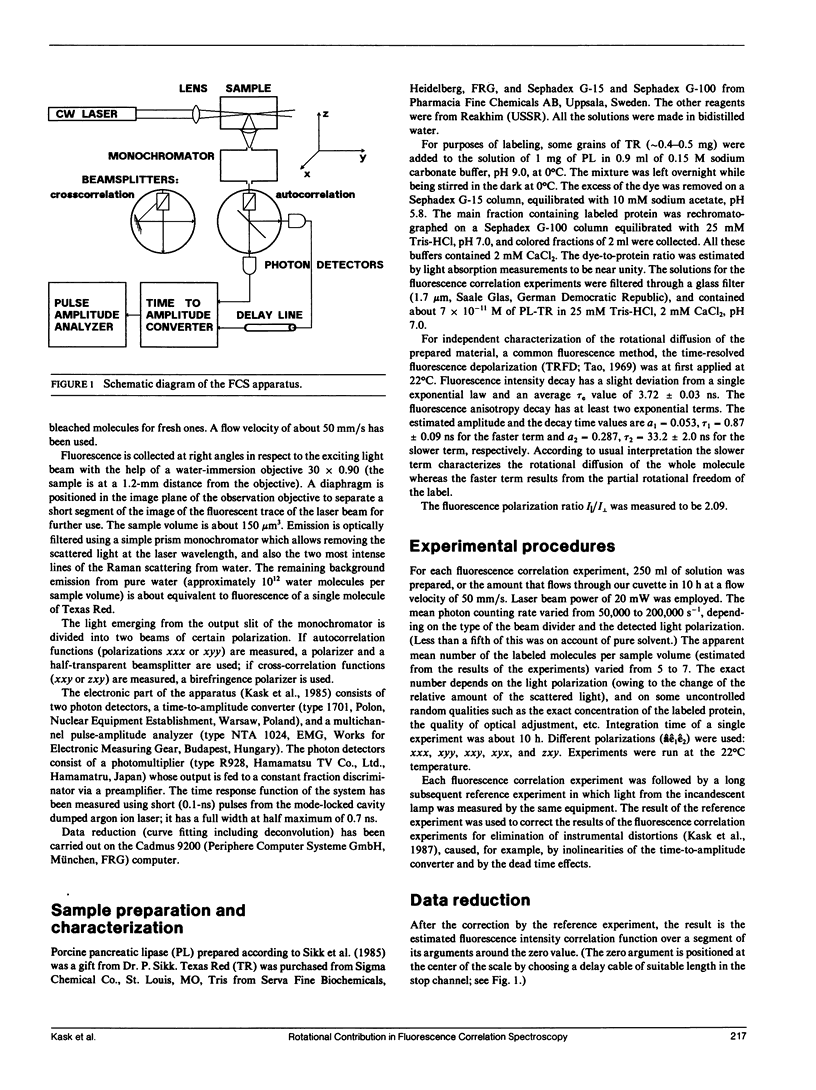
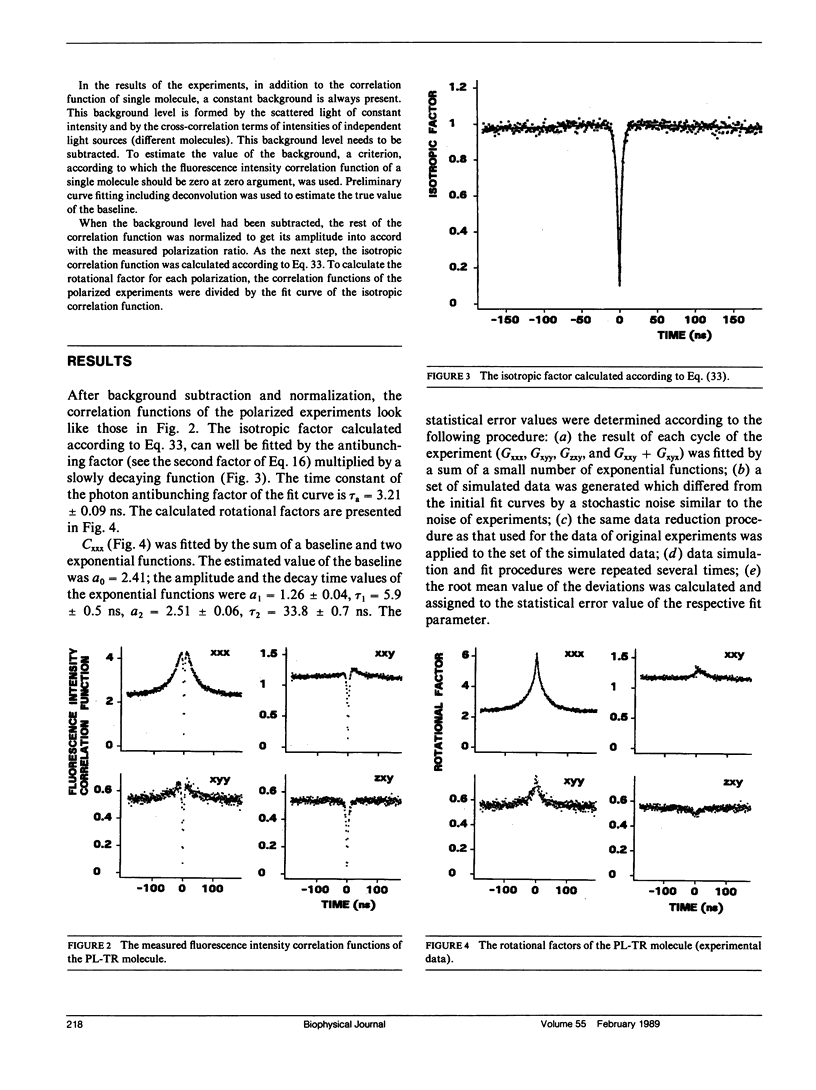
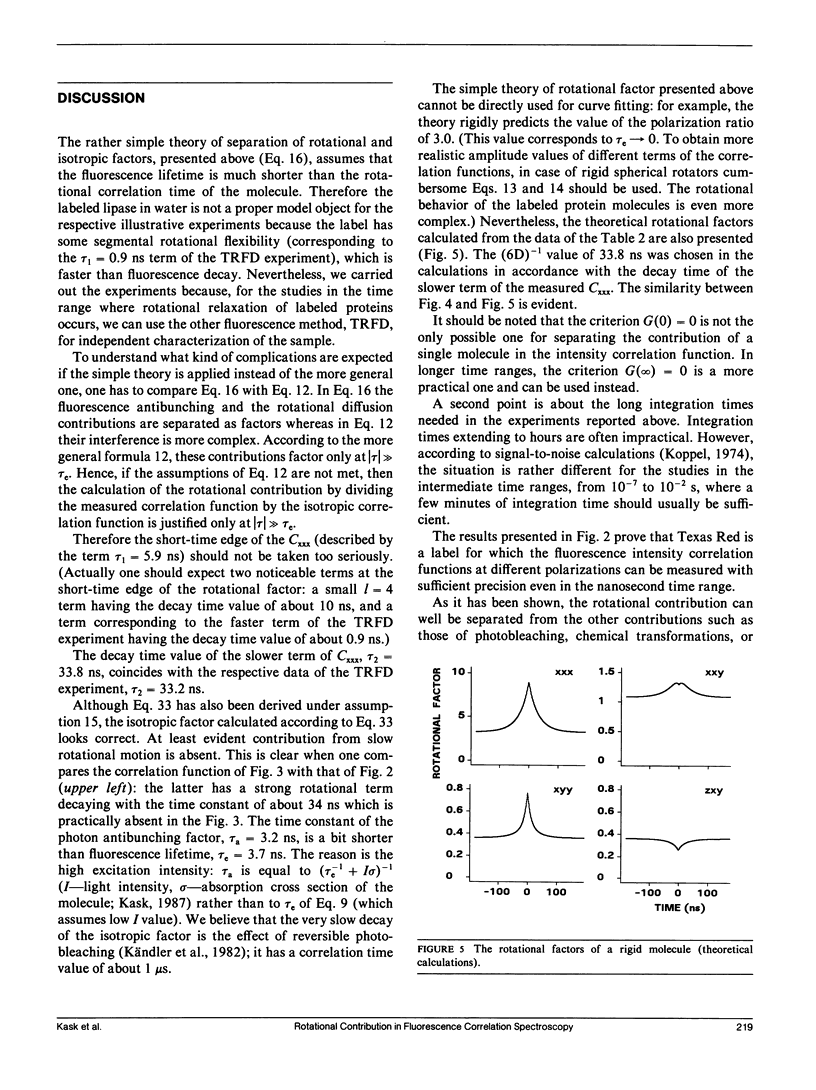
Abstract
The theory of fluorescence correlation spectroscopy is reexamined with the aim of separating the contribution of rotational diffusion. Under constant excitation, fluorescence correlation experiments are characterized by three polarizations: one of the incident beam and two of the two photon detectors. A set of experiments of different polarizations is proposed for study. From the results of the experiments the isotropic factor of the fluorescence intensity correlation functions can be determined, which is independent of the rotational motion of the sample molecule. This function can be used to represent each fluorescence intensity correlation function as the product of the isotropic and the rotational factors. The theory is illustrated by an experiment in which rotational diffusion of porcine pancreatic lipase labeled with Texas Red was observed Texas Red is a label that allows precise fluorescence correlation experiments even in the nanosecond time range.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andries C., Guedens W., Clauwaert J., Geerts H. Photon and fluorescence correlation spectroscopy and light scattering of eye-lens proteins at moderate concentrations. Biophys J. 1983 Sep;43(3):345–354. doi: 10.1016/S0006-3495(83)84358-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borejdo J. Motion of myosin fragments during actin-activated ATPase: fluorescence correlation spectroscopy study. Biopolymers. 1979 Nov;18(11):2807–2820. doi: 10.1002/bip.1979.360181111. [DOI] [PubMed] [Google Scholar]
