Abstract
Oral candidiasis is a collective name for a group of disorders caused by the dimorphic fungus Candida albicans. Host defenses against C. albicans essentially fall into two categories: specific immune mechanisms and local oral mucosal epithelial cell defenses. Since oral epithelial cells secrete a variety of cytokines and chemokines in response to oral microorganisms and since C. albicans is closely associated with oral epithelial cells as a commensal organism, we wanted to determine whether interleukin-18 (IL-18) and gamma interferon (IFN-γ) were produced by oral epithelial cells in response to C. albicans infection and lipopolysaccharide (LPS) stimulation. Our results showed that IL-18 mRNA and protein were constitutively expressed by oral epithelial cells and were down-regulated by Candida infections but increased following LPS stimulation. Both C. albicans and LPS significantly decreased pro-IL-18 (24 kDa) levels and increased active IL-18 (18 kDa) levels. This effect was IL-1β-converting-enzyme dependent. The increase in active IL-18 protein levels promoted the production of IFN-γ by infected cells. No effect was obtained with LPS. Although produced only at an early stage, secreted IFN-γ seemed to be a preferential response by oral epithelial cells to C. albicans growth. These results provide additional evidence for the contribution of oral epithelial cells to local (direct contact) and systemic (IL-18 and IFN-γ production) defense against exogenous stimulation such as C. albicans infection or LPS stimulation.
Candida species are the most frequent cause of life-threatening invasive fungal infections in the immunocompromised host and are responsible for 10% of all nosocomial bloodstream infections (19, 27). The leading cause of candidiasis is Candida albicans. This fungus colonizes different body sites, including the oral cavity. C. albicans colonizes the oral mucosa of approximately 80% of normal individuals as a commensal organism, causing no apparent damage and inducing no apparent inflammation in the surrounding tissue (1, 8). However, under a number of predisposing conditions, C. albicans multiplies and penetrates the host tissue, causing inflammation and tissue destruction (43, 49). Host resistance mechanisms to C. albicans have been investigated in systemic and mucocutaneous candidiases (10, 39), and although it is generally accepted that different mechanisms operate in systemic versus superficial candidiasis, the mechanisms of host defense and the pathogenesis of candidiasis are not completely understood. Adherence of C. albicans to oral epithelial cells is the first step in the initiation of infection, as this enables the organism to overcome the normal flushing mechanisms of body secretions (14, 36).
The oral mucosal epithelium acts as the major barrier to physical, microbial, and chemical agents that may cause local cell injury (38). Recent studies have shown that epithelial cells may function as immobile immunocytes (5, 34). During the evolution of the inflammatory response, leukocyte subsets are recruited and extravasate through the endothelium, a process that is promoted by local cytokine secretion (30). The activation of nonspecific and specific immune cells such as macrophages and T cells during the inflammatory response elicits a concomitant release of cytokines (50). A newly described cytokine, interleukin-18 (IL-18), also known as gamma interferon (IFN-γ) inducing factor, has been recently shown to be a potent inducer of IFN-γ production by activated T cells (35). Since its discovery, IL-18 has been found to contribute to protective immunity against a variety of pathogens, including Cryptococcus, Leishmania, and Salmonella species and Mycobacterium tuberculosis (33).
IL-18 is related to the IL-1 family both structurally and functionally (3). Like IL-1, IL-18 is synthesized as a biologically inactive 24-kDa precursor protein. Cleavage to the active form is mediated by the IL-1 converting enzyme (ICE), also called caspase-1 (18, 20). Cleavage of IL-18 by ICE is essential for IL-18 to become biologically active. This protein is produced by activated macrophages, dermal keratinocytes, osteoblasts, adrenal cortex cells, and intestinal epithelial cells (12, 17, 44), implying that it plays other physiological roles in addition to immune regulation.
Oral epithelial cells are involved in the proinflammatory process through the production of cytokines either constitutively or after a variety of stimuli (26), implying that they may potentially participate in controlling oral infections through an inflammatory (5, 34) process involving different interleukins, such as IL-1β and IL-18. Given the biological role of oral epithelial cells as active immunocytes (5, 32) and the role of IL-18 as a proinflammatory cytokine, we hypothesized that oral epithelial cells, through IL-18, maintain the dynamic equilibrium between the oral microbial community (free microorganisms and dental plaque bacteria) and the host. We looked at whether oral epithelial cells constitutively expressed IL-18 and its converting enzyme (ICE). We determined whether IL-18 production was potentiated when oral epithelial cells were stimulated with LPS or live C. albicans. We also assessed the IFN-γ induction potential of the IL-18 (18 kDa) produced by the oral epithelial cells. In this study, we obtained evidence for the first time that human oral epithelial cells act against C. albicans through an IFN-γ pathway in an IL-18-dependent manner.
MATERIALS AND METHODS
Isolation and culture of oral epithelial cells.
Small pieces of palatal mucosa were biopsied from gingival graft patients after obtaining their informed consent. Biopsy specimens were treated with thermolysin (500 μg/ml) to separate the epithelium from the lamina propria. Epithelial cell suspensions were obtained following treatment with a 0.05% trypsin-0.01 M EDTA solution. Freshly isolated epithelial cells (9 × 103 cells/cm2) were cultured in Dulbecco's Modified Eagle-Ham's F-12 (3:1) (DMEM) (Flow Laboratories, Mississauga, Ontario, Canada) supplemented medium (40). Before their use, cells were characterized using specific antibodies (40). After being characterized, oral epithelial cells were used at passage 3 to realize this study.
Candida.
One C. albicans isolate was used in this study. It is an original clinical isolate (Candida-associated stomatitis) that has been characterized very thoroughly using numerous experimental approaches (4) and was kindly provided by N. Deslauriers (GREB, Laval University, Quebec, Canada). The yeast was grown on Sabouraud dextrose agar (Becton Dickinson, Cockeysville, Md.) at 30°C. For C. albicans suspensions, one colony was used to inoculate 10 ml of phytone-peptone medium (Becton Dickinson) supplemented with 0.1% glucose. The culture was grown to the stationary phase for 18 h at 25°C in a shaking water bath. The blastoconidia were collected, washed with phosphate-buffered saline (PBS), and enumerated using a hemacytometer (Reichert, Buffalo, N.Y.) and trypan blue dye exclusion (24). Viable cells were adjusted to 108 C. albicans cells/ml and used to infect gingival epithelial cell cultures.
Stimulation of epithelial cells with LPS or C. albicans.
Epithelial cells were detached from 75-cm2 culture flasks using trypsin. They were washed twice in culture medium, counted, seeded into six-well tissue culture plates (Falcon, Becton Dickinson, Lincoln Park, N.J.) at 2.5 × 105 cells/well, and then incubated in an 8% CO2 atmosphere at 37°C. The epithelial cells were cultured to about 90% confluence, which was obtained after 5 days of culture. They were then stimulated with C. albicans (105 cells/cm2) or lipopolysaccharide (LPS) (5 μg/ml) extracted from Porphyromonas gingivalis. LPS was extracted and purified as described previously (11, 37). The LPS was kindly provided by D. Grenier (GREB, Laval University, Quebec, Canada). Stimulated and unstimulated cells were cultured for 3, 6, 12, and 24 h. At the end of each contact period, the culture supernatants were harvested and stored at −80°C. The cells were detached as described above and used to extract either total mRNA or proteins.
