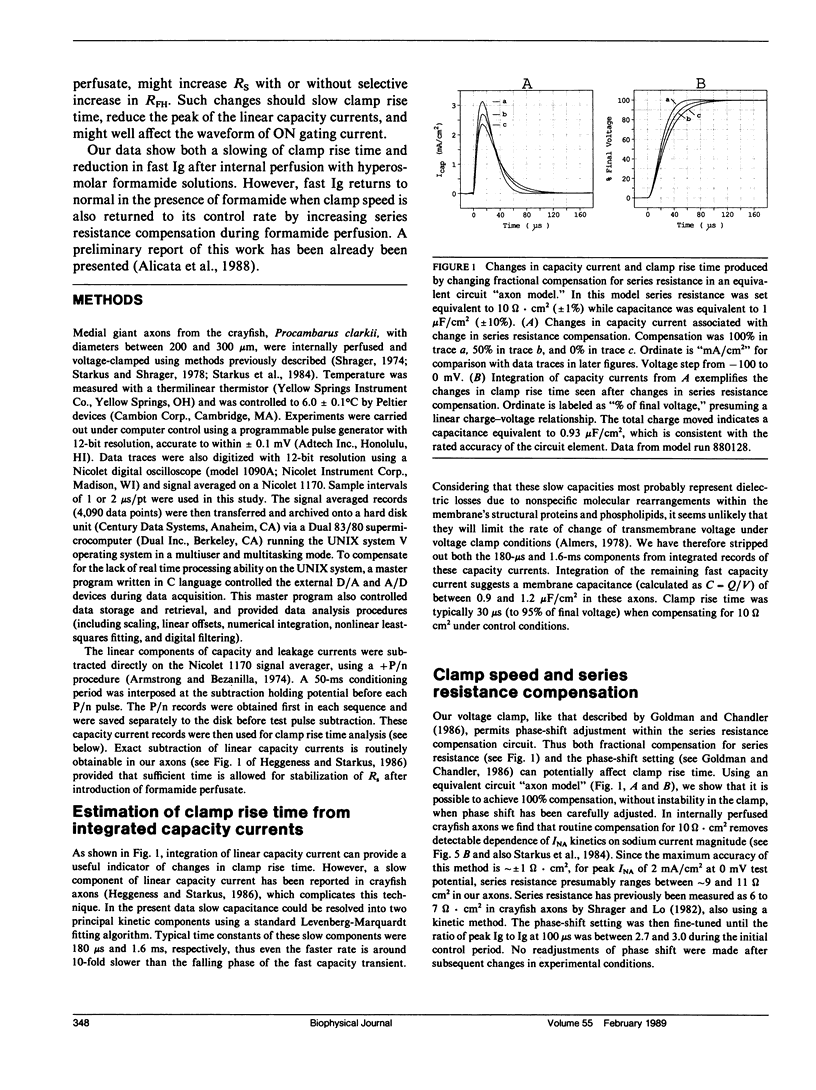
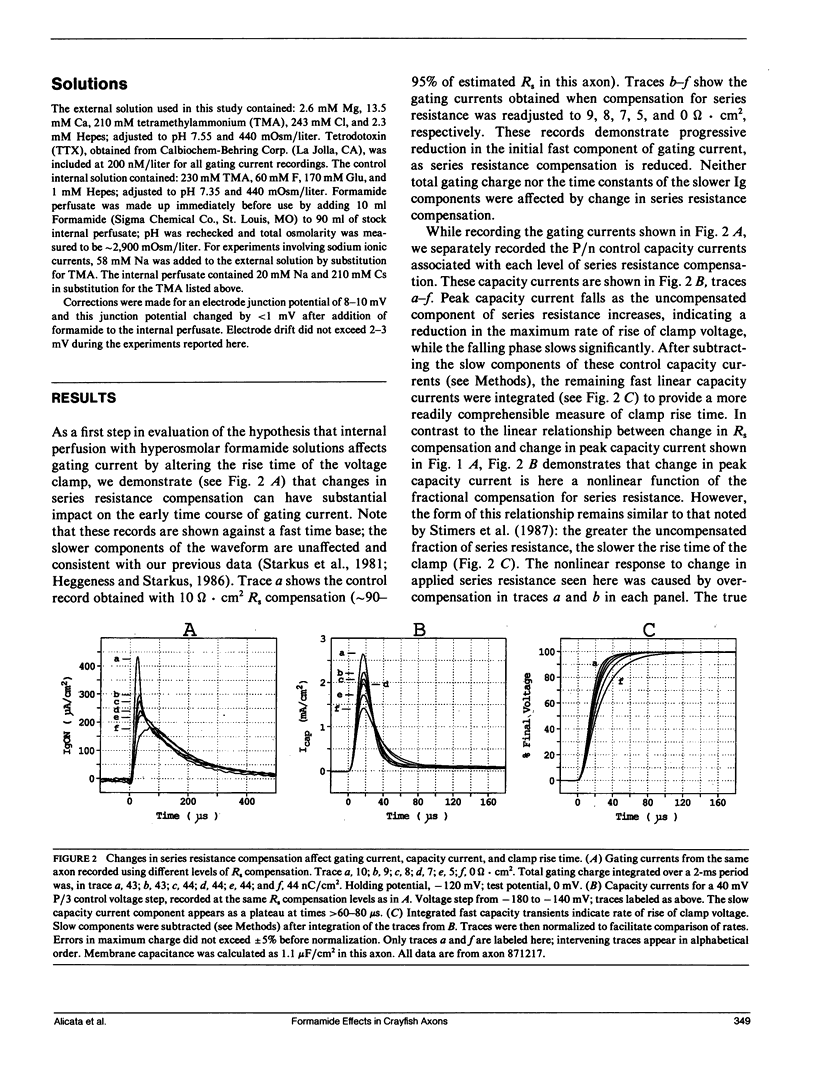
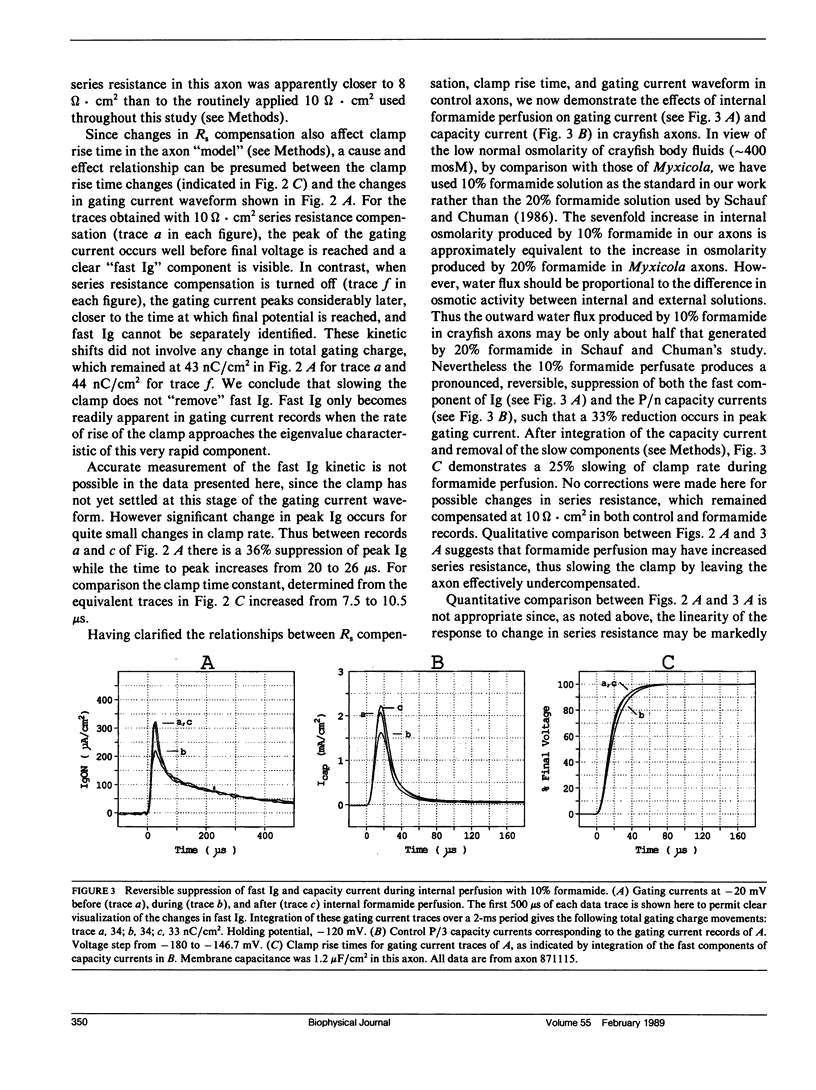
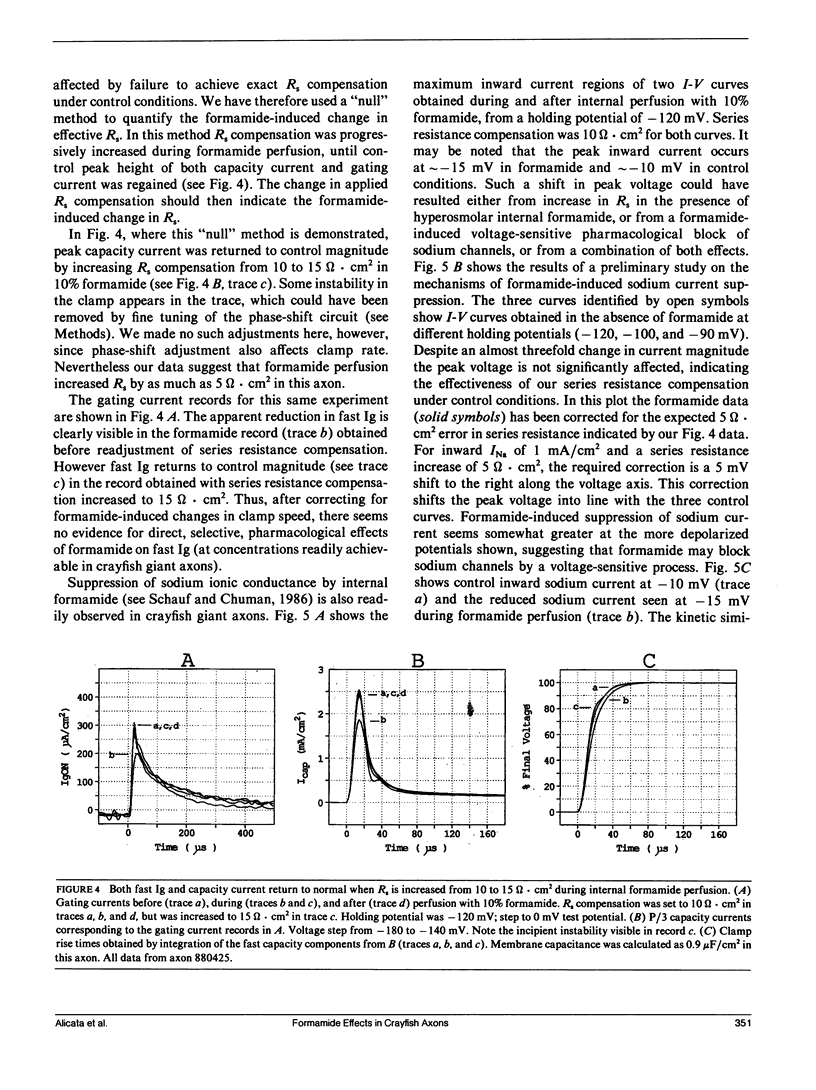
Abstract
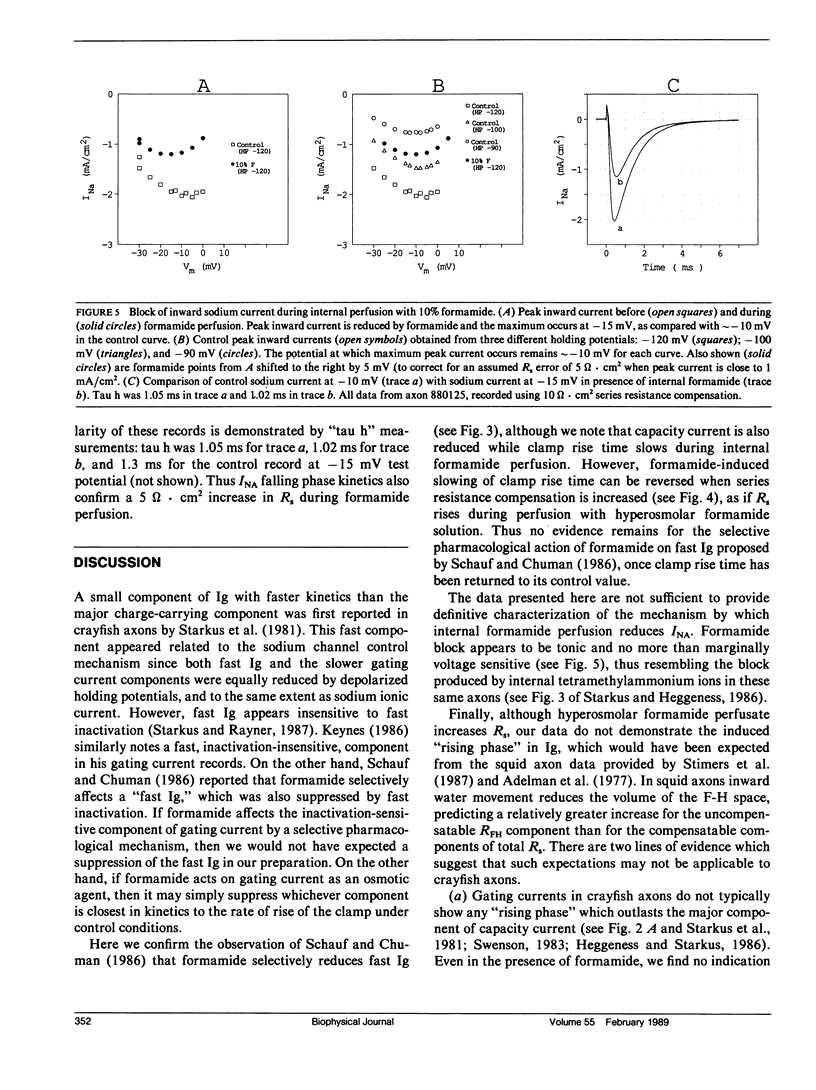
Internal perfusion with solutions made hyperosmolar by 10% formamide selectively reduces the initial fast component of ON gating current (fast Ig) in crayfish axons. This result parallels the effects of formamide perfusion seen in Myxicola giant axons (Schauf, C. L., and M. A. Chuman. 1986. Neural Membranes. Alan R. Liss, Inc., New York. 3-23). However, our findings do not confirm their conclusion that internal formamide has a specific pharmacological effect on fast Ig. Formamide-induced suppression of fast Ig is always associated with changes in linear capacity current, indicating a reduction in the rate of rise of the voltage clamp. Furthermore, this suppression of fast Ig can be reversed when clamp rise time is returned to its control rate by increasing compensation for series resistance (Rs) during formamide perfusion. Increases in Rs during 10% formamide