Abstract
Despite extensive public health efforts, there are presently 200 to 400 million malaria infections and 1 to 2 million deaths each year due to the Plasmodium parasite. A prime target for malaria vaccine development is the circumsporozoite (CS) protein, which is expressed on the extracellular sporozoite and the intracellular hepatic stages of the parasite. Previous studies in rodent malaria models have shown that CS repeat B-cell epitopes expressed in a recombinant hepatitis B virus core (HBc) protein can elicit protective immunity. To design a vaccine for human use, a series of recombinant HBc proteins containing epitopes of Plasmodium falciparum CS protein were assayed for immunogenicity in mice [A. Birkett, B. Thornton, D. Milich, G. A. Oliveira, A. Siddique, R. Nussenzweig, J. M. Calvo-Calle, and E. H. Nardin, abstract from the 50th Annual Meeting of the American Society of Tropical Medicine and Hygiene 2001, Am. J. Trop. Med. Hyg. 65(Suppl. 3):258, 2001; D. R. Milich, J. Hughes, J. Jones, M. Sallberg, and T. R. Phillips, Vaccine 20:771-788, 2001]. The present paper summarizes preclinical analyses of the optimal P. falciparum HBc vaccine candidate, termed ICC-1132, which contains T- and B-cell epitopes from the repeat region and a universal T-cell epitope from the C terminus of the CS protein. The vaccine was highly immunogenic in mice and in Macaca fascicularis (cynomolgus) monkeys. When formulated in adjuvants suitable for human use, the vaccine elicited antisporozoite antibody titers that were logs higher than those obtained in previous studies. Human malaria-specific CD4+-T-cell clones and T cells of ICC-1132-immunized mice specifically recognized malaria T-cell epitopes contained in the vaccine. In addition to inducing strong malaria-specific immune responses in naïve hosts, ICC-1132 elicited potent anamnestic antibody responses in mice primed with P. falciparum sporozoites, suggesting potential efficacy in enhancing the sporozoite-primed immune responses of individuals living in areas where malaria is endemic.
The complex life cycle of the malaria parasite is initiated by infective sporozoites that are injected into the mammalian host by the mosquito vector. Immunization with irradiated sporozoites can protect mice, monkeys, and human volunteers against sporozoite challenge (reviewed in references 34 and 37). Individuals and experimental animals immunized with irradiated sporozoites develop protective humoral and cellular immune effector mechanisms that specifically target the preerythrocytic stages of the parasite. Since sporozoites cannot be cultivated in vitro, intensive research efforts have focused on the development of malaria subunit vaccines that can simulate sporozoite-induced protective immunity.
The circumsporozoite (CS) protein is a primary target of protective immune responses in sporozoite-immunized experimental hosts (31, 37). Antibodies specific for an immunodominant B-cell epitope in the central repeat region of the CS protein can immobilize sporozoites and block invasion of host hepatocytes. The Plasmodium falciparum protective B-cell epitope consists of multiple tandem repeats of the tetramer NANP sequence (35, 52). The first phase I and II trials of a peptide-protein conjugate containing only P. falciparum (NANP)3 repeats demonstrated the potential of CS subunit vaccines to protect against sporozoite challenge (16).
Additional studies in the rodent malaria model demonstrated that irradiated sporozoites elicited not only neutralizing anti-CS antibodies but also potent cellular immunity that targeted the hepatic exoerythrocytic forms (EEF) of the parasite. However, the CS NANP repeat region lacks strong Th cell epitopes, and little or no parasite-specific T-cell response was elicited following immunization with CS repeat vaccines (10, 11, 15, 16). Second-generation CS peptide vaccines have included parasite-specific CD4+-T-cell epitopes to insure that memory Th cells are elicited. These are important both for anamnestic antibody responses and for lymphokines, primarily gamma interferon (IFN-γ), to inhibit development of hepatic EEFs (reviewed in reference 34).
Recently, promising results were obtained in phase I and II trials of a truncated P. falciparum CS protein expressed in a recombinant hepatitis B virus (HBV) surface antigen, termed RTS,S. Immunized volunteers developed high levels of antibodies and Th1-type cellular responses (2, 17, 48). More importantly, when administered in a complex adjuvant formulation, this vaccine protected approximately 50% of immunized volunteers against P. falciparum sporozoite challenge. Vaccine-induced protective immunity, however, was relatively short-lived in the malaria-naïve volunteers, as well as in vaccinees living in areas of malaria endemicity (2, 47).
Similar to the hepatitis surface antigen, recombinant HBV core (HBc) protein spontaneously assembles into subviral particles composed of 180 to 240 monomers (38). Recombinant core particles were found to be significantly more immunogenic than recombinant surface antigen at both the B- and T-cell levels (24). Highly potent immunogens can be produced by strategic insertion of heterologous B- and T-cell epitopes derived from bacterial, viral, and protozoan pathogens (reviewed in references 38 and 50). Optimal immunogenicity was observed when B-cell epitopes were inserted into an immunodominant loop region located at the tip of the surface “spikes” on HBc particles, while fusion to the C terminus elicited lower antibody response (41).
In earlier studies, hybrid recombinant HBc particles containing CS repeats of Plasmodium berghei and Plasmodium yoelii rodent malarias elicited high levels of antisporozoite antibodies and protection in mice (42, 43). However, similar hybrid HBc particles containing P. falciparum (NANP)4 repeats elicited antibody titers that were orders of magnitude lower and were poorly reactive with P. falciparum sporozoites (43). An optimal P. falciparum CS-HBc immunogen should elicit not only high levels of antisporozoite antibodies but also malaria-specific T cells to target both extracellular and intracellular parasite stages.
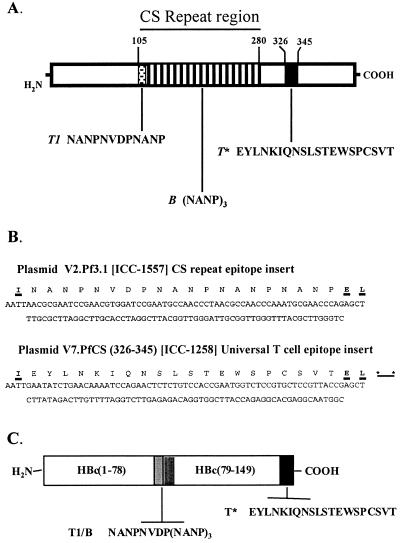
The (NANP)3 B-cell epitope in combination with various P. falciparum CS T-cell epitopes has also been tested as synthetic peptide vaccines in recent phase I clinical trials (32, 36). The two T-cell epitopes used in these vaccines were originally identified by CD4+-T-cell clones derived from P. falciparum sporozoite-immunized volunteers (27, 33). One T-cell epitope, termed T1, is located in alternating NVDP and NANP tetramers of the CS minor repeat region, while a second epitope, termed T∗, is located in the C terminus of the P. falciparum CS protein (Fig. 1A).
FIG. 1.
(A) P. falciparum (NF54) CS protein showing the B-cell epitope (NANP)n within the major repeat region (bars), the T1 epitope within the 5′ minor repeat region (stippled), and the universal T∗ epitope in the C terminus (black). (B) The synthetic dsDNA fragment encoding the malaria epitope inserted in each plasmid is shown. Amino acid insertions contributed by the restriction sites are underlined, and the stop codons are indicated by asterisks. (C) Hybrid HBc protein showing the CS T1 and B repeat epitopes (hatched box) inserted between HBc amino acids 78 and 79, a region located at the tip of the core particle surface spikes (3, 8), and the T∗ epitope fused to amino acid Val149 at the C terminus of the truncated HBc protein (solid box).
In these clinical trials, the synthetic multiple antigen peptide (MAP) vaccine, containing T1- and B-cell epitopes from the CS repeat region, elicited high anti-P. falciparum sporozoite antibody titers (>104) in human volunteers (36). The high-responder phenotype correlated with class II genotypes found in 30 to 40% of the population, indicating a limited HLA restriction of the P. falciparum CS repeat response in humans, as found previously in murine studies (10, 15, 30). A second synthetic peptide vaccine, (T1BT∗)4 polyoxime, combined the T1 and B repeat epitopes with the C-terminal T∗ epitope. Unlike the T1 epitope, the T∗ epitope binds to multiple HLA class II molecules in vitro and elicits Th cells in a broad range of murine major histocompatibility complex (MHC) backgrounds (4, 28). Consistent with the presence of a universal Th cell epitope, immunization with the (T1BT∗)4 vaccine overcame genetic restriction and induced antibodies in volunteers of diverse HLA haplotypes (32). The majority of the volunteers developed T∗-specific CD4+ T cells and high antibody titers (103 to 104). These findings have encouraged further efforts to optimize the qualitative and quantitative responses to malaria CS vaccines.