RNA extraction and reverse transcription (RT)-PCR analysis.
Total cellular RNA was prepared from unstimulated and LPS- and C. albicans-stimulated epithelial cells using the RNeasy Total RNA kit (Qiagen Inc., Valencia, Calif.) and quantified by fluorescence using Ribogreen (Molecular Probes Inc., Eugene, Oreg.). Total RNA was reverse transcribed into cDNA using the Moloney murine leukemia virus reverse transcriptase (Canadian Life Technologies, Gaithersburg, Md.) and random hexamers (Amersham Pharmacia Biotech Inc., Quebec, Canada). One microliter of each cDNA product was added to a 50-μl PCR mixture containing Taq polymerase (Qiagen Inc.) and forward and reverse primers (Keystone Laboratories Inc., Camarillo, Calif.). All reactions were performed in a DNA thermal cycler (Perkin-Elmer Cetus, Norwalk, Conn.) over 35 cycles, each consisting of 1 min at 95°C, 30 s at 52°C, and 1 min at 72°C for. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA was measured in each sample as a control. The following primers were used for the PCR: IL-18 (forward), 5′-GCTTGAATCTAAATTATCAGTC-3′; IL-18 (reverse), 5′-GAAGATTCAAATTGCATCTTAT-3′; ICE (forward), 5′-ATCCGTTCCATGGGTGAAGGTACA-3′; ICE (reverse), 5′-CAAATGCCTCCAGCTCTGTAATCA-3′; GAPDH (forward), 5′-ATGCAACGGATTTGGTCGTAT-3′; and GAPDH (reverse), 5′-TCTCGCTCCTGGAAGATGGTG-3′. The predicted sizes of the PCR products were 320 bp for pro-IL-18, 620 bp for ICE, and 220 bp for GAPDH. After the PCR procedure, 10 μl of the products were separated on a 2% agarose gel containing ethidium bromide and visualized by UV light. The gels were photographed, and the relative intensity of the bands was measured on digitized images using a Macintosh computer and the public domain NIH Image program.
Western blotting and immunodetection of intracellular IL-18.
Western blotting analyses were performed as described previously (31). Unstimulated and LPS- and C. albicans-stimulated oral epithelial cells were lysed in Tris buffer containing 2 mM EDTA, 2% Triton X-100, and an antiprotease mix. The lysates were boiled in sample buffer (200 mM Tris [pH 6.8], 20% glycerol, 2% SDS, 0.1% bromophenol blue, and 10% β-mercaptoethanol), and equal amounts of total protein were loaded onto a sodium dodecyl sulfate-10% polyacrylamide gel. Recombinant human IL-18 and monocyte lysate were used as positive controls for IL-18 and ICE protein detection. After electrophoretic separation, the proteins were transferred to a nylon polyvinylidene difluoride membrane (Millipore, Bedford, Mass.), blocked overnight with TBS-T (100 mM Tris [pH 7.5], 0.9% NaCl, and 0.1% Tween 20), and complemented with 4% skim milk (BLOTTO). The membrane was incubated for 1 h at room temperature with goat anti-human IL-18 monoclonal antibody (MBL, Naka-ku Nagoya, Japan) diluted 1/1,000 in BLOTTO or with affinity-purified anti-human ICE rabbit polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, Calif.) diluted 1/1,000 in BLOTTO. Anti-human IL-18 monoclonal antibody reacts with pro-IL-18 and IL-18. Anti-human ICE polyclonal antibody reacts with a 45-kDa precursor, procaspase-1, also designated ICE. The membrane was washed (once for 10 min and once for 5 min) in TBS-T and then incubated for 30 min at room temperature with horseradish peroxidase-labeled sheep anti-mouse immunoglobulin G (Cedarlane Laboratories Ltd., Ontario, Canada). After washing with TBS-T (three times for 5 min and once for 2 min), the blots were developed using an enhanced chemiluminescence kit (Amersham International, Little Chalfont, United Kingdom).
Measurement of IL-18 and IFN-γ secreted by oral epithelial cells.
The supernatants collected from unstimulated and LPS- and C. albicans-stimulated oral epithelial monolayer cultures were used to determine the levels of secreted IL-18 and IFN-γ. Cytokine measurements were performed in triplicate samples using an enzyme-linked immunosorbent assay (ELISA) for IL-18 (Medical & Biological Laboratories Co., Ltd., Nagoya, Japan) and IFN-γ (Cell Sciences, Norwood, Mass.). Supernatants were filtered through 0.22-μm-pore-size filters and used to quantify IL-18 and IFN-γ according to the manufacturer's instructions. A 50-μl volume of undiluted supernatant was used to perform the quantitative analysis. Plates were read at 450 nm and analyzed using an EAR-400 Easy Reader (SLT-Lab Instrumentation, Grödig, Austria). The sensitivity of the IL-18 ELISA kit was better than 13 pg/ml and the sensitivity of the IFN-γ ELISA kit was better than 1 pg/ml according to the manufacturer. The experiments were repeated three times, and the means ± standard deviations (SD) were plotted.
Effect of C. albicans on epithelial cell viability and growth.
Immediately after each contact period with C. albicans, the epithelial cell cultures were washed three times with culture medium and then detached using a 0.05% trypsin-0.01 M EDTA solution for 20 min at 37°C. After the enzyme treatment, the cells were washed twice with DMEM-supplemented culture medium. The pellets were resuspended in 1 ml of DMEM, and cell viability was determined using a dye exclusion test (41). A 100-μl volume of each epithelial cell suspension was mixed with the same volume of trypan blue solution and incubated on ice for 5 min. This step was performed three times for each cell suspension. The total number of cells in each sample and their viability were then determined by trypan blue exclusion. The percentage of viable cells was then calculated, and the results were reported as means ± SD of six different experiments.
Evaluation of C. albicans adherence to and entry into epithelial cells.
To determine whether C. albicans adheres to and enters the cytoplasm of epithelial cells, glass coverslips were placed in each well of six-well tissue culture plates prior to seeding with epithelial cells. The cells were grown in the tissue culture plates for 72 h at 37°C in an 8% CO2 atmosphere as described above. After contact with C. albicans for 3, 6, 12, and 24 h, the monolayers were washed six times with PBS and fixed with 40% methanol-60% acetone for 10 min. The cells were washed twice with PBS and stained with Masson trichrome. Stained samples were examined under an optical microscope (Leitz), and photographs were taken using Kodak color ISO 100 film. To quantify the number of C. albicans cells adhering to the monolayer and entering the cells, infected cultures were washed six times with Hanks' balanced salts solution. The cells then were lysed with 1% (vol/vol) Triton X-100 in PBS, and C. albicans viability and numbers were estimated by trypan blue dye exclusion as described previously (24). This assay quantifies the total number of fungus cells bound to the outside of and internalized by the epithelial cells. The adherence rates are the means of six different experiments.
