Abstract
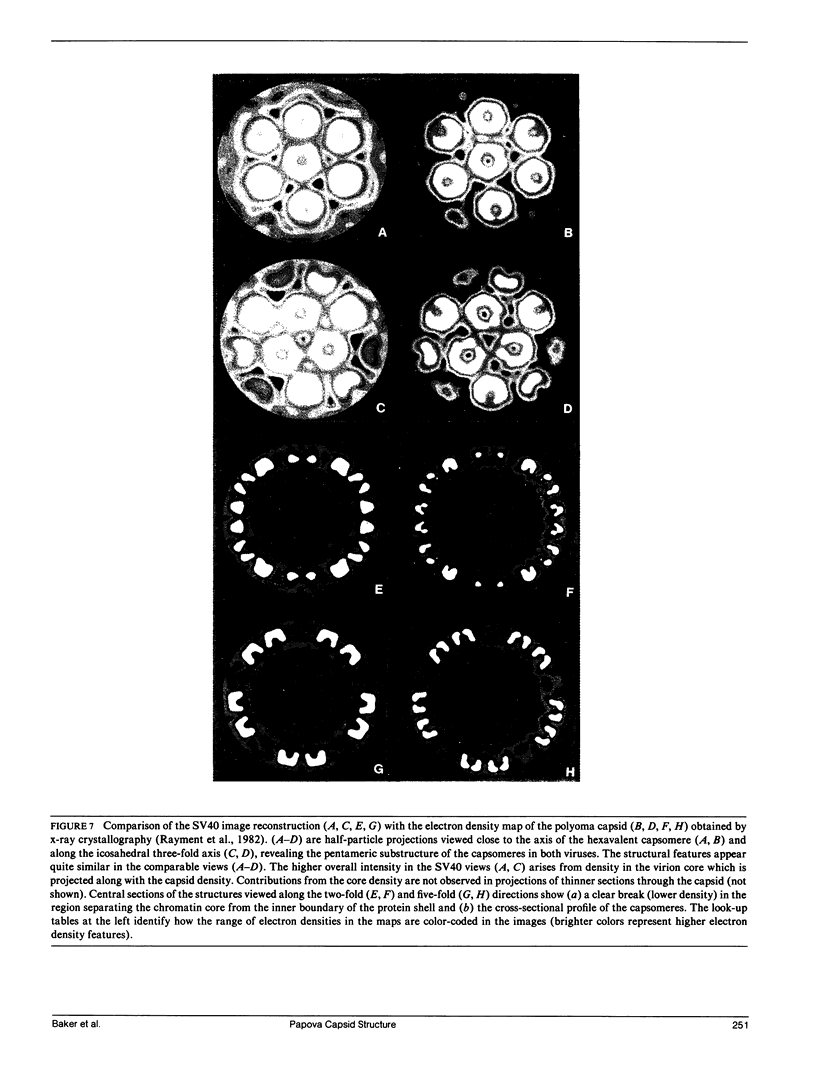
The three-dimensional structure of the simian virus 40 capsid is remarkably similar to the structure of the polyoma empty capsid. This similarity is apparent despite striking differences in the methods used to determine the two structures: image analysis of electron micrographs of frozen-hydrated samples (SV40 virions) and an unconventional x-ray crystallographic analysis (polyoma empty capsids). Both methods have clearly resolved the 72 prominent capsomere units which comprise the T = 7d icosahedral capsid surface lattice. The 12 pentavalent and 60 hexavalent capsomeres consist of pentameric substructures. A pentameric morphology for hexavalent capsomeres clearly shows that the conserved bonding specificity expected from the quasi-equivalence theory is not present in either SV40 or polyoma capsids. Determination of the SV40 structure from cryo-electron microscopy supports the correctness of the polyoma structure solved crystallographically and establishes a strong complementarity of the two techniques. Similarity between the SV40 virion and the empty polyoma capsid indicates that the capsid is not detectably altered by the loss of the nucleohistone core. The unexpected pentameric substructure of the hexavalent capsomeres and the arrangement of the 72 pentamers in the SV40 and polyoma capsid lattices may be characteristic features of all members of the papova virus family, including the papilloma viruses such as human wart and rabbit papilloma.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian M., Dubochet J., Lepault J., McDowall A. W. Cryo-electron microscopy of viruses. Nature. 1984 Mar 1;308(5954):32–36. doi: 10.1038/308032a0. [DOI] [PubMed] [Google Scholar]
- Anderer F. A., Schlumberger H. D., Koch M. A., Frank H., Eggers H. J. Structure of simian virus 40. II. Symmetry and components of the virus particle. Virology. 1967 Jul;32(3):511–523. doi: 10.1016/0042-6822(67)90303-0. [DOI] [PubMed] [Google Scholar]
- Baker T. S., Amos L. A. Structure of the tubulin dimer in zinc-induced sheets. J Mol Biol. 1978 Jul 25;123(1):89–106. doi: 10.1016/0022-2836(78)90378-9. [DOI] [PubMed] [Google Scholar]
- Baker T. S., Caspar D. L., Murakami W. T. Polyoma virus 'hexamer' tubes consist of paired pentamers. Nature. 1983 Jun 2;303(5916):446–448. doi: 10.1038/303446a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker T. S., Drak J., Bina M. Reconstruction of the three-dimensional structure of simian virus 40 and visualization of the chromatin core. Proc Natl Acad Sci U S A. 1988 Jan;85(2):422–426. doi: 10.1073/pnas.85.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner I., Kuhn C., Fanning E. Identification and characterization of fast-sedimenting SV40 nucleoprotein complexes. Virology. 1979 Jul 15;96(1):54–63. doi: 10.1016/0042-6822(79)90172-7. [DOI] [PubMed] [Google Scholar]
- Bina M., Beecher S., Blasquez V. Stability and components of mature simian virus 40. Biochemistry. 1982 Jun 22;21(13):3057–3063. doi: 10.1021/bi00256a004. [DOI] [PubMed] [Google Scholar]
- Blasquez V., Beecher S., Bina M. Simian virus 40 morphogenetic pathway. An analysis of assembly-defective tsB201 DNA protein complexes. J Biol Chem. 1983 Jul 10;258(13):8477–8484. [PubMed] [Google Scholar]