- Borejdo J., Putnam S., Morales M. F. Fluctuations in polarized fluorescence: evidence that muscle cross bridges rotate repetitively during contraction. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6346–6350. doi: 10.1073/pnas.76.12.6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenberg M., Rigler R. Fluorescence correlation spectroscopy applied to rotational diffusion of macromolecules. Q Rev Biophys. 1976 Feb;9(1):69–81. doi: 10.1017/s003358350000216x. [DOI] [PubMed] [Google Scholar]
- Hoshikawa H., Asai H. On the rotational brownian motion of a bacterial idle motor. II. Theory of fluorescence correlation spectroscopy. Biophys Chem. 1985 Aug;22(3):167–172. doi: 10.1016/0301-4622(85)80039-9. [DOI] [PubMed] [Google Scholar]
- Kask P., Piksarv P., Mets U., Pooga M., Lippmaa E. Fluorescence correlation spectroscopy in the nanosecond time range: rotational diffusion of bovine carbonic anhydrase B. Eur Biophys J. 1987;14(4):257–261. doi: 10.1007/BF00256359. [DOI] [PubMed] [Google Scholar]
- Magde D., Elson E. L., Webb W. W. Fluorescence correlation spectroscopy. II. An experimental realization. Biopolymers. 1974 Jan;13(1):29–61. doi: 10.1002/bip.1974.360130103. [DOI] [PubMed] [Google Scholar]
- Palmer A. G., 3rd, Thompson N. L. Molecular aggregation characterized by high order autocorrelation in fluorescence correlation spectroscopy. Biophys J. 1987 Aug;52(2):257–270. doi: 10.1016/S0006-3495(87)83213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen N. O. Diffusion and aggregation in biological membranes. Can J Biochem Cell Biol. 1984 Nov;62(11):1158–1166. doi: 10.1139/o84-149. [DOI] [PubMed] [Google Scholar]
- Schlessinger J., Koppel D. E., Axelrod D., Jacobson K., Webb W. W., Elson E. L. Lateral transport on cell membranes: mobility of concanavalin A receptors on myoblasts. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2409–2413. doi: 10.1073/pnas.73.7.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikk P., Osa A., Aaviksaar A. Irreversible inhibition of pancreatic lipase by bis-p-nitrophenyl methylphosphonate. FEBS Lett. 1985 May 20;184(2):193–196. doi: 10.1016/0014-5793(85)80605-0. [DOI] [PubMed] [Google Scholar]