A series of recombinant CS-HBc proteins containing the malaria T1, B, and T∗ epitopes in various combinations and configurations were assayed for immunogenicity in mice [23; A. Birkett, B. Thornton, D. Milich, G. A. Oliveira, A. Siddique, R. Nussenzweig, J. M. Calvo-Calle, and E. H. Nardin, abstract from the 50th Annual Meeting of the American Society of Tropical Medicine and Hygiene 2001, Am. J. Trop. Med. Hyg. 65(Suppl. 3):258, 2001]. One particle, ICC-1132, contained the T1 and B repeat epitopes inserted in the HBc immunodominant loop and the universal T-cell epitope (T∗) fused to the C terminus of the truncated HBc protein (Fig. 1C). In preparation for a phase I clinical trial, the present studies have utilized rodent and simian hosts to assay the immunogenicity of ICC-1132 formulated in various adjuvants suitable for human use. In addition, the antigenicity of hybrid core particles for human cells was assayed in vitro using peripheral blood lymphocytes (PBL) and malaria-specific human CD4+ T cells.
MATERIALS AND METHODS
Construction of expression vectors.
The ICC-1132 recombinant protein was expressed in Escherichia coli transfected with the expression plasmid V12.Pf3.1. This plasmid encodes a truncated HBc gene (amino acids 1 to 149) with the malaria CS T∗ epitope fused after the C-terminal Val149 and the CS repeat epitopes inserted in the HBc loop region between amino acids 78 and 79 [23; Birkett et al., Am. J. Trop. Med. Hyg. 65(Suppl. 3):258, 2001]. HBc particles engineered to present heterologous epitopes have historically been truncated at, or around, amino acid 149 to avoid incorporation of host RNA by the protamine-rich C-terminal tail (amino acids 150 to 183).
To construct this hybrid vaccine, a plasmid (V2) containing the truncated HBc gene was first digested at the EcoRI and SacI restriction sites, which had been strategically introduced between the codons for D78 and P79 of the loop region. A synthetic double-stranded DNA (dsDNA) fragment encoding the repeat sequence NANPNVDP(NANP)3, modified by the addition of 5′ EcoRI and 3′ SacI overhangs, was then inserted to yield plasmid V2.Pf3.1 (Fig. 1B). V2.Pf3.1 was used to direct the expression of a particle containing only the CS repeats (ICC-1557).
A second plasmid, V7.PfCS(326-345), was used to direct the expression of a particle containing only the T∗ sequence fused to the C-terminal Val149 of the truncated HBc (ICC-1258). The plasmid was constructed in a similar manner, using a plasmid (V7) containing a HBc gene that had been modified to accept C-terminal fusions after amino acid Val149 at EcoRI-SacI restriction sites. The plasmid was first digested with EcoRI and SacI restriction enzymes, and a synthetic dsDNA fragment encoding the T∗ sequence, modified by the addition of EcoRI-SacI restriction site overhangs, was inserted to produce plasmid V7.PfCS(326-345) (Fig. 1B).
An expression vector, V12.Pf3.1, was then constructed to encode a hybrid HBc protein containing CS repeats in the immunodominant loop and T∗ at the C terminus (ICC-1132). To do this, the carboxyl terminus of the V7.PfCS(326-345) plasmid was amplified using two PCR primers to produce a dsDNA fragment corresponding to HBc amino acids 79 to 149 plus the T∗ epitope, flanked with SacI and HindIII restriction sites. The PCR primers used for amplification were HBc-P79/SacI-F (5′-CGCGAGCTCCCAGCGTCTAGAGACCTAG) and PfCS(326-345)-H3-R (5′GCCAAGCTTACTAGGTAACGGAGCACGGA); PfCS(326-345)-H3-R was designed to facilitate deletion of the 2 amino acids (Glu-Leu) coded by the EcoRI restriction site following the T∗ epitope. To yield the final expression vector V12.Pf3.1, the PCR product was cut with SacI and HindIII and then cloned into the V2.Pf3.1 vector, which had been prepared by cutting with the same two restriction enzymes. The nucleotide sequences of all plasmids were verified by DNA sequencing.
Expression and purification of recombinant particles.
E. coli strain TB1 was transformed with either the V12.Pf3.1 (ICC-1132), V2.Pf3.1 (ICC-1557), or V7.PfCS(326-345) (ICC-1258) vector and selected on Luria-Bertani plates containing ampicillin (50 μg/ml). After 16 to 24 h of incubation at 37°C, a single colony was picked, expanded overnight, and used to inoculate a 500-ml culture (tryptone-yeast extract-NaCl [TYN] medium supplemented with 1 g of glucose/liter and 50 μg of ampicillin/ml). After 16 to 20 h, cells were harvested by centrifugation, resuspended in 50 to 100 ml of Tris-EDTA buffer (50 mM Tris-HCl, 10 mM EDTA, pH 8.0), and lysed by a single passage at 16,000 lb/in2 through a French pressure cell (ThermoSpectronic, Cambridge, United Kingdom). After centrifugation, the proteins in the supernatant were precipitated with ammonium sulfate and the precipitate was resuspended in a minimal volume of Tris-EDTA buffer. The resuspended protein pellet was dialyzed extensively against the same buffer and centrifuged, and the supernatant was recovered.
All particles were purified by gel filtration chromatography on a Sepharose CL-4B column (Pharmacia, Piscataway, N.J.). Fractions eluted from the column were pooled and loaded onto an hydroxyapatite column (Clarkson, South Williamsport, Pa.). The column was washed with 50 mM sodium phosphate, pH 6.8, and the nonbinding hydroxyapatite eluent was applied to a Mono-Q anion-exchange column (Pharmacia) and eluted with a linear gradient of 0 to 1.2 M NaCl. The protein was concentrated by a second ammonium sulfate precipitation and dialyzed against 20 mM sodium phosphate, pH 6.8, and the concentration was determined using a standard bicinchoninic acid assay.
Immunogenicity. (i) Murine studies.
BALB/c and (B6 × 129)F1 mice were obtained from Jackson Laboratory, Bar Harbor, Maine. MHC class II-deficient mice (I-Aβb−/−) were a kind gift of Albert Bendelac, Princeton University. Mice were immunized intraperitoneally on days 0, 21, and 42 with 10 μg of ICC-1132 or HBc149. Endotoxin units were <0.5/μg as determined by Limulus amebocyte lystate assay. Mice were primed with antigen emulsified in Freund's complete adjuvant followed by boosters with antigen in Freund's incomplete adjuvant. Serum samples were collected 21 days after each immunizing dose.
Cellular studies were carried out using lymph node cells obtained from BALB/c mice primed subcutaneously with ICC-1132 in Freund's adjuvant. Additional cytokine studies used spleen cells from mice primed with 10 μg of ICC-1132 formulated in 70% Montanide ISA 720, a metabolizable water-in-oil adjuvant (SEPPIC, Paris, France), followed by booster injection at 42 days with ICC-1132 either in saline, adsorbed to alum (Alhydrogel, Superfos, Frederikssund, Denmark), or emulsified in Montanide ISA 720. Untreated mice served as control. Five days after the booster inoculation, mice were sacrificed and spleen and lymph nodes were removed for T-cell analysis.
(ii) M. fascicularis (cynomolgus) monkeys.
The immunogenicity of ICC-1132 formulated in different adjuvants was also assayed in Macaca fascicularis (cynomolgous) monkeys (maintained by Sierra Biomedical, Sparks, Nev.). Two monkeys (one male and one female) per group were immunized intramuscularly on days 0 and 42 with 20 μg of ICC-1132 in saline or formulated in different adjuvants. Two alum preparations were tested, aluminum phosphate gel (Adju-Phos) with low binding affinity for ICC-1132 and aluminum hydroxide gel (Alhydrogel) with high binding affinity (Superfos). Two additional groups of monkeys received ICC-1132 in an oil-in-water emulsion (SE vehicle; Corixa, Seattle, Wash.) or in the water-in-oil adjuvant, Montanide ISA 720.
Serological assays. (i) ELISA.