Statistical analysis.
All experiments in this study were performed at least three times. The data shown are representative results. Experimental values are given as means ± SD. The statistical significance of differences between two means was evaluated by Student's unpaired t test. Results were considered significant if P was <0.05.
RESULTS
IL-18 mRNA expression by human oral epithelial cells modulated by C. albicans and LPS.
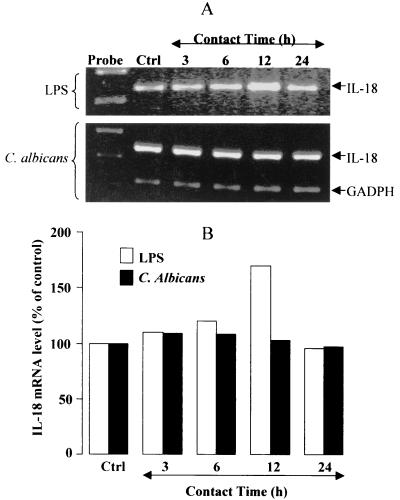
We first examined the profile of constitutive and induced IL-18 mRNA expression by cultured human oral epithelial cells. Oral epithelial cells displayed robust constitutive IL-18 mRNA expression (Fig. 1). There was a significant increase in the IL-18 message following a 12-h stimulation with LPS (Fig. 1). Interestingly, C. albicans down-regulated the IL-18 mRNA expression(Fig. 1). Indeed, in 24-h-infected tissue, we found a significant (P ≤ 0.05) reduction of IL-18 mRNA expression.
FIG. 1.
IL-18 mRNA is transiently modulated by LPS and C. albicans stimulation. (A) Total cellular RNA (1 μg) from unstimulated and C. albicans (105 cell/cm2)- and LPS (5 μg/ml)-stimulated oral epithelial cells was analyzed by RT-PCR with primers specific for IL-18 (320 bp) and GAPDH (220 bp). The stimulation period is indicated at the top of each lane. Lane Ctrl contains unstimulated oral epithelial cell cultures. Expression of GAPDH mRNA is shown as a loading control for the experiments. The gel shown is a representative of three different experiments. (B) The increase in IL-18 was assessed by the public domain NIH Image software package as the mean ± SD of three different experiments.
IL-18 production by human oral epithelial cells decreased by C. albicans and LPS.
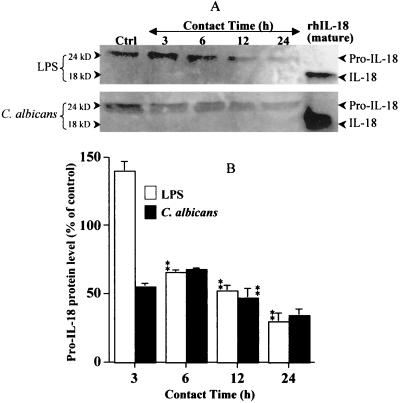
Since IL-18 mRNA is constitutively expressed by epithelial cells, and since the gene expression may or may not be modulated by stimulating agents, we thus looked at whether IL-18 mRNA was translated into IL-18 protein. Western blot analyses using cell lysates showed that unstimulated oral epithelial cells constitutively expressed IL-18 protein (Fig. 2). Unstimulated oral epithelial cells contained pro-IL-18 (24 kDa) but not IL-18 (18 kDa). In stimulated cultures, both C. albicans and LPS induced an important decrease in pro-IL-18 protein. This down-regulation of pro-IL-18 by C. albicans and LPS was time dependent. Oral epithelial cells seemed to have stable high levels of IL-18 mRNA and Pro-IL-18 protein that dropped following contact with C. albicans or LPS. These results suggested that the drop in pro-IL-18 in stimulated cells may be due to the cleavage of inactive IL-18 to active IL-18 and that this mobilizes the cell to produce high levels of IL-18 mRNA in order to renew its stock of protein. These observations raised the question as to whether oral epithelial cells express the converting enzyme involved in processing inactive IL-18 to active IL-18 protein.
FIG. 2.
IL-18 protein is constitutively expressed by oral epithelial cells but significantly decreased by LPS and C. albicans stimulation. (A) Total protein (100 μg) from lysates of unstimulated (Ctrl) and C. albicans (105 cell/cm2)- and LPS (5 μg/ml)-stimulated oral epithelial cells was analyzed by Western blotting for the presence of inactive-IL-18 (24 kDa) protein. The stimulation period is indicated at the top of each lane. The position of IL-18 protein is indicated. Recombinant human IL-18 (10 ng) was used as a positive control. The gel shown is a representative of three different experiments. (B) The decrease in inactive IL-18 protein was determined by band scanning using the public domain NIH Image software program. Data are the means + SDs (error bars) of three different experiments. The statistical differences (∗∗, P ≤ 0.01) were obtained by comparing the value obtained at 3 h to the value obtained at 6 h or the value obtained at 6 h to that obtained at 12 h, etc., for the same stimuli (LPS or C. albicans).
ICE mRNA and protein modulation by C. albicans and LPS.
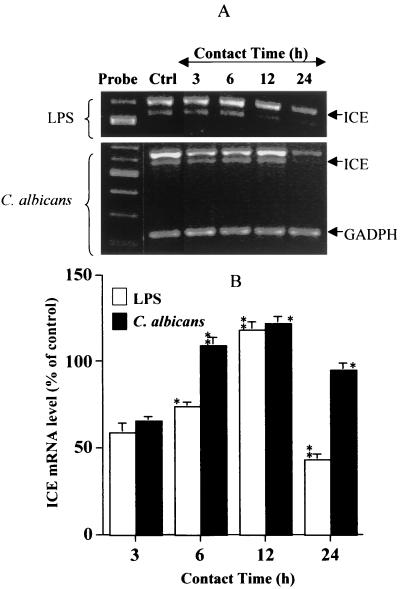
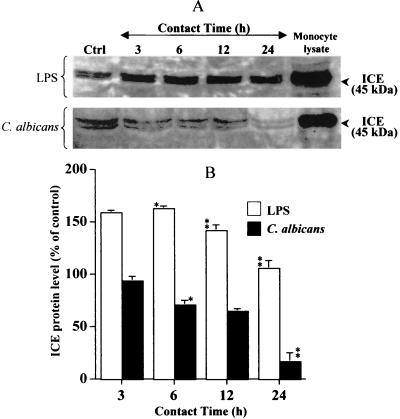
To be converted to active IL-18 protein, inactive pro-IL-18, a 24-kDa protein, has to be cleaved by a specific ICE, also known as caspase-1. Inactive-IL-1 and IL-18 have been identified as substrates for ICE (18, 20). Since oral epithelial cells express the IL-18 protein, we next looked at whether cultured human oral epithelial cells expressed ICE mRNA. Total RNA analyses using RT-PCR showed (Fig. 3) that oral epithelial cells constitutively expressed the converting enzyme. The basal levels of ICE expression were significantly (P ≤ 0.01, P ≤ 0.05) modified by C. albicans or LPS stimulation. The question was thus whether the cells expressed the ICE protein involved in pro-IL-18 cleavage. Western blot analyses of cell lysates showed (Fig. 4) that oral epithelial cells constitutively expressed the pro-ICE protein (45 kDa). When the cells were stimulated with LPS, no significant changes were noticed relative to ICE protein expression. Interestingly, when cells were infected with C. albicans, ICE protein levels decreased in a time-dependent manner, nearing almost zero after a 24-h contact between the epithelial cells and C. albicans. The ICE decrease seemed to follow the pro-IL-18 decrease reported in Fig. 2, suggesting that pro-IL-18 was cleaved by ICE to the active form (18 kDa). It was therefore important to determine whether oral epithelial cells secrete the active form of IL-18 into the culture medium.