perfusion of up to 5 omega.cm2 were measured by evaluating the increase in Rs compensation required to return the following parameters to their control levels: (a) peak capacity current, (b) peak gating current, (c) the voltage maximum of the /Na-V curve, and (d) "tau h". We conclude that hyperosmolar internal formamide increases Rs, reduces clamp speed, and thus selectively suppresses fast Ig. On the other hand, the reversible block of sodium ionic current by internal formamide, reported by Schauf and Chuman, is not eliminated by correcting for series resistance changes during formamide perfusion.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman W. J., Jr, Moses J., Rive R. V. An anatomical basis for the resistance and capacitance in series with excitable membrane of the squid giant axon. J Neurocytol. 1977 Dec;6(6):621–646. doi: 10.1007/BF01176377. [DOI] [PubMed] [Google Scholar]
- Almers W. Gating currents and charge movements in excitable membranes. Rev Physiol Biochem Pharmacol. 1978;82:96–190. doi: 10.1007/BFb0030498. [DOI] [PubMed] [Google Scholar]
- Armstrong C. M., Bezanilla F. Charge movement associated with the opening and closing of the activation gates of the Na channels. J Gen Physiol. 1974 May;63(5):533–552. doi: 10.1085/jgp.63.5.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M., Gilly W. F. Fast and slow steps in the activation of sodium channels. J Gen Physiol. 1979 Dec;74(6):691–711. doi: 10.1085/jgp.74.6.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F., Taylor R. E. Temperature effects on gating currents in the squid giant axon. Biophys J. 1978 Sep;23(3):479–484. doi: 10.1016/S0006-3495(78)85464-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The after-effects of impulses in the giant nerve fibres of Loligo. J Physiol. 1956 Feb 28;131(2):341–376. doi: 10.1113/jphysiol.1956.sp005467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman L., Chandler R. E. Geographical distribution and inactivation kinetics in internally perfused Myxicola giant axons. Biophys J. 1986 Mar;49(3):761–766. doi: 10.1016/S0006-3495(86)83702-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heggeness