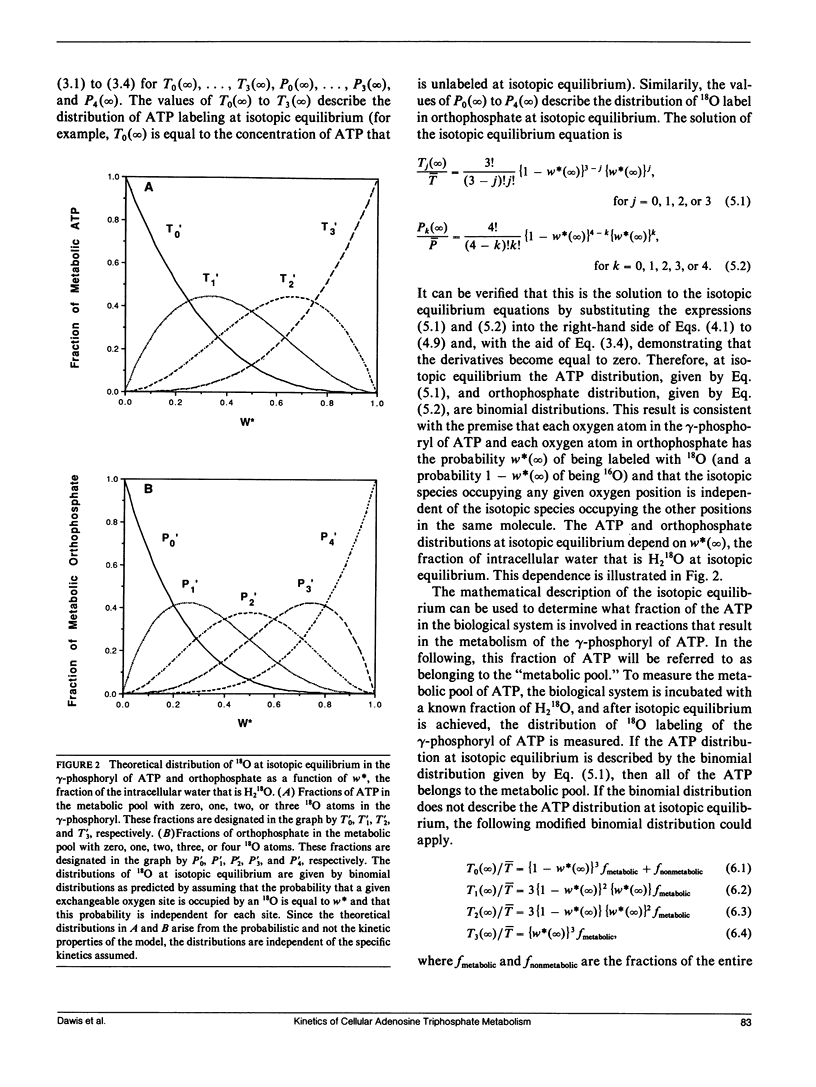
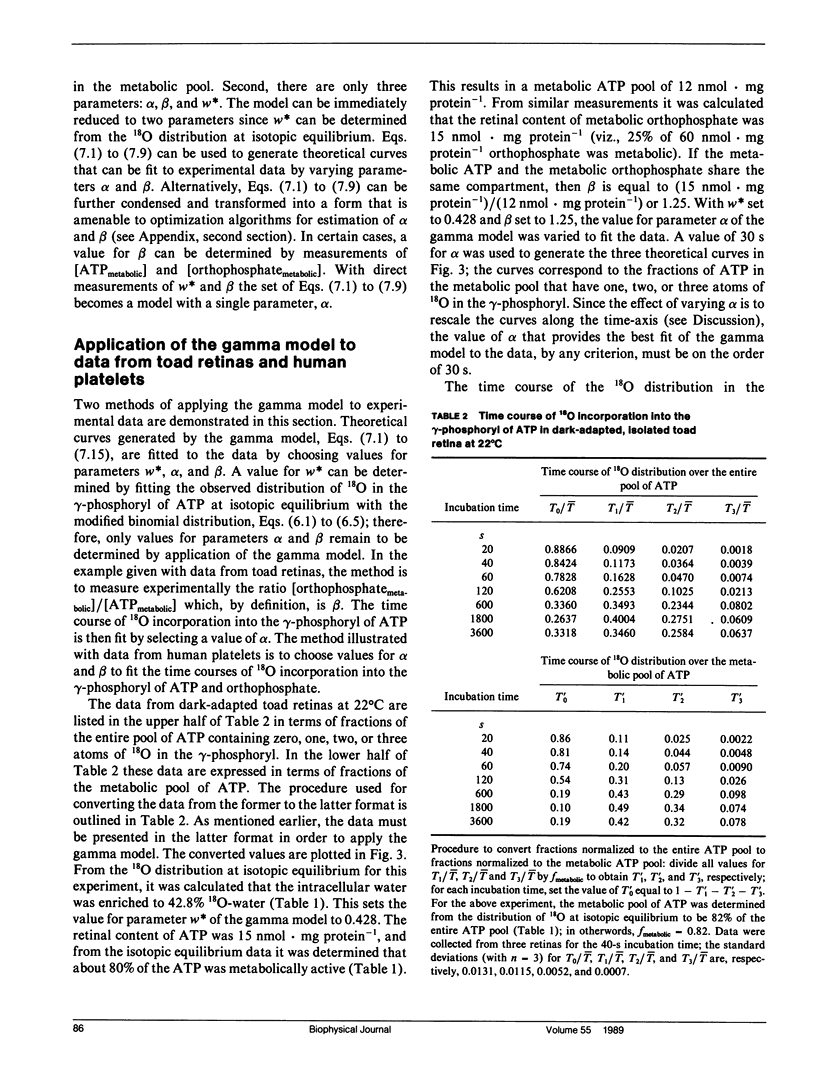
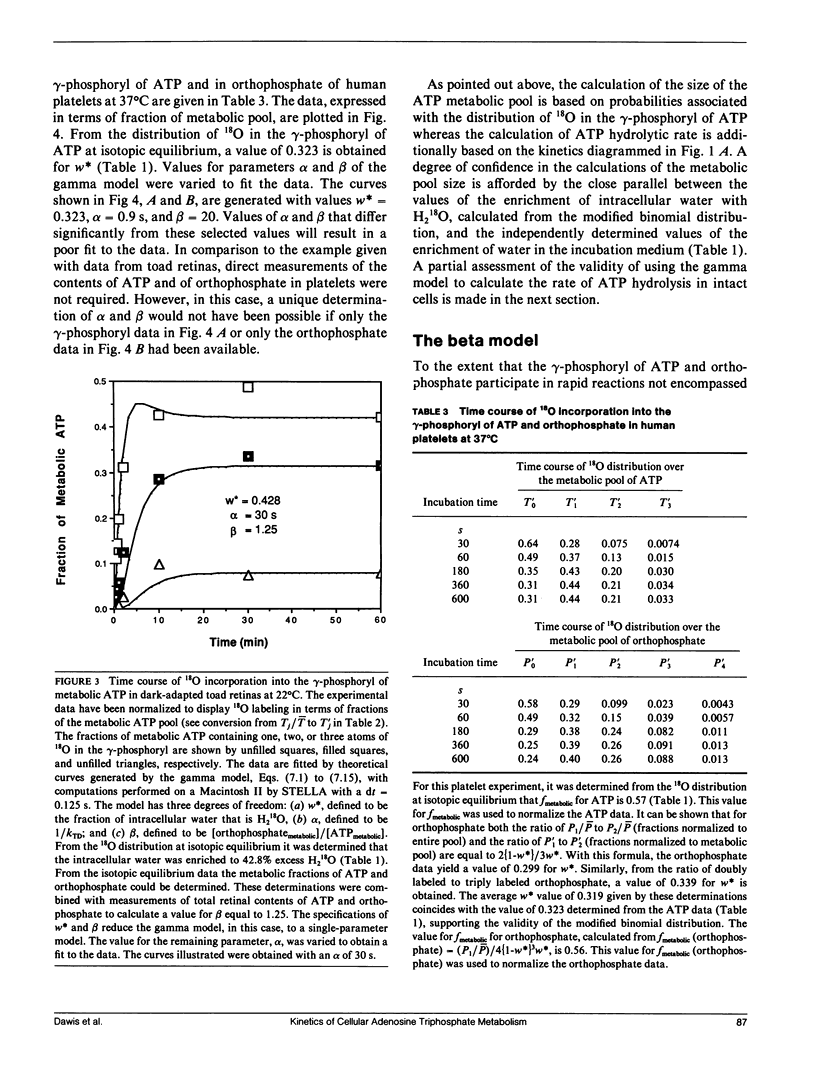
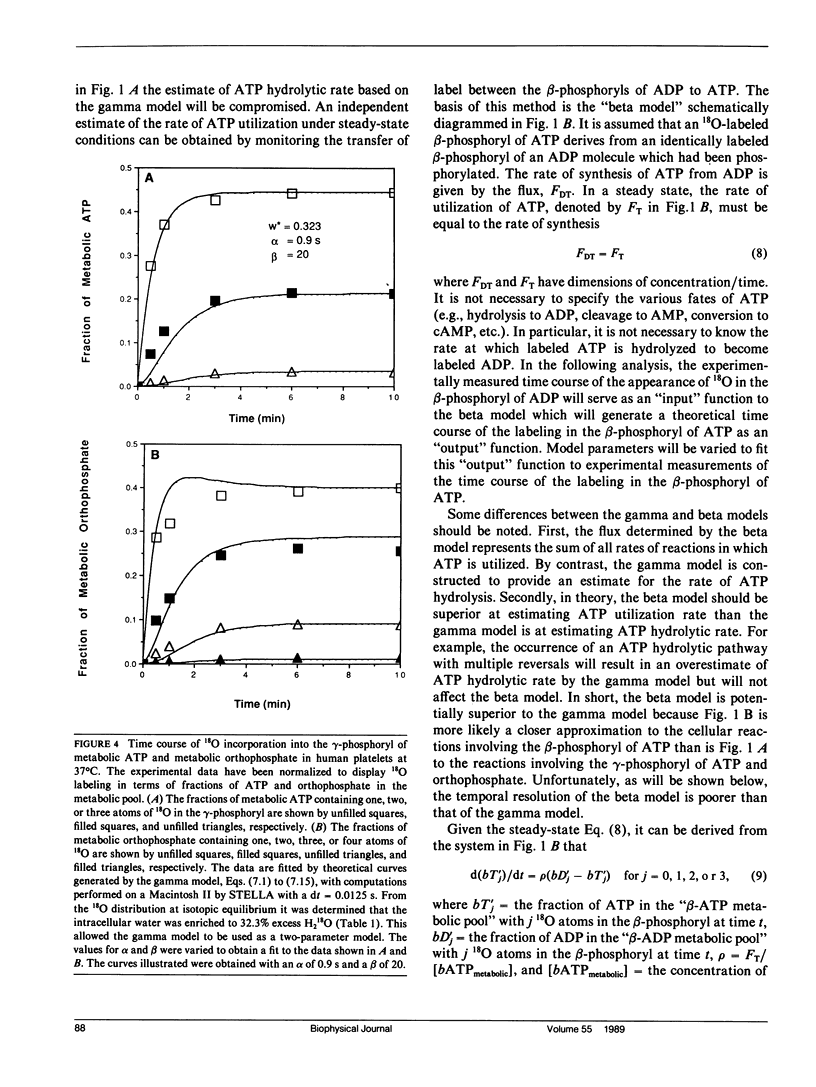
Abstract
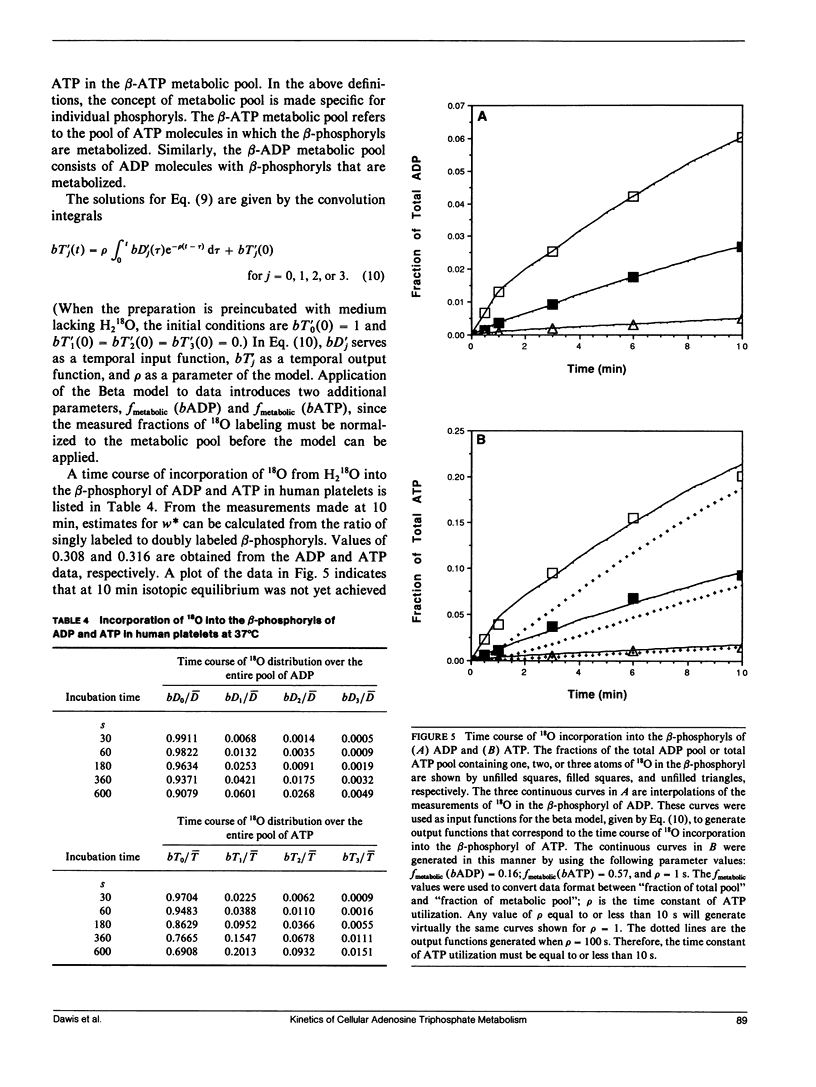
The hydrolytic rates and metabolic pool sizes of ATP were determined in intact cells by monitoring the time courses of 18O incorporation from 18O-water into the gamma-phosphoryl of ATP and orthophosphate. To calculate the rate of ATP hydrolysis, a kinetic model is used to fit the time course of the 18O labeling. The size of the metabolic pool of ATP is calculated from the 18O distribution after isotopic equilibrium has been achieved. Metabolic pools have a binomial distribution of 18O whereas nonmetabolic pools exhibit negligible 18O labeling. The application and limitations of this approach are illustrated with data from isolated toad retinas and human platelets. At 22 degrees C, the time constant of ATP hydrolysis in the dark-adapted toad retina is about 30 s. Under these conditions, over 80% of the retinal ATP is involved in high-energy phosphate metabolism. It is calculated that when cGMP metabolic flux in the photoreceptors is maximally stimulated by light, it accounts for 10% of the ATP utilization by the entire retina. The time constant of ATP hydrolysis in human platelets at 37 degrees C is approximately 1 s, and 60% of the platelet ATP is involved in energy metabolism.