- CASPAR D. L., KLUG A. Physical principles in the construction of regular viruses. Cold Spring Harb Symp Quant Biol. 1962;27:1–24. doi: 10.1101/sqb.1962.027.001.005. [DOI] [PubMed] [Google Scholar]
- Chiu W. Electron microscopy of frozen, hydrated biological specimens. Annu Rev Biophys Biophys Chem. 1986;15:237–257. doi: 10.1146/annurev.bb.15.060186.001321. [DOI] [PubMed] [Google Scholar]
- Crowther R. A., Amos L. A., Finch J. T., De Rosier D. J., Klug A. Three dimensional reconstructions of spherical viruses by fourier synthesis from electron micrographs. Nature. 1970 May 2;226(5244):421–425. doi: 10.1038/226421a0. [DOI] [PubMed] [Google Scholar]
- Crowther R. A., Amos L. A. Harmonic analysis of electron microscope images with rotational symmetry. J Mol Biol. 1971 Aug 28;60(1):123–130. doi: 10.1016/0022-2836(71)90452-9. [DOI] [PubMed] [Google Scholar]
- Crowther R. A. Procedures for three-dimensional reconstruction of spherical viruses by Fourier synthesis from electron micrographs. Philos Trans R Soc Lond B Biol Sci. 1971 May 27;261(837):221–230. doi: 10.1098/rstb.1971.0054. [DOI] [PubMed] [Google Scholar]
- DeRosier D. J., Moore P. B. Reconstruction of three-dimensional images from electron micrographs of structures with helical symmetry. J Mol Biol. 1970 Sep 14;52(2):355–369. doi: 10.1016/0022-2836(70)90036-7. [DOI] [PubMed] [Google Scholar]
- Eisenberg D. A problem for the theory of biological structure. Nature. 1982 Jan 14;295(5845):99–100. doi: 10.1038/295099a0. [DOI] [PubMed] [Google Scholar]
- Fernandez-Munoz R., Coca-Prados M., Hsu M. T. Intracellular forms of simian virus 40 nucleoprotein complexes. I. Methods of isolation and characterization in CV-1 cells. J Virol. 1979 Feb;29(2):612–623. doi: 10.1128/jvi.29.2.612-623.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch J. T., Klug A. The structure of viruses of the papilloma-polyoma type 3. Structure of rabbit papilloma virus, with an appendix on the topography of contrast in negative-staining for electron-microscopy. J Mol Biol. 1965 Aug;13(1):1–12. doi: 10.1016/s0022-2836(65)80075-4. [DOI] [PubMed] [Google Scholar]
- Finch J. T. The surface structure of polyoma virus. J Gen Virol. 1974 Aug;24(2):359–364. doi: 10.1099/0022-1317-24-2-359. [DOI] [PubMed] [Google Scholar]
- Friedmann T., David D. Structural roles of polyoma virus proteins. J Virol. 1972 Oct;10(4):776–782. doi: 10.1128/jvi.10.4.776-782.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller S. D. The T=4 envelope of Sindbis virus is organized by interactions with a complementary T=3 capsid. Cell. 1987 Mar 27;48(6):923–934. doi: 10.1016/0092-8674(87)90701-x. [DOI] [PubMed] [Google Scholar]
- Garber E. A., Seidman M. M., Levine A. J. The detection and characterization of multiple forms of SV40 nucleoprotein complexes. Virology. 1978 Oct 15;90(2):305–316. doi: 10.1016/0042-6822(78)90315-x. [DOI] [PubMed] [Google Scholar]
- Griffith J. D. Chromatin structure: deduced from a minichromosome. Science. 1975 Mar 28;187(4182):1202–1203. doi: 10.1126/science.187.4182.1202. [DOI] [PubMed] [Google Scholar]
- HORNE R. W., WILDY P. Symmetry in virus architecture. Virology. 1961 Nov;15:348–373. doi: 10.1016/0042-6822(61)90366-x. [DOI] [PubMed] [Google Scholar]
- Horne R. W., Wildy P. An historical account of the development and applications of the negative staining technique to the electron microscopy of viruses. J Microsc. 1979 Sep;117(1):103–122. doi: 10.1111/j.1365-2818.1979.tb00234.x. [DOI] [PubMed] [Google Scholar]