Antibodies were measured by enzyme-linked immunoabsorbent assay (ELISA) using HBc proteins or malaria peptides as coating antigens [29, 30; Birkett et al., Am. J. Trop. Med. Hyg. 65(Suppl. 3):258, 2001]. Antirepeat antibodies were assayed using the branched peptide, (T1B)4 MAP, or linear (NANP)5 peptide; similar results were obtained with both antigens. Coated wells were blocked and incubated with twofold dilutions of sera in phosphate-buffered saline-0.05% Tween 20-bovine serum albumin (BSA). Binding of antibodies was detected by ELISA using enzyme-labeled antibodies specific for murine immunoglobulin G (IgG) (Kirkegaard & Perry, Gaithersburg, Md.) or rabbit anti-monkey Ig (Accurate, Westbury, N.Y.) and peroxidase-labeled anti-rabbit antibodies. Following addition of the enzyme substrate ABTS (2,2′-azino[bis-3-ethylbenzthiazolinesulfonic acid])-H2O2, the endpoint titer was determined as the dilution of immune sera giving an optical density greater than the mean + 2 standard deviations obtained with normal sera or two times greater than the optical density obtained with BSA-coated wells.
(ii) IFA.
The indirect immunofluorescence assay (IFA) was carried out using P. falciparum (NF54) sporozoites dissected from the salivary glands of Anopheles mosquitoes infected by feeding on gametocyte cultures. Multiwell slides containing air-dried sporozoites were incubated with twofold dilutions of sera, and the bound antibody was detected by reaction with fluorescein isothiocyanate-labeled antibodies specific for murine IgG (Kirkegaard & Perry) or with rabbit anti-monkey IgG (Accurate) followed by fluorescein isothiocyanate-labeled anti-rabbit antibody (29, 30).
Cellular proliferation and cytokine assays. (i) Murine.
Triplicate wells containing lymph node or spleen cells of naïve or immunized BALB/c mice were stimulated with ICC-1132 or HBc149 particles (10 μg/ml) or malaria CS peptide (20 μg/ml). Proliferation in 6-day cultures was measured by [3H]thymidine incorporation, and results were expressed as stimulation indices (SI) obtained by dividing mean counts per minute in wells with antigen by mean counts per minute in wells without antigen.
Culture supernatants were collected at 48 to 72 h for cytokine analysis. Interleukin 2 (IL-2) was measured by bioassay using an IL-2-dependent cell line, and results were expressed as supernatant-induced SI (32). Additional cytokines were measured by using commercial kits for IFN-γ, IL-4, and IL-10, and results were expressed as picograms per milliliter (BD Pharmingen, San Diego, Calif.).
(ii) Human.
CD4+-T-cell lines (TCL) were established from PBL of volunteers immunized with (T1B)4 MAP or (T1BT∗)4 polyoxime (32, 36). TCL (2 × 104) were cultured with 5 × 104 irradiated PBL antigen-presenting cells (APC) in triplicate wells. The cells were stimulated with 10-fold dilutions of ICC-1132 or HBc149 particles starting at 10 μg/ml or CS peptides (20 μg/ml), recombinant CS proteins (20 μg/ml), or extracts of 105 P. falciparum sporozoites as positive controls. Proliferation in 4-day cultures and IL-2 levels in 24-h supernatants were measured as described for murine assays. Human IFN-γ in 72-h supernatants was measured by using a commercial kit (BD Diagnostics).
RESULTS
Humoral responses induced by P. falciparum ICC-1132 CS-HBc particles. (i) Immunogenicity in mice.
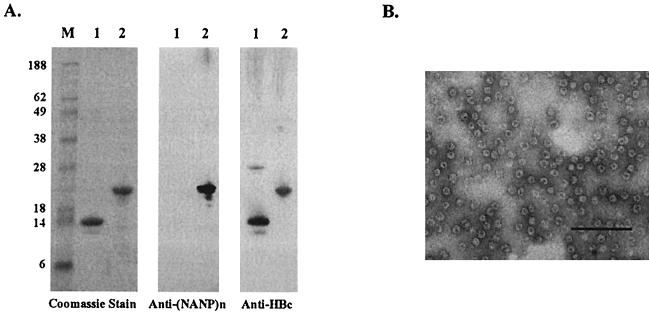
The ICC-1132 particles, containing the P. falciparum repeat (NANP)3 B-cell epitope and two CS-derived T-helper cell epitopes, T1 and T∗ (Fig. 1C), can be produced under good manufacturing practices (GMP) conditions and purified to clinical levels by column chromatography. The purified ICC-1132 and the control HBc149 particle that lacks CS inserts were of the expected molecular weight when analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting (Fig. 2A). The presence of intact particles of an approximate diameter of 32 nm, with a dense central core typical of virus capsids, was demonstrated by electron microscopy (Fig. 2B).
FIG. 2.
(A) Physiochemical characterization of truncated HBc149 protein (lane 1) and purified ICC-1132 (lane 2) by sodium dodecyl sulfate-polyacrylamide gel electrophoresis under reducing conditions. Coomassie blue-stained gels and Western blots, using anti-CS or anti-HBc antibodies, demonstrate migration at the expected molecular mass of the reduced ICC-1132, 22,000 Da, and of the C-terminally truncated HBc149, 17,000 Da (M, molecular mass markers). In the Western blots, a monoclonal antirepeat antibody (MAB 2A10) stained ICC-1132 (lane 2) but not HBc149, which lacks the CS repeats (lane 1). (B) Electron microscopy of purified ICC-1132 particles. Images were generated using 1% ammonium acetate as a negative stain. Magnification, ×40,000; scale bar = 250 nm.
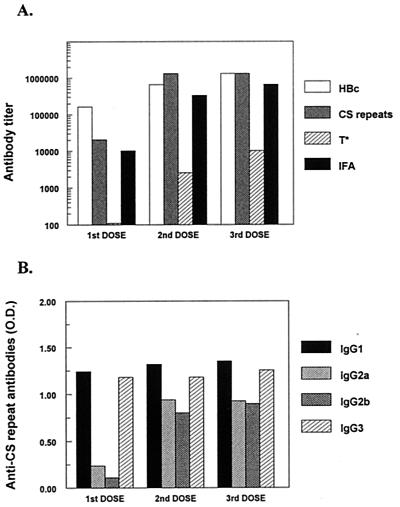
Consistent with previous studies with other murine strains [23; Birkett et al., Am. J. Trop. Med. Hyg. 65(Suppl. 3):258, 2001], administration of two doses of ICC-1132 in Freund's adjuvant elicited extremely high antirepeat antibody titers (geometric mean titer [GMT] = 1.3 × 106) in BALB/c mice (Fig. 3A). Antibody responses were not further increased by a third immunization. The antirepeat peptide titers directly correlated with strong reactivity with P. falciparum sporozoites, reaching peak IFA titers of 655,360. Serum from mice immunized with HBc149 did not react with P. falciparum sporozoites or malaria peptides in either the IFA or ELISA (data not shown).
FIG. 3.
Antibody titers in BALB/c mouse sera obtained 3 weeks after each dose of ICC-1132-Freund's adjuvant. (A) Kinetics and fine specificity of IgG responses measured by IFA using P. falciparum sporozoites and by ELISA using HBc immunogen or malaria peptides as antigen. (B) IgG subtypes of antirepeat antibody response following each immunization. O.D., optical density.
In contrast to the repeat epitope, only low levels of antibody specific for the T∗ epitope were detected in mice immunized with ICC-1132 (Fig. 3A). This is consistent with the poor antibody responses elicited by foreign epitopes inserted at the C terminus of HBc protein (41). The T∗ epitope in the context of the native CS also does not elicit significant antibody responses in sporozoite-immunized mice and human volunteers (5).
In addition to high levels of antirepeat antibodies, anti-HBc titers exceeding 106 were obtained in the mice immunized with ICC-1132 (Fig. 3A) [23; Birkett et al., Am. J. Trop. Med. Hyg. 65(Suppl. 3):258, 2001]. These titers were an order of magnitude lower than those obtained in mice immunized with HBc149 (data not shown). The lower anti-HBc titers result from disruption of the HBc immunodominant B-cell epitope when foreign epitopes are inserted in the loop region [23, 41; Birkett et al., Am. J. Trop. Med. Hyg. 65(Suppl. 3):258, 2001].
Immunization with ICC-1132 elicited repeat-specific antibodies of all IgG subtypes, with a strong Th2-associated IgG1 response (Fig. 3B). In addition, a significant IgG3 response, a subtype frequently elicited in response to T-independent antigens (9, 26, 46), was also noted in the immunized mice (Fig. 3B). Similar results were obtained in (B6 × 129)F1 mice immunized with ICC-1132 in Freund's adjuvant (data not shown). The predominance of IgG1 and IgG3 responses has also been noted in mice immunized with hybrid recombinant core particles formulated in other adjuvants or containing viral epitopes (12, 23).