FIG. 3.
ICE mRNA is significantly modulated by LPS and C. albicans stimulation. (A) Total cellular RNA (1 μg) from unstimulated and C. albicans (105 cell/cm2)- and LPS (5 μg/ml)-stimulated oral epithelial cells was analyzed by RT-PCR with primers specific for ICE (620 bp) and GAPDH (220 bp). The stimulation period is indicated at the top of each lane. Lane Ctrl contains unstimulated oral epithelial cell cultures. The gels shown is a representative of three different experiments. (B) The degree of ICE modulation (increase or decrease) was determined by band scanning using the public domain NIH Image software package. Expression of GAPDH mRNA is shown as a loading control for the experiments. Data are the means + SDs (error bars) of three different experiments. The statistical differences (∗∗, P ≤ 0.01; ∗, P ≤ 0.05) were obtained by comparing the value obtained at 3 h to the value obtained at 6 h or the value obtained at 6 h to that obtained at 12 h, etc., for the same stimuli (LPS or C. albicans).
FIG. 4.
ICE protein is constitutively expressed by oral epithelial cells but significantly decreased by LPS and increased by C. albicans stimulation. (A) Total protein (100 μg) from lysates of unstimulated (Ctrl) and C. albicans- and LPS-stimulated oral epithelial cells was analyzed by Western blotting using an anti-ICE (45 kDa) affinity-purified rabbit polyclonal antibody. The stimulation or infection period with C. albicans is indicated at the top of each lane. Total protein (50 μg) from a rat monocyte lysate was analyzed for ICE protein as a positive control. The gel shown is a representative of three different experiments. (B) The degree of ICE modulation (increase or decrease) was determined by band scanning using the public domain NIH Image software program. Data are the means + SDs (error bars) of three different experiments. The statistical differences (∗∗, P ≤ 0.01; ∗, P ≤ 0.05) were obtained by comparing the value obtained at 3 h to the value obtained at 6 h or the value obtained at 6 h to that obtained at 12 h, etc., for the same stimuli (LPS or C. albicans).
Detection of active IL-18 in culture supernatants of human oral epithelial cells stimulated with C. albicans or LPS.
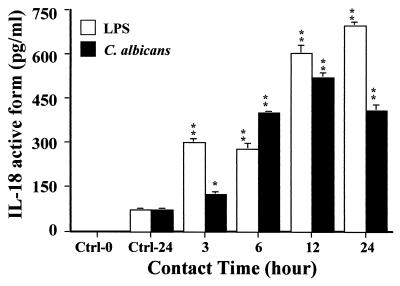
To determine whether oral epithelial cells are able to produce active IL-18 protein, culture supernatants of unstimulated and LPS- and C. albicans-stimulated cells were analyzed by sandwich ELISA using anti-IL-18-specific reagents. A very low basal production of active IL-18 protein was detected in 24-h oral epithelial cell culture supernatants (Fig. 5). These basal levels increased following a C. albicans infection. In infected cultures, IL-18 protein levels reached a maximum after a 12-h contact. The concentration of IL-18 protein varied from 124 ± 7 pg/ml after 3 h to 519 ± 15 pg/ml after 12 h in infected cell cultures. In LPS-stimulated oral epithelial cell cultures, the amount of IL-18 protein increased in a time-dependent manner, reaching 300 ± 11 pg/ml after 3 h and climbing to a maximum of 696 ± 5 pg/ml after 24 h (Fig. 5). Thus, both C. albicans and LPS up-regulated active IL-18 protein production by oral epithelial cells.
FIG. 5.
Enhanced production of active IL-18 protein by oral epithelial cells stimulated with LPS and C. albicans. Oral epithelial cells were isolated from gingival biopsy specimens of healthy persons. Cells were cultured in six-well plates alone or in the presence of C. albicans (blastospore form; 105 C. albicans cells/cm2) or LPS (5 μg/ml) for different periods of time as indicated at the bottom of the figure. Culture medium was collected from each experiment, filtered through a 0.22-μm-pore-size filter, and used to determine the amount of the active form of IL-18. IL-18 concentrations were measured by ELISA. Data are the means + SD (error bars) (n = 3). Note the basal level of IL-18 secretion by oral epithelial cells and the time-dependent increase of this cytokine caused by C. albicans infection and LPS stimulation of oral epithelial cells. The levels of significance for C. albicans and LPS-stimulated oral epithelial cell cultures compared to unstimulated cultures were P < 0.05 (∗) and P < 0.01 (∗∗), respectively. Ctrl-0 is oral epithelial cell culture medium, and Ctrl-24 is supernatant from unstimulated oral epithelial cells cultured for 24 h.
IFN-γ production by human oral epithelial cells stimulated with C. albicans or LPS.
It is well known that IL-18 is a potent inducer of IFN-γ production by activated cells such as T lymphocytes (33). To determine whether the IL-18 protein secreted by oral epithelial cells is functional (by stimulating these epithelial cells to produce IFN-γ), a specific IFN-γ ELISA was performed using oral epithelial cell culture supernatants. Control cells did not express IFN-γ at a level that was detectable by our assay. When cells were stimulated with LPS they produced a limited amount of IFN-γ that varied from 10 to 20 pg/ml. When oral epithelial cell cultures were infected with C. albicans, they produced significant amounts of IFN-γ after a 3-h contact (Table 1). Surprisingly, this apparent release of IFN-γ was not temporally correlated with the accumulation of extracellular IL-18 despite the well-characterized role of IL-18 as the major IFN-γ inducing factor. Indeed, during the subsequent contact periods of oral epithelial cells and C. albicans, IFN-γ production was very weak after 6, 12, and 24 h (Table 1). Two conclusions can be reached from these results: (i) LPS modulated weakly the oral epithelial cell response via an IL-18 independent mechanism, and (ii) C. albicans stimulated oral epithelial cells to produce IFN-γ via an IL-18-dependent mechanism at early events but blocked IFN-γ production later on to escape its toxic effect.
