Abstract
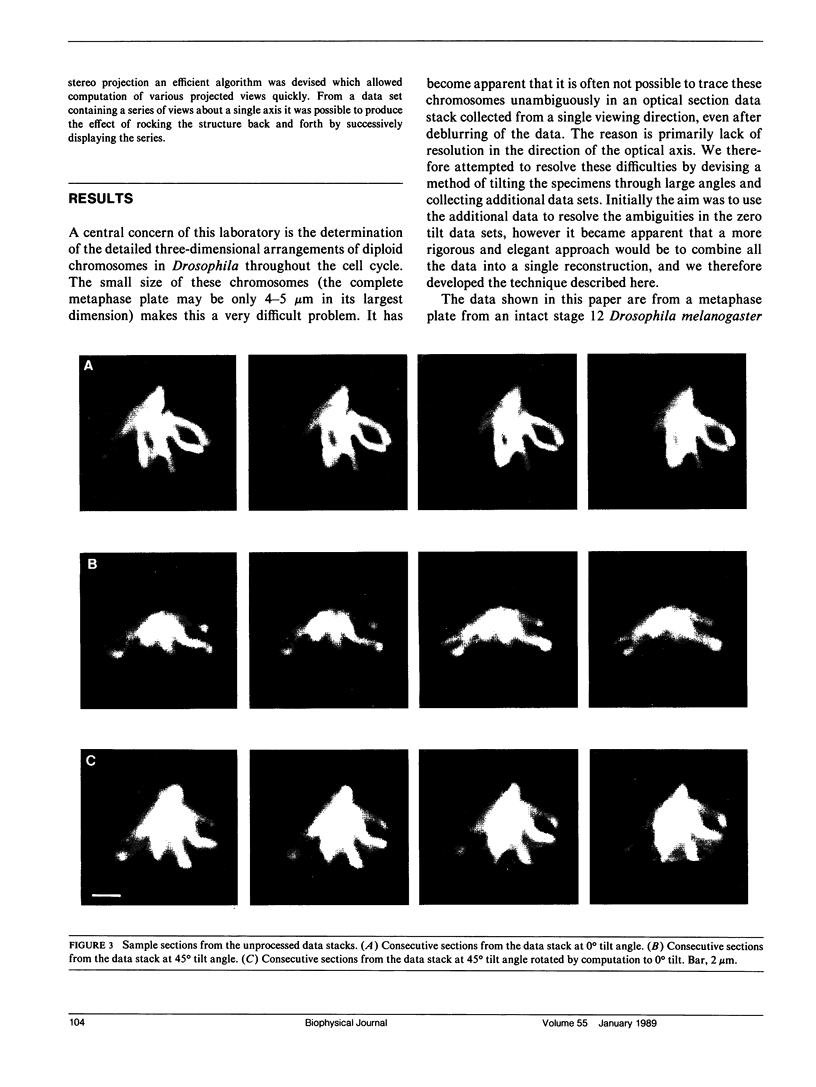
The resolution along the optical axis (z) is much less than the in-plane resolution in any current optical microscope, conventional or otherwise. We have used mutually tilted, through-focal section views of the same object to provide a solution to this problem. A tilting specimen stage was constructed for an optical microscope, which with the use of a coverslip-free water immersion lens, allowed the collection of data sets from intact Drosophila melanogaster embryos at viewing directions up to 90 degrees apart. We have devised an image processing scheme to determine the relative tilt, translation, and sampling parameters of the different data sets. This involves the use of a modified phase cross-correlation function, which produces a very sharp maximum. Finally the data sets are merged using figure-of-merit and local area scaling techniques borrowed from x-ray protein crystallography. We demonstrate the application of this technique to data sets from a metaphase plate in an embryo of Drosophila melanogaster. As expected, the merged reconstruction combined the highest resolution available in the individual data sets. As estimated from the Fourier transform, the final resolution is 0.25 microns in x and y and 0.4 microns in z. In the final reconstruction all ten chromosome arms can be easily delineated; this was not possible in the individual data sets. Within many of the arms the two individual chromatids can be seen. In some cases the chromatids are wrapped around each other helically, in others they lie alongside each other in a parallel arrangement.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agard D. A. Optical sectioning microscopy: cellular architecture in three dimensions. Annu Rev Biophys Bioeng. 1984;13:191–219. doi: 10.1146/annurev.bb.13.060184.001203. [DOI] [PubMed] [Google Scholar]
- Agard D. A., Sedat J. W. Three-dimensional architecture of a polytene nucleus. Nature. 1983 Apr 21;302(5910):676–681. doi: 10.1038/302676a0. [DOI] [PubMed] [Google Scholar]
- Boocock C. A., Brown A. F., Dunn G. A. A simple chamber for observing microscopic specimens in both top and side views. J Microsc. 1985 Jan;137(Pt 1):29–34. doi: 10.1111/j.1365-2818.1985.tb02558.x. [DOI] [PubMed] [Google Scholar]
- Hiraoka Y., Sedat J. W., Agard D. A. The use of a charge-coupled device for quantitative optical microscopy of biological structures. Science. 1987 Oct 2;238(4823):36–41. doi: 10.1126/science.3116667. [DOI] [PubMed] [Google Scholar]
- Mitchison T. J., Sedat J. Localization of antigenic determinants in whole Drosophila embryos. Dev Biol. 1983 Sep;99(1):261–264. doi: 10.1016/0012-1606(83)90275-0. [DOI] [PubMed] [Google Scholar]
- Saxton W. O., Frank J. Motif detection in quantum noise-limited electron micrographs by cross-correlation. Ultramicroscopy. 1977 Apr;2(2-3):219–227. doi: 10.1016/s0304-3991(76)91385-1. [DOI] [PubMed] [Google Scholar]
- Skaer R. J., Whytock S. Interpretation of the three-dimensional structure of living nuclei by specimen tilt. J Cell Sci. 1975 Oct;19(1):1–10. doi: 10.1242/jcs.19.1.1. [DOI] [PubMed] [Google Scholar]
- White J. G., Amos W. B., Fordham M. An evaluation of confocal versus conventional imaging of biological structures by fluorescence light microscopy. J Cell Biol. 1987 Jul;105(1):41–48. doi: 10.1083/jcb.105.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]