(ii) T-cell dependency of antibody response to ICC-1132.
The murine IgG3 subtype is frequently elicited by immunization with T-independent antigens, such as polysaccharides with repeating sugar units (26, 46). The HBc protein, unlike the HBV surface antigen, can elicit T-independent, as well as T-dependent, antibody responses to HBc epitopes and to foreign epitopes contained in recombinant hybrid core proteins (12, 24).
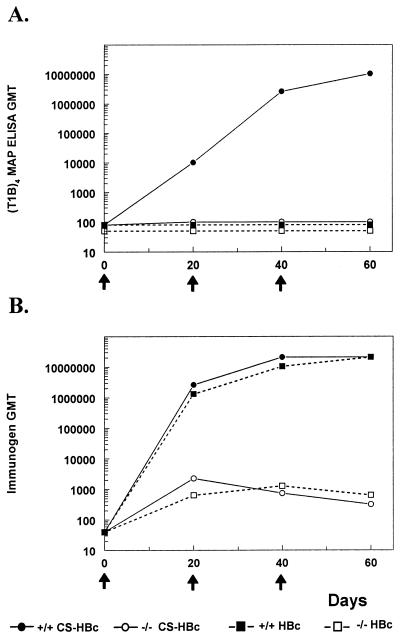
To determine if the strong antimalaria response induced by ICC-1132 was T dependent, transgenic mice lacking class II molecules were immunized. The class II−/− mice failed to develop an IgG response specific for CS repeats at any time point during immunization with ICC-1132 (Fig. 4A, open circles). Parental B6 × 129 controls, expressing normal class II and CD4+-T-cell levels, developed high antirepeat antibody titers following immunization with ICC-1132 (Fig. 4A, closed circles). Mice immunized with HBc149 did not develop antibodies reactive with CS repeats (Fig. 4A, squares).
FIG. 4.
Antibody titers of class II−/− knockout mice (open symbols) or wild-type mice (closed symbols) as measured by ELISA using P. falciparum CS repeats (A) or HBc protein (B) as antigen. Samples were obtained following immunization (arrows) with ICC-1132 (circles) or HBc149 (squares) in Freund's adjuvant.
The parental B6 × 129 mice also developed high anti-HBc titers following immunization with ICC-1132 or HBc149 (Fig. 4B, closed symbols). In contrast to antirepeat antibody response, however, anti-HBc antibodies could be detected in the class II−/− mice, although the magnitude of this response was reduced (Fig. 4B, open symbols). The anti-HBc titers peaked after the first immunization and did not increase following booster inoculations, as is typical of antibody responses to T-independent antigens.
Cellular responses induced by ICC-1132. (i) Induction of malaria-specific T cells in ICC-1132-immunized mice.
The specificity of Th elicited by malaria vaccines is a critical determinant of vaccine efficacy. In mice immunized with ICC-1132, T cells specific for either HBc or CS could provide helper factors for the T-dependent antirepeat antibody response. However, only the T cells specific for CS epitopes can provide malaria-specific memory T cells for anamnestic antibody responses and inhibitory lymphokines that can function in resistance to sporozoite challenge.
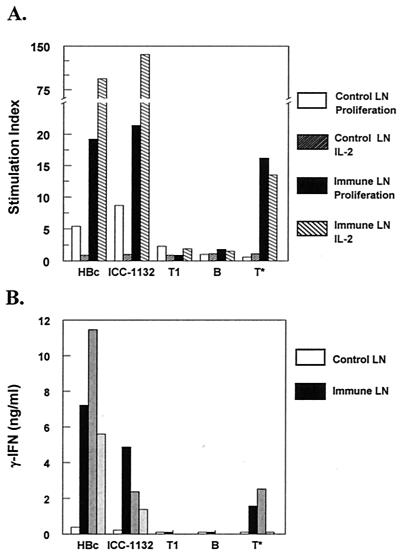
To determine whether CS-specific T cells were elicited by immunization with ICC-1132, lymph node cells of BALB/c mice primed with ICC-1132 were assayed in vitro. Stimulation with HBc particles, with or without CS epitope inserts, elicited strong proliferative and cytokine responses, consistent with the presence of multiple T epitopes in the core antigen (Fig. 5). Importantly, cells from ICC-1132-immunized mice also proliferated and released IL-2 and IFN-γ in response to in vitro challenge with the malaria T∗ peptide. The cells of the ICC-1132-immunized mice did not respond when stimulated with repeat peptides, consistent with the nonresponder phenotype of H-2d mice to CS repeats (4, 10, 15, 30).
FIG. 5.
(A) Lymph node (LN) cells of BALB/c mice immunized with ICC-1132 in Freund's adjuvant or adjuvant-only controls were assayed for proliferation (solid bars) and IL-2 responses (hatched bars). Results are shown as the SI obtained following stimulation with HBc or ICC-1132 core particles (10 μg/ml) or malaria CS peptides T1, B, and T∗ (20 μg/ml). (B) IFN-γ in supernatants of immune lymph node cells (dark bars) stimulated with 10-fold dilutions of antigen, starting at 10 μg/ml for core particles and 20 μg/ml for malaria CS peptides. Results for only the highest antigen concentration are shown for control nonimmune lymph nodes (open bars).
The magnitude of the cellular responses to malaria epitopes differed from the response to HBc epitopes. Core particles, with or without CS epitopes, elicited levels of IL-2 response that were 7- to 10-fold higher than those obtained following stimulation of immune cells with T∗ peptide. In contrast, IFN-γ levels elicited by T∗ peptide were comparable to those induced by ICC-1132 stimulation, suggesting that the Th1 cellular responses may be predominantly directed to the T∗ epitope.
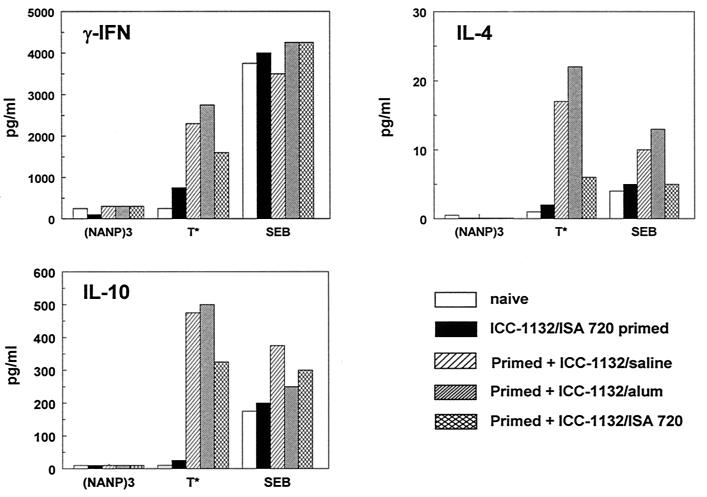
Spleen cells of mice primed with ICC-1132 formulated in Montanide ISA 720 and boosted with vaccine formulated in various adjuvants suitable for human use were also tested (Fig. 6). In all vaccine formulations, T cells specific for T∗ were elicited, indicating that the type of adjuvant did not alter the fine specificity of the CS-specific response. Consistent with the broad range of antibody subtypes detected in ICC-1132-immunized mice, Th1-type as well as Th2-type cytokines could be detected in the culture supernatants. T∗-specific cytokine responses were increased following a booster inoculation of the mice primed with ICC-1132-Montanide ISA 720. Enhanced IFN-γ responses could be obtained by booster with ICC-1132 in saline, as well as with particles formulated in alum or Montanide ISA 720. Consistent with the known Th2 adjuvant properties of alum, higher IL-4 levels were obtained in mice boosted with ICC-1132 adsorbed to alum.
FIG. 6.
Cytokine production by spleen cells of mice primed with a single injection of ICC-1132-Montanide ISA 720 or followed by a booster injection of ICC-1132 either without adjuvant (in saline), adsorbed to alum, or emulsified in Montanide ISA 720. The spleen cells obtained from immunized mice or naïve mice were stimulated with malaria CS peptides or superantigen staphylococcal enterotoxin B (SEB), and levels of cytokine (in picograms per milliliter) were measured in culture supernatants.
(ii) Anamnestic responses in P. falciparum sporozoite-primed mice.
Importantly for field applications, the CS T-cell epitopes contained in the ICC-1132 stimulated strong anamnestic antibody responses in mice primed with P. falciparum sporozoites. A 100-fold increase of antirepeat antibodies and a 250-fold increase in IFA titers were observed in sporozoite-primed mice 10 days after a single injection of ICC-1132 (Table 1). These antibody titers were over 1,000-fold higher than titers in unprimed mice, with a GMT of 103,213 versus one of 101, respectively. The responses were malaria specific, as HBc-lacking CS epitopes did not elicit anamnestic antibody responses in P. falciparum sporozoite-primed mice.