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames A., 3rd, Walseth T. F., Heyman R. A., Barad M., Graeff R. M., Goldberg N. D. Light-induced increases in cGMP metabolic flux correspond with electrical responses of photoreceptors. J Biol Chem. 1986 Oct 5;261(28):13034–13042. [PubMed] [Google Scholar]
- Bagshaw C. R., Trentham D. R. The reversibility of adenosine triphosphate cleavage by myosin. Biochem J. 1973 Jun;133(2):323–328. doi: 10.1042/bj1330323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittl J. A., Ingwall J. S. Reaction rates of creatine kinase and ATP synthesis in the isolated rat heart. A 31P NMR magnetization transfer study. J Biol Chem. 1985 Mar 25;260(6):3512–3517. [PubMed] [Google Scholar]
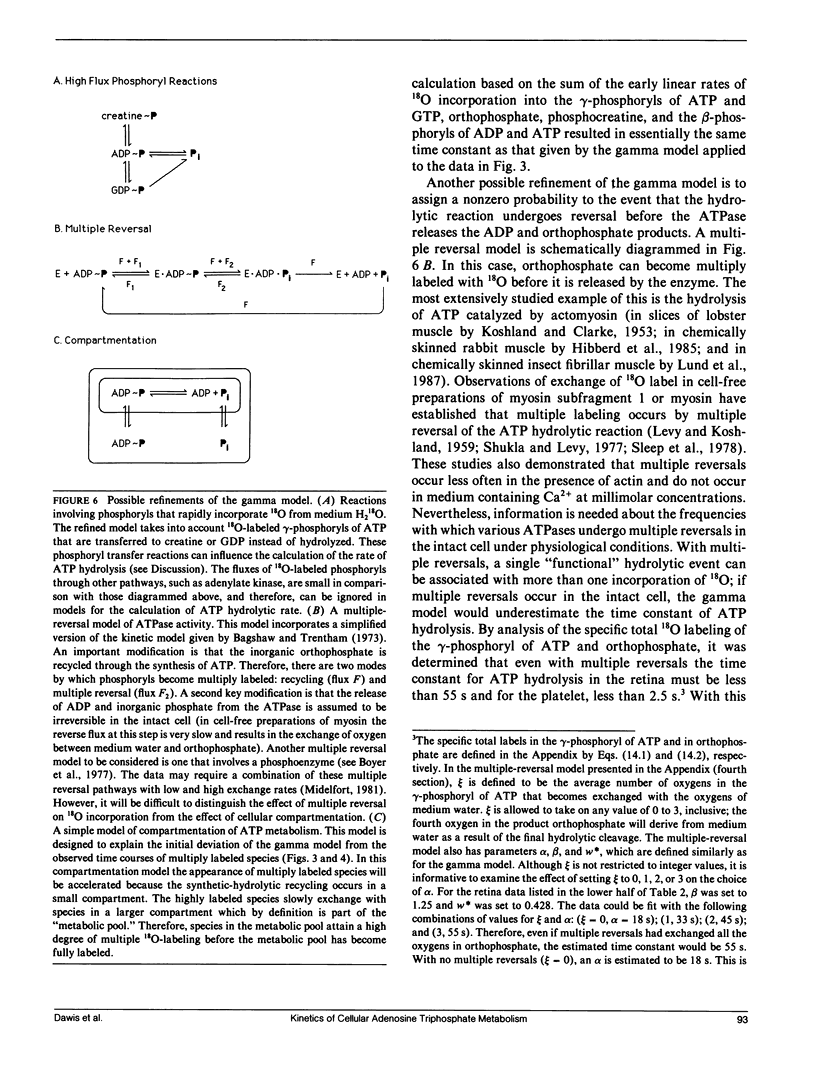
- Boyer P. D., de Meis L., da Gloria Costa Carvalho M., Hackney D. D. Dynamic reversal of enzyme carboxyl group phosphorylation as the basis of the oxygen exchange catalyzed by sarcoplasmic reticulum adenosine triphosphatase. Biochemistry. 1977 Jan 11;16(1):136–140. doi: 10.1021/bi00620a023. [DOI] [PubMed] [Google Scholar]
- Brown T. R., Ugurbil K., Shulman R. G. 31P nuclear magnetic resonance measurements of ATPase kinetics in aerobic Escherichia coli cells. