Abstract
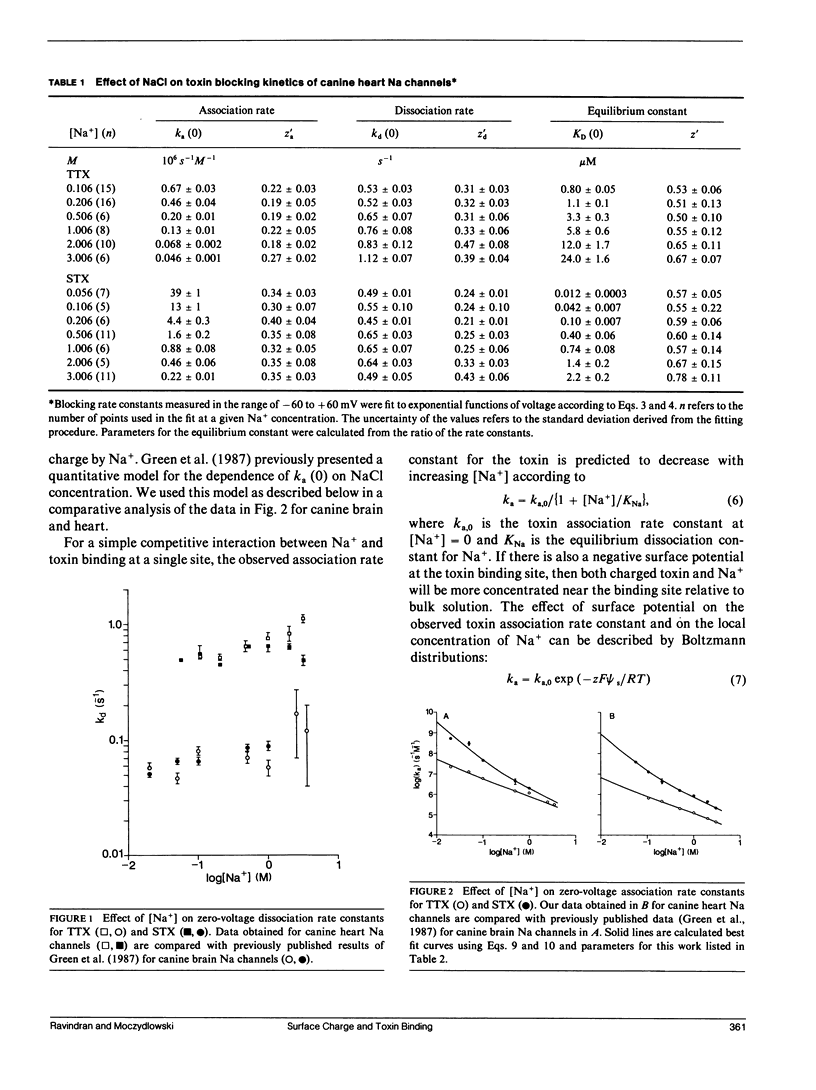
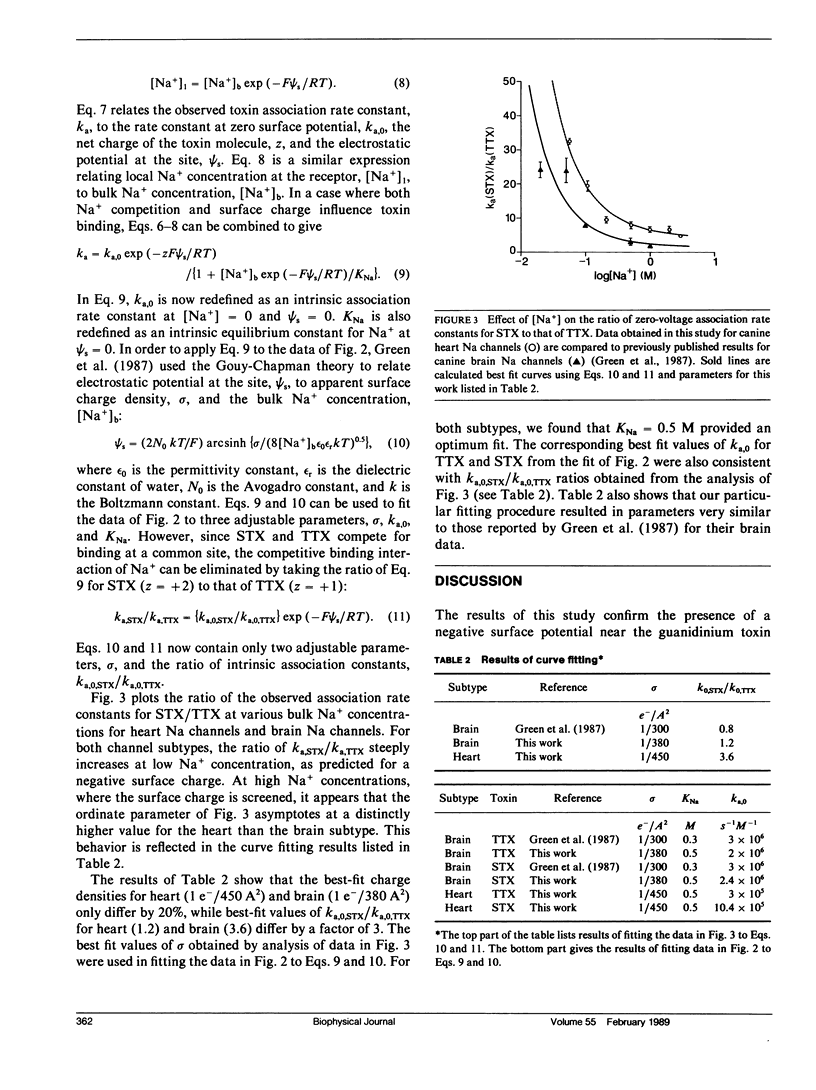
The presence of negative surface charge near the tetrodotoxin/saxitoxin binding site of canine heart Na channels was revealed by analysis of the kinetics of toxin block of single batrachotoxin-activated Na channels in planar bilayers as a function of [NaCl]. The voltage-dependence of toxin binding and the toxin dissociation rate are nearly constant as [NaCl] is varied from 0.05 to 3 M. In contrast, the association rate constant of the toxins is inversely dependent on [NaCl], with the rate for the divalent toxin, saxitoxin2+, affected more steeply than that of the monovalent toxin, tetrodotoxin1+. These results for toxin-insensitive Na channels from canine heart parallel previous findings for toxin-sensitive Na channels from canine brain. The model of Green et al. (Green, W. N., L. B. Weiss, and O. S. Anderson. 1987. J. Gen. Physiol. 89:873-903), which includes Na+ competition and Gouy-Chapman screening of surface charge, provided an excellent fit to the data. The results suggest that the two canine Na channel subtypes have a similar density of negative surface charge (1 e-/400 A2) and a similar dissociation constant for Na+ competition (0.5 M) at the toxin binding site. Thus, negative surface charge is a conserved feature of channel function of these two subtypes. The difference in toxin binding affinities arises from small differences in intrinsic association and dissociation rates.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez O., Brodwick M., Latorre R., McLaughlin A., McLaughlin S., Szabo G. Large divalent cations and electrostatic potentials adjacent to membranes. Experimental results with hexamethonium. Biophys J. 1983 Dec;44(3):333–342. doi: 10.1016/S0006-3495(83)84307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Rubinson K. A. Chemical modification of crab nerves can make them insensitive to the local anaesthetics tetrodotoxin and saxitoxin. Nature. 1975 Oct 2;257(5525):412–414. doi: 10.1038/257412a0. [DOI] [PubMed] [Google Scholar]
- Barchi R. L., Weigele J. B. Characteristics of saxitoxin binding to the sodium channel of sarcolemma isolated from rat skeletal muscle. J Physiol. 1979 Oct;295:383–396. doi: 10.1113/jphysiol.1979.sp012975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnie S., McLaughlin S. Large divalent cations and electrostatic potentials adjacent to membranes. A theoretical calculation. Biophys J. 1983 Dec;44(3):325–332. doi: 10.1016/S0006-3495(83)84306-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani J. A. Ion-channel entrances influence permeation. Net charge, size, shape, and binding considerations. Biophys J. 