TABLE 1.
ICC-1132 elicits anamnestic antibody responses in P. falciparum sporozoite-primed micea
| Priming | Boost | IgG titer for:
|
|
|---|---|---|---|
| ELISA | IFA | ||
| P. falciparum sporozoites | ICC-1132 | 103,213 | 327,680 |
| P. falciparum sporozoites | HBc149 | 1,280 | 2,560 |
| None | ICC-1132 | 101 | 320 |
BALB/c mice were primed with a single injection of 105 P. falciparum sporozoites. Approximately 3 months later, when ELISA GMTs were 970 (range, 320 to 2,560) and pooled sera IFA titer was 1,280, the sporozoite-primed mice were injected with 5 μg of ICC-1132 or HBc. Naïve mice received a single injection of ICC-1132 as control. IgG ELISA and IFA titers were determined 10 days after the boost.
(iii) Immunogenicity of ICC-1132 in cynomolgus monkeys.
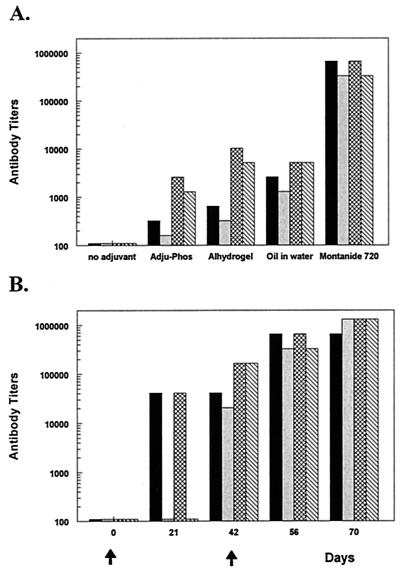
While there have been numerous murine studies, the immunogencity of HBc particles, either with or without foreign epitope inserts, has not been examined in nonhuman primates. The antibody response to ICC-1132 formulated in different adjuvants was therefore tested in Old World cynomolgus monkeys.
Significant differences were noted in the primate antibody responses to ICC-1132 compared to those in previous murine studies using the same vaccine formulations [23; Birkett et al., Am. J. Trop. Med. Hyg. 65(Suppl. 3):258, 2001]. ICC-1132 without adjuvant was substantially less immunogenic in the cynomolgus monkeys (Fig. 7A) than in mice. Similarly, alum formulations that bound ICC-1132 with high affinity (Alhydrogel) or low affinity (Adju-Phos) were poorly immunogenic in the monkeys and elicited antibody titers that were < 104 by ELISA and IFA. The primate responses to ICC-1132 emulsified in oil in water (Corixa vehicle) were similar to those obtained with alum formulations.
FIG. 7.
Cynomolgous monkeys were immunized with ICC-1132 formulated with adjuvants acceptable for human use: Adju-Phos, Alhydrogel, oil-in-water emulsion, or Montanide ISA 720. Two monkeys were immunized per group. (A) ELISA and IFA titers for the first monkey of each group are shown as black and grey bars, respectively, and for the second monkey as hatched and diagonal bars. (B) Kinetics of ELISA and IFA responses in the two cynomolgous monkeys immunized with ICC-1132 in Montanide ISA 720.
Optimal antibody responses in the cynomolgus monkeys, as in mice, were obtained using the water-in-oil emulsion Montanide ISA 720 (Fig. 7A). Antirepeat antibodies were detectable 20 days after a single dose of ICC-1132 in Montanide ISA 720, and both IFA and ELISA titers reached 105 by 40 days postinjection (Fig. 7B). A booster inoculation of the same formulation significantly increased antibody responses, reaching peak titers of 9 × 105 in ELISA and 1.3 × 106 by IFA. In all adjuvant formulations, the antirepeat antibody responses directly correlated with antisporozoite antibody titers.
Antigenicity of hybrid HBc particles for human cells.
The results obtained in the murine and primate host have supported progression to human trials to assay safety and immunogenicity. In preparation for these studies, ICC-1132 particles were assayed in vitro using naïve human PBL and human malaria-specific T-cell clones.
(i) Naïve PBL.
To determine the nonspecific inhibitory properties of HBc particles, naïve PBL were cultured with HBc particles and stimulated with phytohemagglutinin mitogen. Similar levels of proliferation and IL-2 production were elicited by mitogen in cultures with or without ICC-1132 and HBc149, indicating that the particles were not directly toxic for human PBL (data not shown).
Naïve PBL cultured with ICC-1132 or HBc particles in medium alone, without mitogen, demonstrated significant dose-dependent nonspecific proliferation, with SI ranging from 2 to 47. Despite the presence of high levels of proliferation in some individuals, IL-2 could not be detected in the culture supernatants, suggesting proliferation of B rather than T cells (data not shown).
Consistent with B-cell stimulatory properties, lymph node cells from nonimmune mice proliferated when cocultured with high concentrations of either ICC-1132 or HBc particles (Fig. 5A), but T-cell cytokines IL-2, IFN, IL-4, and IL-10 were not detected in the culture supernatants (Fig. 5B and 6). Previous studies have shown that HBc particles without foreign epitope inserts can stimulate naïve murine B cells, as well as naïve human B cells transferred to transgenic SCID mice (6, 19, 22). This property is believed to play a role in the T-independent antibody responses elicited by core particles and to contribute to their potent immunogenicity as vaccine carriers.
(ii) Human malaria-specific CD4+-T-cell clones.
Human CD4+-T-cell clones, specific for T* or repeat epitopes (32; J. M. Calvo-Calle, unpublished data), were used to determine whether the malaria epitopes, expressed either in the loop region or C terminus of the HBc core protein, were antigenic. The CD4+ T cells specifically recognized the ICC-1132 particles but not HBc particles, indicating that these T-cell epitopes were correctly processed and presented by HLA class II molecules (Table 2). Stimulation levels were comparable to those obtained with extracts of P. falciparum sporozoites (clone 9-2D4) or a recombinant P. falciparum CS protein. The T-cell responses were malaria specific as recombinant vivax CS protein expressed in yeast, murine dihydrofolate reductase expressed in E. coli, or extracts of P. berghei sporozoites did not elicit proliferation or IL-2 production. The HBc particles, with or without CS epitopes, did not stimulate irradiated APC or human CD4+-TCL of other specificities (data not shown).
TABLE 2.
Human CD4+ T-cell clones recognize P. falciparum CS repeat and universal T∗ epitopes contained in ICC-1132a
| Antigen | Assay results for:
|
|||||
|---|---|---|---|---|---|---|
| T-cell clone 9-2D4
|
T-cell clone 9-2E10
|
|||||
| Proliferation (SI) | IL-2 (SI) | IFN-γ (pg/ml) | Proliferation (SI) | IL-2 (SI) | IFN-γ (pg/ml) | |
| T∗ peptide | 1 | 1 | <5 | 124 | 402 | 2,374 |
| Repeat peptide | 21 | 195 | 106 | 1 | 1 | <5 |
| ICC-1132 | 10 | 44 | 22 | 128 | 364 | 2,073 |
| HBc149 | 1 | 1 | <5 | 2 | 3 | <5 |
| P. falciparum rCS | 12 | 107 | ND | 83 | 202 | 1045 |
| Control protein | 1 | 1 | <5 | 1 | 2 | <5 |
| P. falciparum sporozoites | 6 | 116 | 122 | ND | ND | ND |
| P. berghei sporozoites | 1 | 1 | <5 | ND | ND | ND |
T-cell clones were derived from a volunteer immunized with a malaria polyoxime vaccine (32; Calvo-Calle, unpublished). The T cells were stimulated with 10 to 20 μg of CS peptides, core particles, or recombinant CS protein (rCS) per ml or freeze-thawed extracts of 105 sporozoites, using irradiated PBL as APC. Proliferation and IL-2 assay results are expressed as SI. ND, not determined.
In addition, human CD4+-TCL specific for the T1 epitope, derived from volunteers immunized with MAP vaccine (36), also recognized HBc particles containing the CS repeat epitopes (ICC-1557) but not HBc149 (data not shown). These CD4+ T cells are restricted by class II molecules encoded by DRB1∗0401, DRB1∗1101, or DQB1∗0603 (36), indicating that the T1 epitope contained in core particles can be processed for presentation by multiple HLA class II isotypes. ICC-1132 elicited proliferation, IL-2, and IFN-γ levels similar to levels obtained when T1-specific TCL were stimulated with MAP or recombinant P. falciparum CS protein (data not shown). TCL stimulated with HBc149, or with a recombinant CS protein lacking the repeat region, did not proliferate or produce cytokines.