TABLE 1.
IFN-γ produced by epithelial cells following contact with C. albicans or LPS
| Contact time (h) | IFN-γ (pg/ml) produced (mean ± SD)a following contact with:
|
|
|---|---|---|
| C. albicans (105Candida cells/cm2) | LPS (5 μg/ml) | |
| Controlb | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 3 | 264.5 ± 10.07 | 10.40 ± 2.5 |
| 6 | 3.5 ± 0.3 | 19.60 ± 1.9 |
| 12 | 31.5 ± 2.09 | 15.40 ± 1.3 |
| 24 | 0.0 ± 0.0 | 13.90 ± 0.7 |
(n = 3).
Cultured supernatant of unstimulated oral epithelial cells.
Epithelial cell viability after infection with C. albicans.
To determine whether epithelial cells were still alive 3 h after being infected with C. albicans and whether the high amount of IFN-γ obtained after 3 h was not the result of cell death and lysis-inducing protein liberation in the culture medium, we assessed the cell viability of C. albicans-infected cultures. As reported in Table 2, no significant differences were detected between infected and uninfected cultures, either in terms of cell viability or total numbers of viable cells. This indicated that the increase level of IL-18 observed after a 3-h contact between oral epithelial cells and C. albicans was not promoted by the C. albicans infection. The high level of IFN-γ produced after a 3-h infection period was thus a biological response of the oral epithelial cells to the C. albicans infection.
TABLE 2.
Total oral epithelial cell number and viability following contact with C. albicansa
| Contact time (h) | Mean % cell viability ± SD | Mean total no. of cells ± SD (106) |
|---|---|---|
| Control | 95 ± 2.5 | 1.17 ± 0.051 |
| 3 | 94 ± 3.5 | 1.21 ± 0.072 |
| 6 | 95 ± 2.8 | 1.45 ± 0.092 |
| 12 | 95.6 ± 2.01 | 1.57 ± 0.024 |
| 24 | 95.5 ± 2 | 1.67 ± 0.08 |
Results are based on six different experiments.
Effect of human oral epithelial cells on C. albicans adherence, transformation, and growth.
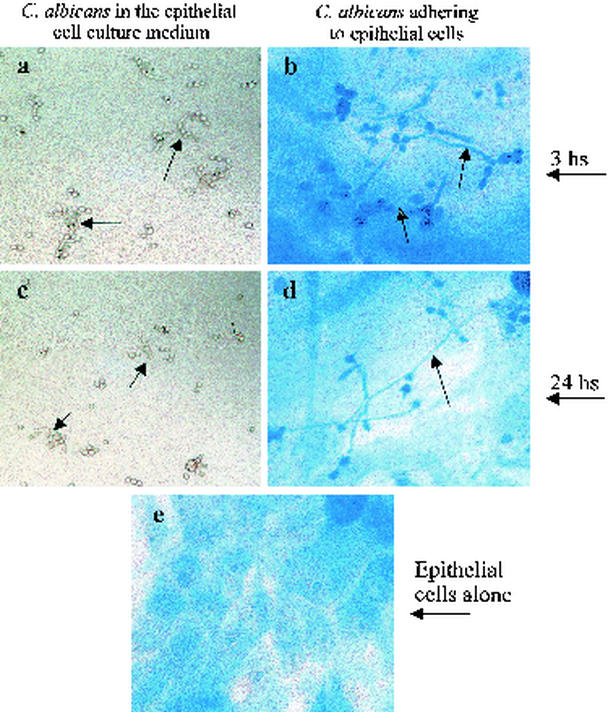
The above observation raised a question concerning the effect of epithelial cells on the adherence, transformation, and growth of C. albicans. We thus separated infected oral epithelial cells from nonadhering C. albicans, and observed and photographed them using an optical microscope. This revealed that C. albicans adhered to the epithelial cells and changed its blastospore form to a hyphal form (Fig. 6). The transformation of C. albicans from blastospores to hyphae was time dependent. The percentage of the hyphal form rose from 22% after a 3-h contact to more than 80% after a 24-h contact (Table 3). Viability and growth analyses showed that all C. albicans that adhered to and penetrated into epithelial cells were alive However, their numbers were significantly reduced, varying from 1.2 × 105/cm2 after a 3-h infection to around 103/cm2 after a 24-h infection. These results suggested that oral epithelial cells inhibited the growth of C. albicans. C. albicans adheres to the cells and changes its commensal form (blastospore) to a virulent form (hyphal), which may help C. albicans escape epithelial cell inhibitory effect. The transformation to the hyphal form requires close contact between C. albicans and oral epithelial cells since free C. albicans cells only change to a pseudohyphal form.
FIG. 6.
C. albicans adhesion to oral epithelial cells and the transition to the hyphal phase from the blastospore phase. Oral epithelial cells were cultured as monolayers in six-well plates. When they reached 80% confluence, they were infected with C. albicans (105 cells/cm2) for 3 and 24 h. The culture supernatants were harvested, observed under an optical microscope, and photographed. The monolayer cultures were stained using Masson trichrome, observed under an optical microscope, and photographed. Phase-contrast photomicrographs show C. albicans harvested from the supernatants of oral epithelial monolayer cultures after 3 h (a) and 24 h (b), when a large number of blastospores can be seen changing to the pseudohyphal (arrows) form. Also shown are C. albicans as a hyphal form adhering to an oral epithelial cell monolayer after 3 h (c) and 24 h (d). (e) Uninfected oral epithelial monolayer culture (e). Note the switch of C. albicans from a budding to a hyphal form when it adheres to the epithelial cells (arrows). Magnification, ×300.
TABLE 3.
Viability, form, and number of C. albicans cells adhering to cultured oral epithelial cells.
| Contact time (h) | Mean no. of C. albicans ± SDa | Mean % viability of C. albicans ± SD | Mean % estimated hyphae ± SD |
|---|---|---|---|
| 3 | 1.2 ± 0.7 | 98 ± 0.01 | 22 ± 3 |
| 6 | 0.7 ± 0.03b | 99 ± 0.02 | 30 ± 4 |
| 12 | 0.2 ± 0.09b | 97 ± 0.32 | 50 ± 1 |
| 24 | 0.06 ± 0.01b | 99 ± 0.37 | 80 ± 7 |
C. albicans was seeded onto epithelial cell culture at 105 cells/cm2.
P ≤ 0.01 is the level of significance for growth inhibition of C. albicans in infected oral epithelial cell cultures compared to the initial C. albicans seeding concentration.
