Abstract
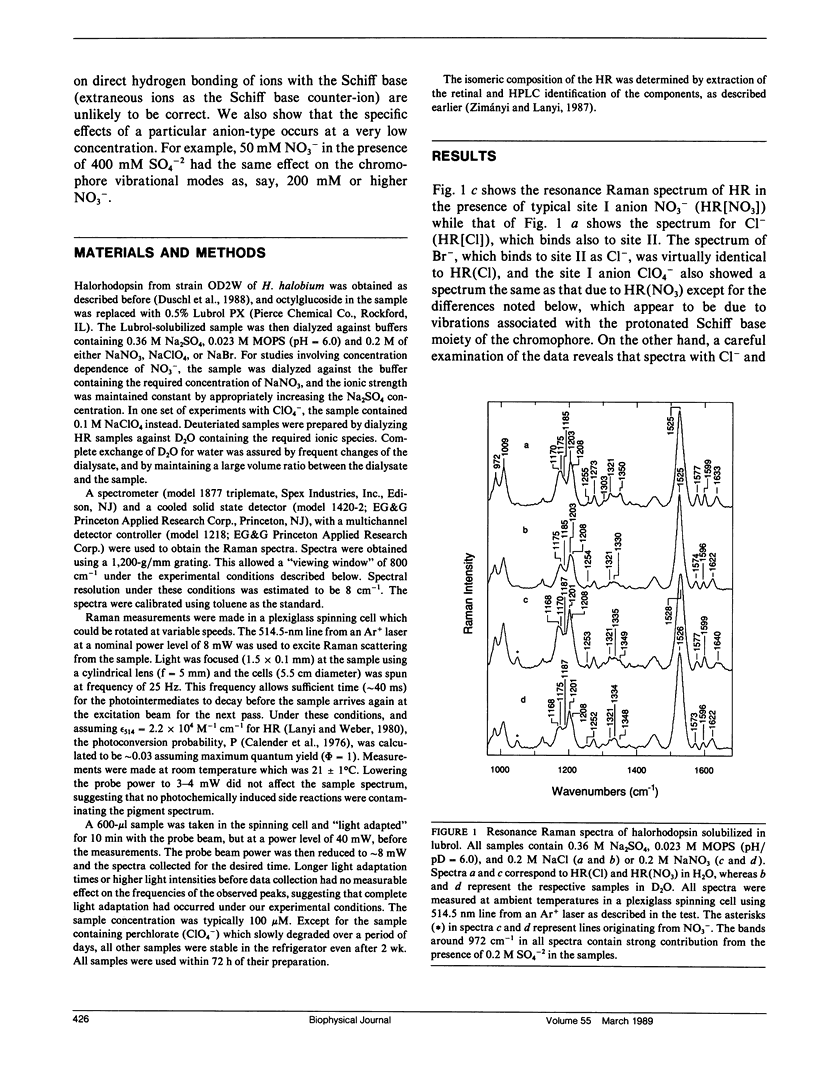
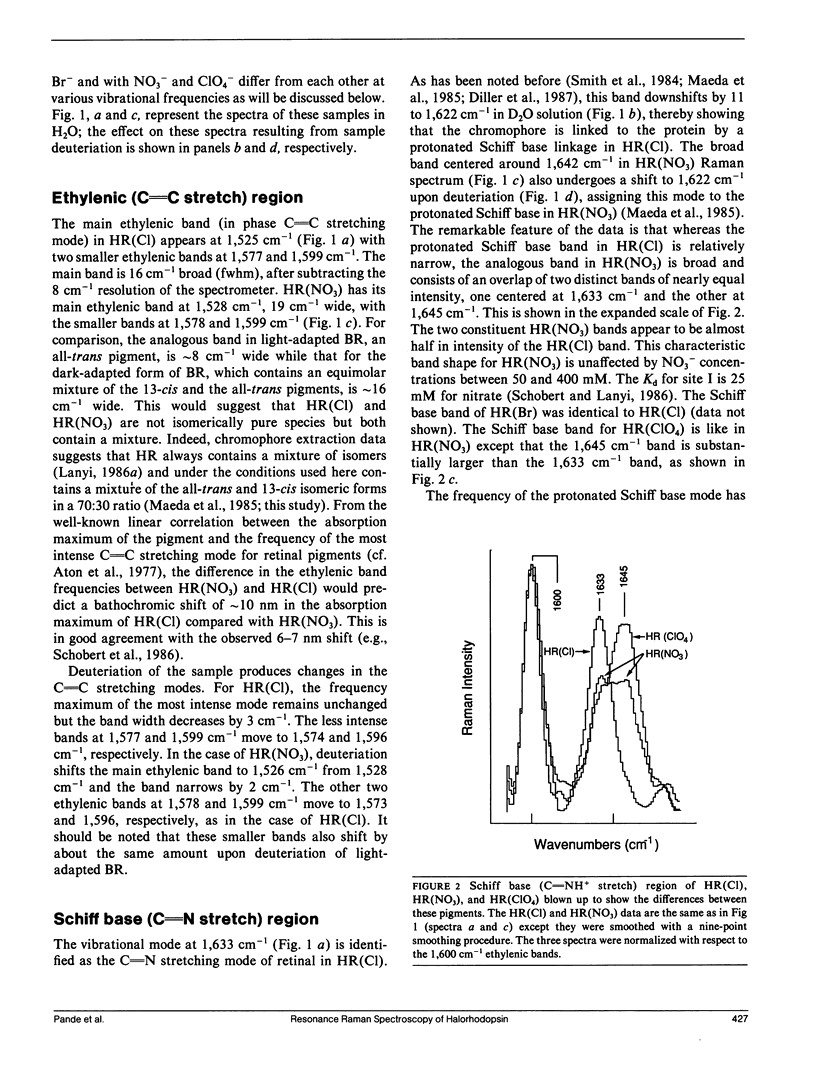
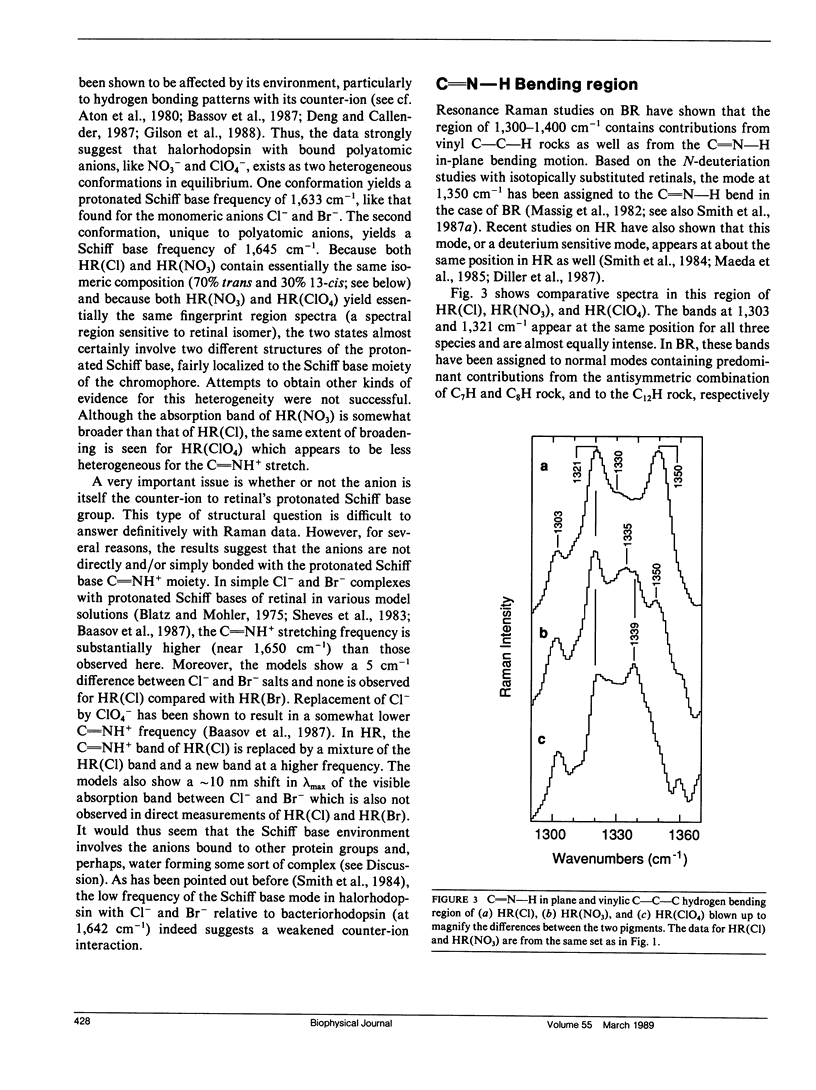
Resonance Raman experiments were conducted to probe and understand the effect of various anions on halorhodopsin. These included monoatomic anions Cl- and Br-, which bind to the so-called halorhodopsin binding sites I and II, and polyatomic anions NO3- and ClO4-, which bind to site I only. The two types of ions clearly show different effects on the vibrational spectrum of the chromophore. The differences are not localized to the Schiff base region of the molecule, but extend to the chromophore structure-sensitive fingerprint region as well. We find that the protonated Schiff base frequency is at 1,633 cm-1 for Cl- and Br- ions, as reported previously for Cl-. However, we find that two Schiff base frequencies characterize halorhodopsin upon binding of the polyatomic anions. One frequency lies at the same location as that found for the monoatomic anions and the other is at 1,645 cm-1. Halorhodopsin with bound NO3- and ClO4- thus may consist of two heterogeneous structures in equilibrium. This heterogeneity does not seem to correlate with a retinal isomeric heterogeneity, which we can also demonstrate in these samples. The results suggest that anions binding to site I do not bind to the Schiff base directly, but can influence chromophore and/or protein conformational states.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aton B., Doukas A. G., Callender R. H., Becher B., Ebrey T. G. Resonance Raman studies of the purple membrane. Biochemistry. 1977 Jun 28;16(13):2995–2999. doi: 10.1021/bi00632a029. [DOI] [PubMed] [Google Scholar]
- Baasov T., Friedman N., Sheves M. Factors affecting the C = N stretching in protonated retinal Schiff base: a model study for bacteriorhodopsin and visual pigments. Biochemistry. 1987 Jun 2;26(11):3210–3217. doi: 10.1021/bi00385a041. [DOI] [PubMed] [Google Scholar]