DISCUSSION
Recombinant HBc particles containing rodent malaria B-cell epitopes had earlier been found to elicit protective immunity in rodent malaria models; however, similar constructs containing P. falciparum CS repeats were significantly less immunogenic (43). A series of hybrid core particles containing P. falciparum CS repeats with or without CS-derived T-cell epitopes were screened to identify a more potent immunogen [23; Birkett et al., Am. J. Trop. Med. Hyg. 65(Suppl. 3):258, 2001]. In the present studies, immunogenicity of the optimal construct, designated ICC-1132, was assayed in vivo in rodents and nonhuman primates and in vitro using naïve and malaria-immune human CD4+ T cells.
The ICC-1132 particle contains the CS repeat epitope inserted into the immunodominant located HBc loop region that forms spikes on the particle surface and the universal T-cell epitope fused to the C terminus of the HBc protein (Fig. 1C). Mice immunized with the ICC-1132 particle developed high antirepeat ELISA titers (1.3 × 106) and, more important, equally high antisporozoite IFA titers of 655,360. These antibody titers represent a 30-fold increase over ELISA titers and a 400-fold increase over antisporozoite IFA titers, obtained in earlier studies with HBc particles containing only (NANP)4 CS (43).
In contrast to the malaria repeat epitopes, the universal T∗ epitope elicited only weak antibody responses, consistent with the poor immunogencity of foreign B-cell epitopes inserted at the C terminus of the core protein (41). The immunodominance of the CS repeat B-cell epitope and the low immunogencity of the nonrepeat T∗ epitope in the mice immunized with ICC-1132 are consistent with the fine specificity of antibody responses in volunteers immunized with P. falciparum sporozoites (5) or synthetic peptide vaccines (32). The multimeric presentation of CS repeat epitopes on the surface of the hybrid HBc particle may effectively mimic native CS protein on the sporozoite surface.
The multiple tandem repeats of the CS protein have been hypothesized to function as a T-independent antigen, similar to the repetitive subunits of bacterial polysaccharides (45). More recent studies, however, have demonstrated that the antirepeat antibody response in sporozoite-immunized mice, and in human volunteers immunized with malaria synthetic peptide vaccines, is T cell dependent (25, 36). Following immunization with ICC-1132, transgenic class II−/− mice failed to develop an antirepeat IgG response, indicating that the repeat epitopes in the context of the core particle also function as a T-dependent antigen (Fig. 4A). Both T-independent and T-dependent anticore antibody responses were elicited in mice immunized with HBc particles with or without CS inserts (Fig. 4B), consistent with previous studies (24).
Although numerous Th cell epitopes are present in the HBc protein, the ability to elicit anamnestic antibody and cytokine responses following sporozoite challenge requires the presence of malaria-specific T cells. ICC-1132 contains two CS-derived T-cell epitopes, T1 and T∗, which are known to be immunogenic in human volunteers immunized with irradiated P. falciparum sporozoites or with synthetic peptide malaria vaccines (28, 32, 33, 36). The present studies demonstrate that these malaria T-cell epitopes were also immunogenic in the context of the HBc particle. High levels of IFN-γ and IL-2 production were observed following T∗ peptide stimulation of cells from mice immunized with ICC-1132 in Freund's adjuvant (Fig. 5A and B). Consistent with the broad spectrum of antibody subtypes observed in immune sera, Th2-type cytokines IL-4 and IL-10 were also detected in the sera of mice immunized with ICC-1132 formulated in alum or Montanide ISA 720 (Fig. 6).
The parasite-specific T-cell epitopes present in ICC-1132 elicited strong anamnestic responses in sporozoite primed mice (Table 1). A single booster inoculation of ICC-1132 increased antibody titers 100- to 150-fold in the sporozoite-primed mice. The presence of strong Th1-type CD4+ T cells, in addition to providing T-cell help for B-cell antibody responses, also provides the potential to target the intracellular hepatic stages through production of IFN-γ (13, 44). Mice immunized with malaria synthetic peptides have been shown to develop CD4+ T cells that can mediate IFN-γ-dependent protective immunity by inhibiting EEF development within hepatic cells (21, 51).
Cynomolgus monkeys immunized with two injections of ICC-1132 in Montanide ISA 720 developed antirepeat and antisporozoite antibody titers in excess of 106, the highest titers obtained to date in simian hosts immunized with a recombinant malaria protein (Fig. 7A and B). Although ICC-1132 in saline or adsorbed to alum was highly immunogenic in mice, these formulations were significantly less immunogenic in the cynomolgus monkeys. Whether these differences relate to the use of rodent versus simian hosts, different immunization protocols, or dose-to-body weight ratios is unknown. No adverse effect or reactogenicity was noted in cynomolgous monkeys immunized with ICC-1132 in any of the adjuvant formulations.
In both mice and monkeys, optimal responses were obtained with Montanide ISA 720 formulations. Malaria CS synthetic peptide vaccines or recombinant blood stage vaccines adjuvanted with Montanide ISA 720 have shown promising results in nonhuman primates and phase I clinical studies (1, 7, 14, 20, 40). Immunization protocols in which primary doses with vaccine formulated in Montanide ISA 720 are followed with a booster inoculation containing the same antigen in a different adjuvant have reduced reactogenicity noted with some antigen-Montanide ISA 720 formulations (39, 49). It is encouraging that the murine immune responses elicited with ICC-1132-Montanide ISA 720 could be efficiently boosted by a second dose of ICC-1132 either without adjuvant or with alum. These findings suggest that highly immunogenic vaccine formulations can be designed that minimize potential reactogenicity in humans.
Important for human vaccine development, the P. falciparum T-cell epitopes contained in ICC-1132 were processed and presented by human APC to malaria specific HLA-restricted T cells. Human CD4+-T-cell clones specific for either the P. falciparum CS repeat or T∗ epitope proliferated and produced cytokines (IL-2 and IFN-γ) when stimulated with ICC-1132 (Table 2). DR- and DQ-restricted CD4+-TCL that were specific for the T1 repeat epitope also recognized recombinant core particles containing CS repeats (data not shown). Therefore, P. falciparum T-cell epitopes expressed in either the immunodominant loop region or the C terminus of the HBc particle can be recognized by malaria-specific human cells in the context of multiple class II isotypes. Stimulation of these human CD4+ T cells with hybrid CS-HBc particles elicited IFN-γ levels comparable to those elicited by recombinant full-length CS protein or P. falciparum sporozoite extract (Table 2). These findings indicate that the hybrid particles can elicit epitope-specific responses relevant to sporozoite immunity.
The ability of ICC-1132 to stimulate malaria-specific human CD4+ T cells and to elicit high levels of P. falciparum-specific immunity in rodent and primate hosts supports phase I testing to assess safety and immunogenicity in human volunteers. Recent clinical trials of the RTS,S recombinant HBsAg malaria vaccine suggest that protective immunity to P. falciparum sporozoites can be mediated by high levels of antirepeat antibodies and CS-specific CD4+ T cells in the absence of CD8+ T cells (18, 48). Although protection was short-lived in these volunteers, as well as in immunized African adults, studies with RTS,S clearly demonstrate the feasibility of CS-based subunit vaccines (2, 47). Based on the persistence of anticore responses in hepatitis infections, it is hoped that ICC-1132 will elicit long-lived antisporozoite immune responses in humans, as found in murine and simian hosts. The presence of minimal CS epitopes in ICC-1132 may also enhance humoral and cellular immunity by focusing the host immune response on rationally defined T- and B-cell epitopes of the P. falciparum CS protein.
Progression to human clinical trials requires advancing the manufacture and batch release of the recombinant particles, as well as of the adjuvant formulation, to more highly regulated and controlled processes. To this end, master and working cell banks for an ICC-1132-producing E. coli clone have been produced under GMP conditions and a scalable fermentation process has been developed with culture media that are devoid of animal products. Likewise, a scalable purification process, which utilized only reagents with associated drug master files, has been developed and used to direct the manufacture of multiple batches of GMP-grade material. These scale-up and purification processes should facilitate application of modified HBc particles in the development of vaccines for other infectious diseases, in addition to malaria.