1986 Mar;49(3):607–618. doi: 10.1016/S0006-3495(86)83688-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French R. J., Worley J. F., 3rd, Krueger B. K. Voltage-dependent block by saxitoxin of sodium channels incorporated into planar lipid bilayers. Biophys J. 1984 Jan;45(1):301–310. doi: 10.1016/S0006-3495(84)84156-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambale F., Montal M. Characterization of the channel properties of tetanus toxin in planar lipid bilayers. Biophys J. 1988 May;53(5):771–783. doi: 10.1016/S0006-3495(88)83157-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green W. N., Weiss L. B., Andersen O. S. Batrachotoxin-modified sodium channels in planar lipid bilayers. Characterization of saxitoxin- and tetrodotoxin-induced channel closures. J Gen Physiol. 1987 Jun;89(6):873–903. doi: 10.1085/jgp.89.6.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissmer S. Effect of various cations and anions on the action of tetrodotoxin and saxitoxin on frog myelinated nerve fibers. Pflugers Arch. 1984 Dec;402(4):353–359. doi: 10.1007/BF00583935. [DOI] [PubMed] [Google Scholar]
- Guo X. T., Uehara A., Ravindran A., Bryant S. H., Hall S., Moczydlowski E. Kinetic basis for insensitivity to tetrodotoxin and saxitoxin in sodium channels of canine heart and denervated rat skeletal muscle. Biochemistry. 1987 Dec 1;26(24):7546–7556. doi: 10.1021/bi00398a003. [DOI] [PubMed] [Google Scholar]
- Henderson R., Ritchie J. M., Strichartz G. R. Evidence that tetrodotoxin and saxitoxin act at a metal cation binding site in the sodium channels of nerve membrane. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3936–3940. doi: 10.1073/pnas.71.10.3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B., Ritchie J. M., Strichartz G. R. The effect of surface charge on the nerve membrane on the action of tetrodotoxin and saxitoxin in frog myelinated nerve. J Physiol. 1975 Aug;250(1):34P–35P. [PubMed] [Google Scholar]
- Huang L. Y., Yatani A., Brown A. M. The properties of batrachotoxin-modified cardiac Na channels, including state-dependent block by tetrodotoxin. J Gen Physiol. 1987 Sep;90(3):341–360. doi: 10.1085/jgp.90.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James W. M., Agnew W. S. Multiple oligosaccharide chains in the voltage-sensitive Na channel from electrophorus electricus: evidence for alpha-2,8-linked polysialic acid. Biochem Biophys Res Commun. 1987 Oct 29;148(2):817–826. doi: 10.1016/0006-291x(87)90949-1. [DOI] [PubMed] [Google Scholar]
- Kao C. Y. Structure-activity relations of tetrodotoxin, saxitoxin, and analogues. Ann N Y Acad Sci. 1986;479:52–67. doi: 10.1111/j.1749-6632.1986.tb15561.x. [DOI] [PubMed] [Google Scholar]