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5551–5553. doi: 10.1073/pnas.74.12.5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman A. G., Atkinson D. E. Adenine nucleotide concentrations and turnover rates. Their correlation with biological activity in bacteria and yeast. Adv Microb Physiol. 1977;15:253–306. doi: 10.1016/s0065-2911(08)60318-5. [DOI] [PubMed] [Google Scholar]
- Dawis S. M., Graeff R. M., Heyman R. A., Walseth T. F., Goldberg N. D. Regulation of cyclic GMP metabolism in toad photoreceptors. Definition of the metabolic events subserving photoexcited and attenuated states. J Biol Chem. 1988 Jun 25;263(18):8771–8785. [PubMed] [Google Scholar]
- Dawson M. J., Gadian D. G., Wilkie D. R. Muscular fatigue investigated by phosphorus nuclear magnetic resonance. Nature. 1978 Aug 31;274(5674):861–866. doi: 10.1038/274861a0. [DOI] [PubMed] [Google Scholar]
- Goldberg N. D., Ames A. A., 3rd, Gander J. E., Walseth T. F. Magnitude of increase in retinal cGMP metabolic flux determined by 18O incorporation into nucleotide alpha-phosphoryls corresponds with intensity of photic stimulation. J Biol Chem. 1983 Aug 10;258(15):9213–9219. [PubMed] [Google Scholar]
- Hackney D. D., Boyer P. D. Evaluation of the partitioning of bound inorganic phosphate during medium and intermediate phosphate in equilibrium water oxygen exchange reactions of yeast inorganic pyrophosphatase. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3133–3137. doi: 10.1073/pnas.75.7.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackney D. D. Theoretical analysis of distribution of [18O]Pi species during exchange with water. Application to exchanges catalyzed by yeast inorganic pyrophosphatase. J Biol Chem. 1980 Jun 10;255(11):5320–5328. [PubMed] [Google Scholar]
- Hibberd M. G., Webb M. R., Goldman Y. E., Trentham D. R. Oxygen exchange between phosphate and water accompanies calcium-regulated ATPase activity of skinned fibers from rabbit skeletal muscle. J Biol Chem. 1985 Mar 25;260(6):3496–3500. [PubMed] [Google Scholar]
- Holmsen H. Nucleotide metabolism of platelets. Annu Rev Physiol. 1985;47:677–690. doi: 10.1146/annurev.ph.47.030185.003333. [DOI] [PubMed] [Google Scholar]
- KOSHLAND D. E., Jr, CLARKE E. Mechanism of hydrolysis of adenosinetriphosphate catalyzed by lobster muscle. J Biol Chem. 1953 Dec;205(2):917–924. [PubMed] [Google Scholar]
- Kimble E. A., Svoboda R. A., Ostroy S. E. Oxygen consumption and ATP changes of the vertebrate photoreceptor. Exp Eye Res. 1980 Sep;31(3):271–288. doi: 10.1016/s0014-4835(80)80037-6. [DOI] [PubMed] [Google Scholar]