Acknowledgments
We gratefully acknowledge the expert technical assistance of Rita Altszuler and Ivette Caro-Aguilar. Electron microscopy of ICC-1132 particles was carried out by R. Nessler, Central Microscopy Research Facility, University of Iowa. We thank Victor Nussenzweig for critical review of the manuscript.
Studies at New York University were supported by NIH grants AI 25085 and AI 45138 to E.N. and by an Apovia unrestricted research grant. Studies at Apovia were supported by a grant from the Malaria Vaccine Initiative at PATH and by NIH grant AI43830 to A.B.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Benmohamed, L., A. Thomas, M. Bossus, K. Brahimi, J. Wubben, H. Gras-Masse, and P. Druilhe. 2000. High immunogenicity in chimpanzees of peptides and lipopeptides derived from four new Plasmodium falciparum pre-erythrocytic molecules. Vaccine 18:2843-2855. [DOI] [PubMed] [Google Scholar]
- 2.Bojang, K. A., P. J. Milligan, M. Pinder, L. Vigneron, A. Alloueche, K. E. Kester, W. R. Ballou, D. J. Conway, W. H. Reece, P. Gothard, L. Yamuah, M. Delchambre, G. Voss, B. M. Greenwood, A. Hill, K. P. McAdam, N. Tornieporth, J. D. Cohen, and T. Doherty. 2001. Efficacy of RTS,S/AS02 malaria vaccine against Plasmodium falciparum infection in semi-immune adult men in The Gambia: a randomised trial. Lancet 358:1927-1934. [DOI] [PubMed] [Google Scholar]
- 3.Bottcher, B., S. A. Wynne, and R. A. Crowther. 1997. Determination of the fold of the core protein of hepatitis B virus by electron cryomicroscopy. Nature 386:88-91. [DOI] [PubMed] [Google Scholar]
- 4.Calvo-Calle, J. M., J. Hammer, F. Sinigaglia, P. Clavijo, Z. R. Moya-Castro, and E. H. Nardin. 1997. Binding of malaria T cell epitopes to DR and DQ molecules in vitro correlates with immunogenicity in vivo: identification of a universal T cell epitope in the Plasmodium falciparum circumsporozoite protein. J. Immunol. 159:1362-1373. [PubMed] [Google Scholar]
- 5.Calvo-Calle, J. M., E. H. Nardin, P. Clavijo, C. Boudin, D. Stuber, B. Takacs, R. S. Nussenzweig, and A. H. Cochrane. 1992. Recognition of different domains of the Plasmodium falciparum CS protein by the sera of naturally infected individuals compared with those of sporozoite-immunized volunteers. J. Immunol. 149:2695-2701. [PubMed] [Google Scholar]
- 6.Cao, T., U. Lazdina, I. Desombere, P. Vanlandschoot, D. R. Milich, M. Sällberg, and G. Leroux-Roels. 2001. Hepatitis B virus core antigen binds and activates naive human B cells in vivo: studies with a human PBL-NOD/SCID mouse model. J. Virol. 75:6359-6366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins, W. E., A. Walduck, J. S. Sullivan, K. Andrews, A. Stowers, C. L. Morris, V. Jennings, C. Yang, J. Kendall, Q. Lin, L. B. Martin, C. Diggs, and A. Saul. 2000. Efficacy of vaccines containing rhoptry-associated proteins RAP1 and RAP2 of Plasmodium falciparum in Saimiri boliviensis monkeys. Am. J. Trop. Med. Hyg. 62:466-479. [DOI] [PubMed] [Google Scholar]
- 8.Crowther, R. A., N. A. Kiselev, B. Bottcher, J. A. Berriman, G. P. Borisova, V. Ose, and P. Pumpens. 1994. Three-dimensional structure of hepatitis B virus core particles determined by electron cryomicroscopy. Cell 77:943-950. [DOI] [PubMed] [Google Scholar]
- 9.Dai, W. J., A. Hemphill, A. Waldvogel, K. Ingold, P. Deplazes, H. Mossmann, and B. Gottstein. 2001. Major carbohydrate antigen of Echinococcus multilocularis induces an immunoglobulin G response independent of αβ+ CD4+ T cells. Infect. Immun. 69:6074-6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Del Giudice, G., J. A. Cooper, J. Merino, A. S. Verdini, A. Pessi, A. R. Togna, H. D. Engers, G. Corradin, and P. H. Lambert. 1986. The antibody response in mice to carrier-free synthetic polymers of Plasmodium falciparum circumsporozoite repetitive epitope is I-Ab− restricted: possible implications for malaria vaccines. J. Immunol. 137:2952-2955. [PubMed] [Google Scholar]
- 11.Etlinger, H. M., A. M. Felix, D. Gillessen, E. P. Heimer, M. Just, J. R. Pink, F. Sinigaglia, D. Sturchler, B. Takacs, A. Trzeciak, and H. Matile. 1988. Assessment in humans of a synthetic peptide-based vaccine against the sporozoite stage of the human malaria parasite, Plasmodium falciparum. J. Immunol. 140:626-633. [PubMed] [Google Scholar]
- 12.Fehr, T., D. Skrastina, P. Pumpens, and R. M. Zinkernagel. 1998. T cell-independent type I antibody response against B cell epitopes expressed repetitively on recombinant virus particles. Proc. Natl. Acad. Sci. USA 95:9477-9481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferreira, A., L. Schofield, V. Enea, H. Schellekens, P. van der Meide, W. E. Collins, R. S. Nussenzweig, and V. Nussenzweig. 1986. Inhibition of development of exoerythrocytic forms of malaria parasites by gamma-interferon. Science 232:881-884. [DOI] [PubMed] [Google Scholar]
- 14.Genton, B., F. Al-Yaman, R. Anders, A. Saul, G. Brown, D. Pye, D. O. Irving, W. R. Briggs, A. Mai, M. Ginny, T. Adiguma, L. Rare, A. Giddy, R. Reber-Liske, D. Stuerchler, and M. P. Alpers. 2000. Safety and immunogenicity of a three-component blood-stage malaria vaccine in adults living in an endemic area of Papua New Guinea. Vaccine 18:2504-2511. [DOI] [PubMed] [Google Scholar]
- 15.Good, M. F., J. A. Berzofsky, W. L. Maloy, Y. Hayashi, N. Fujii, W. T. Hockmeyer, and L. H. Miller. 1986. Genetic control of the immune response in mice to a Plasmodium falciparum sporozoite vaccine. Widespread nonresponsiveness to single malaria T epitope in highly repetitive vaccine. J. Exp. Med. 164:655-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herrington, D. A., D. F. Clyde, G. Losonsky, M. Cortesia, J. R. Murphy, J. Davis, S. Baqar, A. M. Felix, E. P. Heimer, D. Gillessen, E. Nardin, R. S. Nussenzweig, V. Nussenzweig, M. Hollingdale, and M. Levine. 1987. Safety and immunogenicity in man of a synthetic peptide malaria vaccine against Plasmodium falciparum sporozoites. Nature 328:257-259. [DOI] [PubMed] [Google Scholar]
- 17.Kester, K. E., D. A. McKinney, N. Tornieporth, C. F. Ockenhouse, D. G. Heppner, T. Hall, U. Krzych, M. Delchambre, G. Voss, M. G. Dowler, J. Palensky, J. Wittes, J. Cohen, and W. R. Ballou. 2001. Efficacy of recombinant circumsporozoite protein vaccine regimens against experimental Plasmodium falciparum malaria. J. Infect. Dis. 183:640-647. [DOI] [PubMed] [Google Scholar]
- 18.Lalvani, A., P. Moris, G. Voss, A. A. Pathan, K. E. Kester, R. Brookes, E. Lee, M. Koutsoukos, M. Plebanski, M. Delchambre, K. L. Flanagan, C. Carton, M. Slaoui, C. Van Hoecke, W. R. Ballou, A. V. Hill, and J. Cohen. 1999. Potent induction of focused Th1-type cellular and humoral immune responses by RTS,S/SBAS2, a recombinant Plasmodium falciparum malaria vaccine. J. Infect. Dis. 180:1656-1664. [DOI] [PubMed] [Google Scholar]