DISCUSSION
The various properties that make Candida species opportunistic pathogens and the multiple oral diseases they cause are still a matter of debate. The systemic immune mechanisms involved in the control of candidiasis have been thoroughly researched. Nonetheless, the contribution of oral mucosal cells, especially epithelial cells, to immune surveillance remains to be investigated. During the inflammation process, epithelial cells are capable of secreting a variety of proinflammatory cytokines. These cytokines play critical roles in the development of protective immunity against intracellular pathogens (7, 30). IL-18 is a new entry in the vast epithelial cytokine bank. IL-18, which has pleiotropic immunomodulatory functions, is involved in a variety of human inflammatory conditions, including Crohn's disease (32), rheumatoid arthritis (29), and tuberculoid leprosy (16). IL-18 restores defective Th1 immunity to C. albicans in infected caspase-1-deficient mice (18, 48). Oral epithelial cells may contribute to local and systemic immune defenses against oral microorganisms, including C. albicans through IL-18. The present study indicated that oral epithelial cells produced IL-18 mRNA. As reported previously using Salmonella enterica (47), we showed that oral epithelial cells expressed significant basal levels of IL-18 mRNA signals that were increased by LPS stimulation and decreased by C. albicans stimulation, respectively. This is the first time that the interaction between C. albicans and oral epithelial cells via IL-18 has been addressed. The fact that C. albicans has a slight decreasing effect on IL-18 mRNA expression may be explained by the presence of saturating levels of IL-18 transcripts in both infected and uninfected epithelial cells that overcome the transcription inhibitory effect of C. albicans. As to whether IL-18 transcripts expressed by oral epithelial cells are translated into IL-18 protein, Western blot analyses using epithelial cell lysates showed that basal expression of inactive IL-18 protein (24 kDa) was significantly decreased by stimulation with both LPS and C. albicans.
The presence of IL-18 in epithelial cells, which constitute the primary host-pathogen interface, suggests that IL-18 may play an important role in fighting C. albicans infections. Several studies have demonstrated that IL-18 plays a key role in antimicrobial defenses by protecting against cryptococcal, mycobacterial, and Salmonella infections. Furthermore, IL-18 gene-disrupted mice are susceptible to mycobacterial infections (33, 45, 46). However, some pathogens may limit or interfere with host IL-18 responses (15). This may explain the decrease in IL-18 protein production by C. albicans-infected oral epithelial cells. A second explanation could be that the cleavage of the inactive form of IL-18 to the active form (18 kDa) was not detected on the Western blots of the cell lysates. However, this raises the question as to whether oral epithelial cells possess the ICE. Our data indicate that oral epithelial cells express basal levels of both ICE mRNA and protein. ICE protein levels decreased only when the epithelial cells were infected with C. albicans. Similar results were published by Barsig et al. (2), who reported that macrophages infected with Listeria monocytogenes released smaller quantities of IL-1β than those infected with Shigella flexneri, indicating that the activation of ICE was diminished or absent in macrophages harboring Listeria monocytogenes (2). Interestingly, the drop in the level of ICE protein that we observed (Fig. 4) paralleled the decrease of IL-18 protein expression by C. albicans-infected cells (Fig. 2), suggesting that epithelial cells produce enough ICE protein to convert inactive IL-18 to the active form. The involvement of ICE in infections reported here confirms similar results reported in other situations. Indeed, Brennan et al. (6) have shown that Salmonella-infected macrophages are killed by an ICE-dependent mechanism (6). The decrease in inactive IL-18 protein that we observed (Fig. 2) in infected cell lysates might be due to the conversion of pro-IL-18 to the active form. Specific immunoassays revealed that the production of active IL-18 (18 kDa) was promoted by both LPS stimulation and C. albicans infection. These results are in accordance with those previously reported regarding other infections. In a mouse infection model, genetically susceptible BALB/c mice expressed less IL-12 and IL-18 and had a diminished IFN-γ/Th1 response. In contrast, resistant DBA/2 mice exhibited an increased expression of IL-18 (23), indicating a good correlation between clearance of Mycobacterium avium and levels of IL-18. IL-18-deficient C57BL/6 mice have more severe infections, and pathological changes are significantly suppressed by treatment with exogenous IL-18 (46). Our results may then suggest that oral epithelial cells elaborate a defensive strategy against C. albicans infections through an IL-18 pathway. The vital biological role of IL-18 requires the production of IFN-γ (33). However, the much increased production of the active form of IL-18 (Fig. 5) by C. albicans-infected oral epithelial cells could not be linked to the IFN-γ production by these cells (Table 2) in order to control the pathogenic potential of C. albicans. This control seems to occur at an early stage of the infection (3 h or less), and then C. albicans dominates the cells via a complete inhibition of IFN-γ secretion by infected oral epithelial cells even if these cells continue to produce active IL-18. This may explain the high levels of active IL-18 produced by C. albicans-infected epithelial cells after more than 3 h as well as the absence of IFN-γ production by these cells in response to the IL-18 protein. Such a hypothesis needs to be investigated. The early significant production of IFN-γ could be a transient epithelial cell defense mechanism elaborated by these cells to control fungal pathogenesis. This study establishes an independent relationship between the capacity of oral epithelial cells to secrete active IL-18 protein and IFN-γ protein and the control of Candida infection.
In vitro studies have shown the stimulatory effects of IFN-γ on the phagocytosis and the killing of C. albicans by neutrophils and macrophages (13, 28). The administration of IFN-γ to mice infected with C. albicans has a beneficial effect on the outcome of the infection (25). The important role of endogenous IFN-γ in resistance against both gastrointestinal and systemic candidiases has also been demonstrated by the increased susceptibility of knockout mice deficient in IFN-γ or IFN-γ receptors infected with the yeast (9, 22). We found that C. albicans growth was reduced in infected oral epithelial cell cultures (Table 3), but the C. albicans cells that overcame the epithelial cell effect changed from a commensal (blastospore) to a virulent (hyphal) form. This situation may mimic the physiological and pathological situation in the oral cavity.
The identification of Candida as the etiologic agent of candidiasis, especially in immunocompromised hosts, has resulted in a vast range of studies to assess its virulent properties, with a view to elucidating the pathogenesis of the disease. Much progress has been made in our understanding of some features, such as the mechanisms for adherence to host tissue, cell surface hydrophobicity, form switching, secretion of aspartyl proteinases, and phospholipase production (21, 42). Nonetheless, more research is needed to elucidate and fully understand the key events involved in host-yeast interactions and the mechanisms that eventually lead to candidal infections. Studying the role of the epithelial cell in host defenses against Candida infections is crucial to finding new insights into the complex biological and pathological behavior of C. albicans and non-C. albicans Candida species. In such cases, oral epithelial cells may help us unravel the opportunistic process by which this ubiquitous organism becomes a pathogen.
Acknowledgments
This work was supported by grants from the Natural Sciences and Engineering Research Council (M.R.), the Medical Research Council (M.R. and J.C.), and the Fonds de la Recherche en Santé du Québec (M.R.). M.R. and J.C. are research scholars (junior and senior, respectively) supported by the FRSQ program.