- LEVY H. M., KOSHLAND D. E., Jr Mechanism of hydrolysis of adenosinetriphosphate by muscle proteins and its relation to muscular contraction. J Biol Chem. 1959 May;234(5):1102–1107. [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., HASSELBERGER F. X., SCHULZ D. W. EFFECT OF ISCHEMIA ON KNOWN SUBSTRATES AND COFACTORS OF THE GLYCOLYTIC PATHWAY IN BRAIN. J Biol Chem. 1964 Jan;239:18–30. [PubMed] [Google Scholar]
- Lee R. G., Lanir A., Clouse M. E. Liver adenine nucleotide metabolism during ischemia and reperfusion of mice livers studied by phosphorous-31 nuclear magnetic resonance. Invest Radiol. 1987 Jun;22(6):479–483. doi: 10.1097/00004424-198706000-00006. [DOI] [PubMed] [Google Scholar]
- Lund J., Webb M. R., White D. C. Changes in the ATPase activity of insect fibrillar flight muscle during calcium and strain activation probed by phosphate-water oxygen exchange. J Biol Chem. 1987 Jun 25;262(18):8584–8590. [PubMed] [Google Scholar]
- Midelfort C. F. On the mechanism of actomyosin ATPase from fast muscle. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2067–2071. doi: 10.1073/pnas.78.4.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimers H. J., Mustard J. F., Packham M. A. Transfer of adenine nucleotides between the releasable and nonreleasable compartments of rabbit blood platelets. J Cell Biol. 1975 Oct;67(1):61–71. doi: 10.1083/jcb.67.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla K. K., Levy H. M. Mechanism of oxygen exchange in actin-activated hydrolysis of adenosine triphosphate by myosin subfragment 1. Biochemistry. 1977 Jan 11;16(1):132–136. doi: 10.1021/bi00620a022. [DOI] [PubMed] [Google Scholar]