S. T., Starkus J. G. Saxitoxin and tetrodotoxin. Electrostatic effects on sodium channel gating current in crayfish axons. Biophys J. 1986 Mar;49(3):629–643. doi: 10.1016/S0006-3495(86)83690-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynes R. D. Properties of the sodium gating current in the squid giant axon. Ann N Y Acad Sci. 1986;479:431–438. doi: 10.1111/j.1749-6632.1986.tb15586.x. [DOI] [PubMed] [Google Scholar]
- Shrager P. Ionic conductance changes in voltage clamped crayfish axons at low pH. J Gen Physiol. 1974 Dec;64(6):666–690. doi: 10.1085/jgp.64.6.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrager P., Lo M. V. Influence of ionic current on Na+ channel gating in crayfish giant axon. Nature. 1982 Apr 1;296(5856):450–452. doi: 10.1038/296450a0. [DOI] [PubMed] [Google Scholar]
- Shrager P., Starkus J. C., Lo M. V., Peracchia C. The periaxonal space of crayfish giant axons. J Gen Physiol. 1983 Aug;82(2):221–244. doi: 10.1085/jgp.82.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkus J. G., Fellmeth B. D., Rayner M. D. Gating currents in th intact crayfish giant axon. Biophys J. 1981 Aug;35(2):521–533. doi: 10.1016/S0006-3495(81)84807-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkus J. G., Heggeness S. T., Rayner M. D. Kinetic analysis of sodium channel block by internal methylene blue in pronased crayfish giant axons. Biophys J. 1984 Aug;46(2):205–218. doi: 10.1016/S0006-3495(84)84014-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkus J. G., Shrager P. Modification of slow sodium inactivation in nerve after internal perfusion with trypsin. Am J Physiol. 1978 Nov;235(5):C238–C244. doi: 10.1152/ajpcell.1978.235.5.C238. [DOI] [PubMed] [Google Scholar]
- Stimers J. R., Bezanilla F., Taylor R. E. Sodium channel gating currents. Origin of the rising phase. J Gen Physiol. 1987 Apr;89(4):521–540. doi: 10.1085/jgp.89.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson R. P., Jr A slow component of gating current in crayfish giant axons resembles inactivation charge movement. Biophys J. 1983 Mar;41(3):245–249. doi: 10.1016/S0006-3495(83)84434-8. [DOI] [PMC free article] [PubMed] [Google Scholar]