- KLUG A., FINCH J. T. STRUCTURE OF VIRUSES OF THE PAPILLOMA-POLYOMA TYPE. I. HUMAN WART VIRUS. J Mol Biol. 1965 Feb;11:403–423. doi: 10.1016/s0022-2836(65)80066-3. [DOI] [PubMed] [Google Scholar]
- KLUG A. STRUCTURE OF VIRUSES OF THE PAPILLOMA-POLYOMA TYPE. II. COMMENTS ON OTHER WORK. J Mol Biol. 1965 Feb;11:424–431. doi: 10.1016/s0022-2836(65)80067-5. [DOI] [PubMed] [Google Scholar]
- Klug A. Architectural design of spherical viruses. Nature. 1983 Jun 2;303(5916):378–379. doi: 10.1038/303378a0. [DOI] [PubMed] [Google Scholar]
- Klug A., Finch J. T. Structure of viruses of the papilloma-polyoma type. IV. Analysis of tilting experiments in the electron microscope. J Mol Biol. 1968 Jan 14;31(1):1–12. doi: 10.1016/0022-2836(68)90050-8. [DOI] [PubMed] [Google Scholar]
- Lepault J., Leonard K. Three-dimensional structure of unstained, frozen-hydrated extended tails of bacteriophage T4. J Mol Biol. 1985 Apr 5;182(3):431–441. doi: 10.1016/0022-2836(85)90202-5. [DOI] [PubMed] [Google Scholar]
- MATTERN C. F. Polyoma and papilloma viruses: do they have 42 or 92 subunits? Science. 1962 Aug 24;137(3530):612–613. doi: 10.1126/science.137.3530.612. [DOI] [PubMed] [Google Scholar]
- MAYOR H. D., MELNICK J. L. Icosahedral models and viruses: a critical evaluation. Science. 1962 Aug 24;137(3530):613–615. doi: 10.1126/science.137.3530.613. [DOI] [PubMed] [Google Scholar]
- MELNICK J. L. Papova virus group. Science. 1962 Mar 30;135(3509):1128–1130. doi: 10.1126/science.135.3509.1128. [DOI] [PubMed] [Google Scholar]
- Milligan R. A., Brisson A., Unwin P. N. Molecular structure determination of crystalline specimens in frozen aqueous solutions. Ultramicroscopy. 1984;13(1-2):1–9. doi: 10.1016/0304-3991(84)90051-2. [DOI] [PubMed] [Google Scholar]
- Oliver R. M. Negative stain electron microscopy of protein macromolecules. Methods Enzymol. 1973;27:616–672. doi: 10.1016/s0076-6879(73)27029-5. [DOI] [PubMed] [Google Scholar]
- Rayment I., Baker T. S., Caspar D. L., Murakami W. T. Polyoma virus capsid structure at 22.5 A resolution. Nature. 1982 Jan 14;295(5845):110–115. doi: 10.1038/295110a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salunke D. M., Caspar D. L., Garcea R. L. Self-assembly of purified polyomavirus capsid protein VP1. Cell. 1986 Sep 12;46(6):895–904. doi: 10.1016/0092-8674(86)90071-1. [DOI] [PubMed] [Google Scholar]
- Stewart M., Vigers G. Electron microscopy of frozen-hydrated biological material. Nature. 1986 Feb 20;319(6055):631–636. doi: 10.1038/319631a0. [DOI] [PubMed] [Google Scholar]
- Unwin P. N. Electron microscopy of the stacked disk aggregate of tobacco mosaic virus protein. II. The influence of electron irradiation of the stain distribution. J Mol Biol. 1974 Aug 25;87(4):657–670. doi: 10.1016/0022-2836(74)90076-x. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Fisher H. W. Electron microscopy of tobacco mosaic virus under conditions of minimal beam exposure. J Mol Biol. 1970 Aug 28;52(1):121–123. doi: 10.1016/0022-2836(70)90181-6. [DOI] [PubMed] [Google Scholar]
- Yuen L. K., Consigli R. A. Identification and protein analysis of polyomavirus assembly intermediates from infected primary mouse embryo cells. Virology. 1985 Jul 15;144(1):127–138. doi: 10.1016/0042-6822(85)90311-3. [DOI] [PubMed] [Google Scholar]