- Sleep J. A., Hackney D. D., Boyer P. D. Characterization of phosphate oxygen exchange reactions catalyzed by myosin through measurement of the distribution of 18-O-labeled species. J Biol Chem. 1978 Aug 10;253(15):5235–5238. [PubMed] [Google Scholar]
- Stryer L. Cyclic GMP cascade of vision. Annu Rev Neurosci. 1986;9:87–119. doi: 10.1146/annurev.ne.09.030186.000511. [DOI] [PubMed] [Google Scholar]
- Walseth T. F., Gander J. E., Eide S. J., Krick T. P., Goldberg N. D. 18O labeling of adenine nucleotide alpha-phosphoryls in platelets. Contribution by phosphodiesterase-catalyzed hydrolysis of cAMP. J Biol Chem. 1983 Feb 10;258(3):1544–1558. [PubMed] [Google Scholar]
- Walseth T. F., Graeff R. M., Goldberg N. D. Monitoring cyclic nucleotide metabolism in intact cells by 18O labeling. Methods Enzymol. 1988;159:60–74. doi: 10.1016/0076-6879(88)59008-0. [DOI] [PubMed] [Google Scholar]
- Winkler B. S. Glycolytic and oxidative metabolism in relation to retinal function. J Gen Physiol. 1981 Jun;77(6):667–692. doi: 10.1085/jgp.77.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman R., Weiter J. J. Oxygen transport in the bullfrog retina. Exp Eye Res. 1980 Feb;30(2):117–127. doi: 10.1016/0014-4835(80)90106-2. [DOI] [PubMed] [Google Scholar]