- Sorscher S. M., Bartholomew J. C., Klein M. P. The use of fluorescence correlations spectroscopy to probe chromatin in the cell nucleus. Biochim Biophys Acta. 1980 Nov 14;610(1):28–46. doi: 10.1016/0005-2787(80)90053-2. [DOI] [PubMed] [Google Scholar]
- Thompson N. L., Axelrod D. Immunoglobulin surface-binding kinetics studied by total internal reflection with fluorescence correlation spectroscopy. Biophys J. 1983 Jul;43(1):103–114. doi: 10.1016/S0006-3495(83)84328-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus J. A., Haugland R., Sharrow S. O., Segal D. M. Texas Red, a hydrophilic, red-emitting fluorophore for use with fluorescein in dual parameter flow microfluorometric and fluorescence microscopic studies. J Immunol Methods. 1982;50(2):193–204. doi: 10.1016/0022-1759(82)90225-3. [DOI] [PubMed] [Google Scholar]
- Webb W. W. Applications of fluorescence correlation spectroscopy. Q Rev Biophys. 1976 Feb;9(1):49–68. doi: 10.1017/s0033583500002158. [DOI] [PubMed] [Google Scholar]
- Wegener W. A., Rigler R. Separation of translational and rotational contributions in solution studies using fluorescence photobleaching recovery. Biophys J. 1984 Dec;46(6):787–793. doi: 10.1016/S0006-3495(84)84077-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman M., Schindler H., Feher G. Determination of molecular weights by fluctuation spectroscopy: application to DNA. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2776–2780. doi: 10.1073/pnas.73.8.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]