- Blanck A., Oesterhelt D. The halo-opsin gene. II. Sequence, primary structure of halorhodopsin and comparison with bacteriorhodopsin. EMBO J. 1987 Jan;6(1):265–273. doi: 10.1002/j.1460-2075.1987.tb04749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatz P. E., Mohler J. H. Effect of selected anions and solvents on the electron absorption, nuclear magnetic resonance, and infrared spectra of the N-retinylidene-n-butylammonium cation. Biochemistry. 1975 Jun 3;14(11):2304–2309. doi: 10.1021/bi00682a005. [DOI] [PubMed] [Google Scholar]
- Callender R. H., Doukas A., Crouch R., Nakanishi K. Molecular flow resonance Raman effect from retinal and rhodopsin. Biochemistry. 1976 Apr 20;15(8):1621–1629. doi: 10.1021/bi00653a005. [DOI] [PubMed] [Google Scholar]
- Deng H., Callender R. H. A study of the Schiff base mode in bovine rhodopsin and bathorhodopsin. Biochemistry. 1987 Nov 17;26(23):7418–7426. doi: 10.1021/bi00397a033. [DOI] [PubMed] [Google Scholar]
- Engelman D. M., Henderson R., McLachlan A. D., Wallace B. A. Path of the polypeptide in bacteriorhodopsin. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2023–2027. doi: 10.1073/pnas.77.4.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilson H. S., Honig B. H., Croteau A., Zarrilli G., Nakanishi K. Analysis of the factors that influence the C=N stretching frequency of polyene Schiff bases. Implications for bacteriorhodopsin and rhodopsin. Biophys J. 1988 Feb;53(2):261–269. doi: 10.1016/S0006-3495(88)83087-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanyi J. K. Halorhodopsin: a light-driven chloride ion pump. Annu Rev Biophys Biophys Chem. 1986;15:11–28. doi: 10.1146/annurev.bb.15.060186.000303. [DOI] [PubMed] [Google Scholar]