- Miller J. A., Agnew W. S., Levinson S. R. Principal glycopeptide of the tetrodotoxin/saxitoxin binding protein from Electrophorus electricus: isolation and partial chemical and physical characterization. Biochemistry. 1983 Jan 18;22(2):462–470. doi: 10.1021/bi00271a032. [DOI] [PubMed] [Google Scholar]
- Moczydlowski E., Garber S. S., Miller C. Batrachotoxin-activated Na+ channels in planar lipid bilayers. Competition of tetrodotoxin block by Na+. J Gen Physiol. 1984 Nov;84(5):665–686. doi: 10.1085/jgp.84.5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numa S., Noda M. Molecular structure of sodium channels. Ann N Y Acad Sci. 1986;479:338–355. doi: 10.1111/j.1749-6632.1986.tb15580.x. [DOI] [PubMed] [Google Scholar]
- Rando T. A., Strichartz G. R. Saxitoxin blocks batrachotoxin-modified sodium channels in the node of Ranvier in a voltage-dependent manner. Biophys J. 1986 Mar;49(3):785–794. doi: 10.1016/S0006-3495(86)83706-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed J. K., Raftery M. A. Properties of the tetrodotoxin binding component in plasma membranes isolated from Electrophorus electricus. Biochemistry. 1976 Mar 9;15(5):944–953. doi: 10.1021/bi00650a002. [DOI] [PubMed] [Google Scholar]
- Spalding B. C. Properties of toxin-resistant sodium channels produced by chemical modification in frog skeletal muscle. J Physiol. 1980 Aug;305:485–500. doi: 10.1113/jphysiol.1980.sp013377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strichartz G., Rando T., Hall S., Gitschier J., Hall L., Magnani B., Bay C. H. On the mechanism by which saxitoxin binds to and blocks sodium channels. Ann N Y Acad Sci. 1986;479:96–112. doi: 10.1111/j.1749-6632.1986.tb15564.x. [DOI] [PubMed] [Google Scholar]
- Weigele J. B., Barchi R. L. Saxitoxin binding to the mammalian sodium channel. Competition by monovalent and divalent cations. FEBS Lett. 1978 Nov 1;95(1):49–53. doi: 10.1016/0014-5793(78)80049-0. [DOI] [PubMed] [Google Scholar]
- Winiski A. P., McLaughlin A. C., McDaniel R. V., Eisenberg M., McLaughlin S. An experimental test of the discreteness-of-charge effect in positive and negative lipid bilayers. Biochemistry. 1986 Dec 16;25(25):8206–8214. doi: 10.1021/bi00373a013. [DOI] [PubMed] [Google Scholar]
- Woodhull A. M. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973 Jun;61(6):687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worley J. F., 3rd, French R. J., Krueger B. K. Trimethyloxonium modification of single batrachotoxin-activated sodium channels in planar bilayers. Changes in unit conductance and in block by saxitoxin and calcium. J Gen Physiol. 1986 Feb;87(2):327–349. doi: 10.1085/jgp.87.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]