Editor: B. B. Finlay
REFERENCES
- 1.Arendorf, T. M., and D. M. Walker. 1980. The prevalence and intra-oral distribution of Candida albicans in man. Arch. Oral Biol. 25:1-10. [DOI] [PubMed] [Google Scholar]
- 2.Barsig, J., and S. H. Kaufmann. 1997. The mechanism of cell death in Listeria monocytogenes-infected murine macrophages is distinct from apoptosis. Infect. Immun. 65:4075-4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bazan, J. F., J. C. Timans, and R. A. Kastelein. 1996. A newly defined interleukin-1? Nature 379:591.. [DOI] [PubMed] [Google Scholar]
- 4.Beausejour, A., D. Grenier, J. P. Goulet, and N. Deslauriers. 1998. Proteolytic activation of the interleukin-1β precursor by Candida albicans. Infect. Immun. 66:676-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bos, J. D., and M. L. Kapsenberg. 1993. The skin immune system: progress in cutaneous biology. Immunol. Today 14:75-78. [DOI] [PubMed] [Google Scholar]
- 6.Brennan, M. A., and B. T. Cookson. 2000. Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol. Microbiol. 38:31-40. [DOI] [PubMed] [Google Scholar]
- 7.Bretsche, P. A., N. Ismail, J. N. Menon, C. A. Power, J. Uzonna, and G. Wei. 2001. Vaccination against and treatment of tuberculosis, the leishmaniases and AIDS: perspectives from basic immunology and immunity to chronic intracellular infections. Cell. Mol. Life Sci. 58:1879-1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cannon, R. D., and W. L. Chaffin. 1999. Oral colonization by Candida albicans. Crit. Rev. Oral Biol. Med. 10:359-383. [DOI] [PubMed] [Google Scholar]
- 9.Cenci, E., A. Mencacci, G. Del Sero, C. F. d'Ostiani, P. Mosci, A. Bacci, C. Montagnoli, and L. M. Kopf. 1998. Romani. IFN-gamma is required for IL-12 responsiveness in mice with Candida albicans infection. J. Immunol. 161:3543-3550. [PubMed] [Google Scholar]
- 10.Challacombe, S. J. 1994. Immunologic aspects of oral candidiasis. Oral Surg. Oral Med. Oral Pathol. 78:202-210. [DOI] [PubMed] [Google Scholar]
- 11.Darveau, R. P., and R. E. Hancock. 1983. Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimurium strains. J. Bacteriol. 155:831-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dinarello, C. A. 1999. IL-18: a TH1-inducing, proinflammatory cytokine and new member of the IL-1 family. J. Allergy Clin. Immunol 103:11-24. [DOI] [PubMed] [Google Scholar]
- 13.Djeu, J. Y., D. K. Blanchard, D. Halkias, and H. Friedman. 1986. Growth inhibition of Candida albicans by human polymorphonuclear neutrophils: activation by interferon-gamma and tumor necrosis factor. J. Immunol. 137:2980-2984. [PubMed] [Google Scholar]
- 14.Douglas, L. J. 1987. Adhesion of Candida species to epithelial surfaces. Crit. Rev. Microbiol. 15:27-43. [DOI] [PubMed] [Google Scholar]
- 15.Elhofy, A., and K. L. Bost. 1999. Limited interleukin-18 response in Salmonella-infected murine macrophages and in Salmonella-infected mice. Infect. Immun. 67:5021-5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia, V. E., K. Uyemura, P. A. Sieling, M. T. Ochoa, C. T. Morita, H. Okamura, M. Kurimoto, T. H. Rea, and R. L. Modlin. 1999. IL-18 promotes type 1 cytokine production from NK cells and T cells in human intracellular infection. J. Immunol. 162:6114-6121. [PubMed] [Google Scholar]
- 17.Gerdes, N., G. K. Sukhova, P. Libby, R. S. Reynolds, J. L. Young, and U. Schonbeck. 2002. Expression of interleukin (IL)-18 and functional IL-18 receptor on human vascular endothelial cells, smooth muscle cells, and macrophages: implications for atherogenesis. J. Exp. Med. 195:245-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghayur, T., S. Banerjee, M. Hugunin, D. Butler, L. Herzog, A. Carter, L. Quintal, L. Sekut, R. Talanian, M. Paskind, W. Wong, R. Kamen, D. Tracey, and H. Allen. 1997. Caspase-1 processes IFN-gamma-inducing factor and regulates LPS-induced IFN-gamma production. Nature 386:619-623. [DOI] [PubMed] [Google Scholar]
- 19.Groll, A. H., and T. J. Walsh. 2001. Uncommon opportunistic fungi: new nosocomial threats. Clin. Microbiol. Infect. 7:8-24. [DOI] [PubMed] [Google Scholar]
- 20.Gu, Y., K. Kuida, H. Tsutsui, G. Ku, K. Hsiao, M. A. Fleming, N. Hayashi, K. Higashino, H. Okamura, K. Nakanishi, M. Kurimoto, T. Tanimoto, R. A. Flavell, V. Sato, M. W. Harding., D. J. Livingston, and M. S. Su. 1997. Activation of interferon-gamma inducing factor mediated by interleukin-1beta converting enzyme. Science 275:206-209. [DOI] [PubMed] [Google Scholar]
- 21.Hube, B. 1996. Candida albicans secreted aspartyl proteinases. Curr. Top. Med. Mycol. 7:55-69. [PubMed] [Google Scholar]
- 22.Kaposzta, R., P. Tree, L. Marodi, and S. Gordon. 1998. Characteristics of invasive candidiasis in gamma interferon- and interleukin-4-deficient mice: role of macrophages in host defense against Candida albicans. Infect. Immun. 66:1708-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobayashi, K., N. Nakata, M. Kai, T. Kasama, Y. Hanyuda, and Y. Hatano. 1997. Decreased expression of cytokines that induce type 1 helper T cell/interferon-gamma responses in genetically susceptible mice infected with Mycobacterium avium. Clin. Immunol. Immunopathol. 85:112-116. [DOI] [PubMed] [Google Scholar]
- 24.Kucsera, J., K. Yarita, and K. Takeo. 2000. Simple detection method for distinguishing dead and living yeast colonies. J. Microbiol. Methods 41:19-21. [DOI] [PubMed] [Google Scholar]
- 25.Kullberg, B. J., J. W. vant Wout, C. Hoogstraten, and R. van Furth. 1993. Recombinant interferon-gamma enhances resistance to acute disseminated Candida albicans infection in mice. J. Infect. Dis. 168:436-443. [DOI] [PubMed] [Google Scholar]