- Lanyi J. K. Light-dependent trans to cis isomerization of the retinal in halorhodopsin. FEBS Lett. 1984 Oct 1;175(2):337–342. doi: 10.1016/0014-5793(84)80764-4. [DOI] [PubMed] [Google Scholar]
- Lanyi J. K. Photochromism of halorhodopsin. cis/trans isomerization of the retinal around the 13-14 double bond. J Biol Chem. 1986 Oct 25;261(30):14025–14030. [PubMed] [Google Scholar]
- Lanyi J. K., Weber H. J. Spectrophotometric identification of the pigment associated with light-driven primary sodium translocation in Halobacterium halobium. J Biol Chem. 1980 Jan 10;255(1):243–250. [PubMed] [Google Scholar]
- Lanyi J. K., Zimányi L., Nakanishi K., Derguini F., Okabe M., Honig B. Chromophore/protein and chromophore/anion interactions in halorhodopsin. Biophys J. 1988 Feb;53(2):185–191. doi: 10.1016/S0006-3495(88)83080-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schobert B., Lanyi J. K. Electrostatic interaction between anions bound to site I and the retinal Schiff base of halorhodopsin. Biochemistry. 1986 Jul 15;25(14):4163–4167. doi: 10.1021/bi00362a026. [DOI] [PubMed] [Google Scholar]
- Schobert B., Lanyi J. K., Oesterhelt D. Effects of anion binding on the deprotonation reactions of halorhodopsin. J Biol Chem. 1986 Feb 25;261(6):2690–2696. [PubMed] [Google Scholar]
- Smith S. O., Lugtenburg J., Mathies R. A. Determination of retinal chromophore structure in bacteriorhodopsin with resonance Raman spectroscopy. J Membr Biol. 1985;85(2):95–109. doi: 10.1007/BF01871263. [DOI] [PubMed] [Google Scholar]
- Smith S. O., Marvin M. J., Bogomolni R. A., Mathies R. A. Structure of the retinal chromophore in the hR578 form of halorhodopsin. J Biol Chem. 1984 Oct 25;259(20):12326–12329. [PubMed] [Google Scholar]
- Zimányi L., Lanyi J. K. Iso-halorhodopsin: a stable, 9-cis retinal containing photoproduct of halorhodopsin. Biophys J. 1987 Dec;52(6):1007–1013. doi: 10.1016/S0006-3495(87)83293-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