- 26.Lundqvist, C., V. Baranov, S. Teglund, S. Hammarstrom, and M. L. Hammarstrom. 1994. Cytokine profile and ultrastructure of intraepithelial gamma delta T cells in chronically inflamed human gingiva suggest a cytotoxic effector function. J. Immunol. 153:2302-2312. [PubMed] [Google Scholar]
- 27.Lundstrom, T., and J. Sobel. 2001. Nosocomial candiduria: a review. Clin. Infect. Dis. 32:1602-1607. [DOI] [PubMed] [Google Scholar]
- 28.Marodi, L., S. Schreiber, D. C. Anderson, R. P. MacDermott, H. M. Korchak, and R. B. Johnston. 1993. Enhancement of macrophage candidacidal activity by interferon-gamma. Increased phagocytosis, killing, and calcium signal mediated by a decreased number of mannose receptors. J. Clin. Investig. 91:2596-2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McInnes, I. B., J. A. Gracie, and F. Y. Liew. 2001. Interleukin-18: a novel cytokine in inflammatory rheumatic disease. Arthritis Rheum. 44:1481-1483. [DOI] [PubMed] [Google Scholar]
- 30.Mencacci, A., E. Cenci, A. Bacci, C. Montagnoli, F. Bistoni, and L. Romani. 2000. Cytokines in candidiasis and aspergillosis. Curr. Pharm. Biotechnol. 3:235-251. [DOI] [PubMed] [Google Scholar]
- 31.Molet, S., Q. Hamid, F. Davoine, E. Nutku, R. Taha, N. Page, R. Olivenstein, J. Elias, and J. Chakir. 2001. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J. Allergy Clin. Immunol. 108:430-438. [DOI] [PubMed] [Google Scholar]
- 32.Monteleone, G., F. Trapasso, T. Parrello, L. Biancone, A. Stella, R. Iuliano, F. Luzza, A. Fusco, and F. Pallone. 1999. Bioactive IL-18 expression is up-regulated in Crohn's disease. J. Immunol. 163:143-147. [PubMed] [Google Scholar]
- 33.Nakanishi, K., T. Yoshimoto, H. Tsutsui, and H. Okamura. 2001. Interleukin-18 regulates both Th1 and Th2 responses. Annu. Rev. Immunol. 19:423-474. [DOI] [PubMed] [Google Scholar]
- 34.Nickoloff, B. J., and L. A. Turka. 1994. Immunological functions of non-professional antigen-presenting cells: new insights from studies of T-cell interactions with keratinocytes. Immunol. Today 10:464-469. [DOI] [PubMed] [Google Scholar]
- 35.Okamura, H., H. Tsutsi, T. Komatsu, M. Yutsudo, A. Hakura, T. Tanimoto, K. Torigoe, T. Okura, Y. Nukada, K. Hattori, et al. 1995. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature 378:88-91. [DOI] [PubMed] [Google Scholar]
- 36.Pendrak, M. L., and S. A. Klotz. 1995. Adherence of Candida albicans to host cells. FEMS. Microbiol. Lett. 129:103-113. [DOI] [PubMed] [Google Scholar]
- 37.Pollanen, M. T., J. I. Salonen, D. Grenier, and V.-J. Uitto. 2000. Epithelial cell response to challenge of bacterial lipoteichoic acids and lipopolysaccharides in vitro. Microbial Pathogenesis J. Med. Microbiol. 49:245-254. [DOI] [PubMed] [Google Scholar]
- 38.Presland, R. B., and B. A. Dale. 2000. Epithelial structural proteins of the skin and oral cavity: function in health and disease. Crit. Rev. Oral Biol. Med. 11:383-408. [DOI] [PubMed] [Google Scholar]
- 39.Romani, L. 1999. Immunity to Candida albicans: Th1, Th2 cells and beyond. Curr. Opin. Microbiol. 2:363-367. [DOI] [PubMed] [Google Scholar]
- 40.Rouabhia, M., and N. Deslauriers. 2002. Production and characterization of an in vitro engineered human oral mucosa. Biochem. Cell. Biol. 80:189-195. [DOI] [PubMed] [Google Scholar]
- 41.Rouabhia, M., J. Chakir, O. Othmane, and P. A. Deschaux. 1989. Interaction between immune and endocrine systems: effect of luteotrophic hormone (LH) and thymic hormone on surface antigens (Thy1-2, Lyt1 and Lyt2) expression. Thymus 14:205-212. [PubMed] [Google Scholar]
- 42.San-Blas, G., L. R. Travassos, B. C. Fries, D. L. Goldman, A. Casadevall, A. K. Carmona, T. F. Barros, R. Puccia, M. K. Hostetter, S. G. Shanks, V. M. Copping, Y. Knox, and N. A. Gow. 2000. Fungal morphogenesis and virulence. Med. Mycol. 38:79-86. [PubMed] [Google Scholar]
- 43.Shibuya, K., W. F. Coulson, J. S. Wollman, M. Wakayama, T. Ando, T. Oharaseki, K. Takahashi, and S. Naoe. 2001. Histopathology of cryptococcosis and other fungal infections in patients with acquired immunodeficiency syndrome. Int. J. Infect. Dis. 5:78-85. [DOI] [PubMed] [Google Scholar]
- 44.Stoll, S., G. Muller, M. Kurimoto, J. Saloga, T. Tanimoto, H. Yamauchi, H. Okamura, J. Knop, and A. H. Enk. 1997. Production of IL-18 (IFN-gamma-inducing factor) messenger RNA and functional protein by murine keratinocytes. J. Immunol. 159:298-302. [PubMed] [Google Scholar]
- 45.Sugawara, I. 2000. Interleukin-18 (IL-18) and infectious diseases, with special emphasis on diseases induced by intracellular pathogens. Microbes Infect. 2:1257-1263. [DOI] [PubMed] [Google Scholar]
- 46.Sugawara, I., H. Yamada, H. Kaneko, S. Mizuno, K. Takeda, and S. Akira. 1999. Role of interleukin-18 (IL-18) in mycobacterial infection in IL-18-gene-disrupted mice. Infect. Immun. 67:2585-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sugawara, S., A. Uehara, T. Nochi, T. Yamaguchi, H. Ueda, A. Sugiyama, K. Hanzawa, K. Kumagai, H. Okamura, and H. Takada. 2001. Neutrophil proteinase 3-mediated induction of bioactive IL-18 secretion by human oral epithelial cells. J. Immunol. 167:6568-6575. [DOI] [PubMed] [Google Scholar]
- 48.Takeda, K., H. Tsutsui, T. Yoshimoto, O. Adachi, N. Yoshida, T. Kishimoto, H. Okamura, K. Nakanishi, and S. Akira. 1998. Defective NK cell activity and Th1 response in IL-18deficient mice. Immunity 8:383-390. [DOI] [PubMed] [Google Scholar]
- 49.Vazquez, J. A. 2000. Therapeutic options for the management of oropharyngeal and esophageal candidiasis in HIV/AIDS patients. HIV Clin. Trials 1:47-59. [DOI] [PubMed] [Google Scholar]
- 50.Vazquez-Torres, A., and E. Balish. 1997. Macrophages in resistance to candidiasis. Microbiol. Mol. Biol. Rev. 61:170-192. [DOI] [PMC free article] [PubMed] [Google Scholar]