- 19.Lazdina, U., T. Cao, J. Steinbergs, M. Alheim, P. Pumpens, D. L. Peterson, D. R. Milich, G. Leroux-Roels, and M. Sällberg. 2001. Molecular basis for the interaction of the hepatitis B virus core antigen with the surface immunoglobulin receptor on naive B cells. J. Virol. 75:6367-6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez, J. A., C. Weilenman, R. Audran, M. A. Roggero, A. Bonelo, J. M. Tiercy, F. Spertini, and G. Corradin. 2001. A synthetic malaria vaccine elicits a potent CD8(+) and CD4(+) T lymphocyte immune response in humans. Implications for vaccination strategies. Eur. J. Immunol. 31:1989-1998. [DOI] [PubMed] [Google Scholar]
- 21.Migliorini, P., B. Betschart, and G. Corradin. 1993. Malaria vaccine: immunization of mice with a synthetic T cell helper epitope alone leads to protective immunity. Eur. J. Immunol. 23:582-585. [DOI] [PubMed] [Google Scholar]
- 22.Milich, D. R., M. Chen, F. Schodel, D. L. Peterson, J. E. Jones, and J. L. Hughes. 1997. Role of B cells in antigen presentation of the hepatitis B core. Proc. Natl. Acad. Sci. USA 94:14648-14653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Milich, D. R., J. Hughes, J. Jones, M. Sallberg, and T. R. Phillips. 2001. Conversion of poorly immunogenic malaria repeat sequences into a highly immunogenic vaccine candidate. Vaccine 20:771-788. [DOI] [PubMed] [Google Scholar]
- 24.Milich, D. R., and A. McLachlan. 1986. The nucleocapsid of hepatitis B virus is both a T-cell-independent and a T-cell-dependent antigen. Science 234:1398-1401. [DOI] [PubMed] [Google Scholar]
- 25.Molano, A., S. H. Park, Y. H. Chiu, S. Nosseir, A. Bendelac, and M. Tsuji. 2000. Cutting edge: the IgG response to the circumsporozoite protein is MHC class II-dependent and CD1d-independent: exploring the role of GPIs in NK T cell activation and antimalarial responses. J. Immunol. 164:5005-5009. [DOI] [PubMed] [Google Scholar]
- 26.Mond, J. J., Q. Vos, A. Lees, and C. M. Snapper. 1995. T cell independent antigens. Curr. Opin. Immunol. 7:349-354. [DOI] [PubMed] [Google Scholar]
- 27.Moreno, A., P. Clavijo, R. Edelman, J. Davis, M. Sztein, D. Herrington, and E. Nardin. 1991. Cytotoxic CD4+ T cells from a sporozoite-immunized volunteer recognize the Plasmodium falciparum CS protein. Int. Immunol. 3:997-1003. [DOI] [PubMed] [Google Scholar]
- 28.Moreno, A., P. Clavijo, R. Edelman, J. Davis, M. Sztein, F. Sinigaglia, and E. Nardin. 1993. CD4+ T cell clones obtained from Plasmodium falciparum sporozoite-immunized volunteers recognize polymorphic sequences of the circumsporozoite protein. J. Immunol. 151:489-499. [PubMed] [Google Scholar]
- 29.Moreno, C. A., R. Rodriguez, G. A. Oliveira, V. Ferreira, R. S. Nussenzweig, Z. R. Moya Castro, J. M. Calvo-Calle, and E. Nardin. 1999. Preclinical evaluation of a synthetic Plasmodium falciparum MAP malaria vaccine in Aotus monkeys and mice. Vaccine 18:89-99. [DOI] [PubMed] [Google Scholar]
- 30.Munesinghe, D. Y., P. Clavijo, M. C. Calle, R. S. Nussenzweig, and E. Nardin. 1991. Immunogenicity of multiple antigen peptides (MAP) containing T and B cell epitopes of the repeat region of the P. falciparum circumsporozoite protein. Eur. J. Immunol. 21:3015-3020. [DOI] [PubMed] [Google Scholar]
- 31.Nardin, E., F. Zavala, V. Nussenzweig, and R. S. Nussenzweig. 1999. Pre-erythrocytic malaria vaccine: mechanisms of protective immunity and human vaccine trials. Parassitologia 41:397-402. [PubMed] [Google Scholar]
- 32.Nardin, E. H., J. M. Calvo-Calle, G. A. Oliveira, R. S. Nussenzweig, M. Schneider, J. M. Tiercy, L. Loutan, D. Hochstrasser, and K. Rose. 2001. A totally synthetic polyoxime malaria vaccine containing Plasmodium falciparum B cell and universal T cell epitopes elicits immune responses in volunteers of diverse HLA types. J. Immunol. 166:481-489. [DOI] [PubMed] [Google Scholar]
- 33.Nardin, E. H., D. A. Herrington, J. Davis, M. Levine, D. Stuber, B. Takacs, P. Caspers, P. Barr, R. Altszuler, P. Clavijo, and R. S. Nussenzweig. 1989. Conserved repetitive epitope recognized by CD4+ clones from a malaria-immunized volunteer. Science 246:1603-1606. [DOI] [PubMed] [Google Scholar]
- 34.Nardin, E. H., and R. S. Nussenzweig. 1993. T cell responses to pre-erythrocytic stages of malaria: role in protection and vaccine development. Annu. Rev. Immunol. 11:687-727. [DOI] [PubMed] [Google Scholar]
- 35.Nardin, E. H., V. Nussenzweig, R. S. Nussenzweig, W. E. Collins, K. T. Harinasuta, P. Tapchaisri, and Y. Chomcharn. 1982. Circumsporozoite proteins of human malaria parasites Plasmodium falciparum and Plasmodium vivax. J. Exp. Med. 156:20-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nardin, E. H., G. A. Oliveira, J. M. Calvo-Calle, Z. R. Castro, R. S. Nussenzweig, B. Schmeckpeper, B. F. Hall, C. Diggs, S. Bodison, and R. Edelman. 2000. Synthetic peptide malaria vaccine elicits high levels of antibodies in vaccinees of defined HLA genotypes. J. Infect. Dis. 182:1486-1496. [DOI] [PubMed] [Google Scholar]
- 37.Nussenzweig, V., and R. S. Nussenzweig. 1989. Rationale for the development of an engineered sporozoite malaria vaccine. Adv. Immunol. 45:283-334. [DOI] [PubMed] [Google Scholar]
- 38.Pumpens, P., and E. Grens. 1999. Hepatitis B core particles as a universal display model: a structure-function basis for development. FEBS Lett. 442:1-6. [DOI] [PubMed] [Google Scholar]
- 39.Raya, N. E., D. Quintana, Y. Carrazana, C. E. Gomez, and C. A. Duarte. 1999. A prime-boost regime that combines Montanide ISA720 and Alhydrogel to induce antibodies against the HIV-1 derived multiepitope polypeptide TAB9. Vaccine 17:2646-2650. [DOI] [PubMed] [Google Scholar]
- 40.Saul, A., G. Lawrence, A. Smillie, C. M. Rzepczyk, C. Reed, D. Taylor, K. Anderson, A. Stowers, R. Kemp, A. Allworth, R. F. Anders, G. V. Brown, D. Pye, P. Schoofs, D. O. Irving, S. L. Dyer, G. C. Woodrow, W. R. Briggs, R. Reber, and D. Sturchler. 1999. Human phase I vaccine trials of 3 recombinant asexual stage malaria antigens with Montanide ISA720 adjuvant. Vaccine 17:3145-3159. [DOI] [PubMed] [Google Scholar]
- 41.Schodel, F., A. M. Moriarty, D. L. Peterson, J. A. Zheng, J. L. Hughes, H. Will, D. J. Leturcq, J. S. McGee, and D. R. Milich. 1992. The position of heterologous epitopes inserted in hepatitis B virus core particles determines their immunogenicity. J. Virol. 66:106-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schodel, F., D. Peterson, D. R. Milich, Y. Charoenvit, J. Sadoff, and R. Wirtz. 1997. Immunization with hybrid hepatitis B virus core particles carrying circumsporozoite antigen epitopes protects mice against Plasmodium yoelii challenge. Behring Inst. Mitt. 98:114-119. [PubMed] [Google Scholar]
- 43.Schodel, F., R. Wirtz, D. Peterson, J. Hughes, R. Warren, J. Sadoff, and D. Milich. 1994. Immunity to malaria elicited by hybrid hepatitis B virus core particles carrying circumsporozoite protein epitopes. J. Exp. Med. 180:1037-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schofield, L., A. Ferreira, R. Altszuler, V. Nussenzweig, and R. S. Nussenzweig. 1987. Interferon-gamma inhibits the intrahepatocytic development of malaria parasites in vitro. J. Immunol. 139:2020-2025. [PubMed] [Google Scholar]
- 45.Schofield, L., M. J. McConville, D. Hansen, A. S. Campbell, B. Fraser-Reid, M. J. Grusby, and S. D. Tachado. 1999. CD1d-restricted immunoglobulin G formation to GPI-anchored antigens mediated by NKT cells. Science 283:225-229. [DOI] [PubMed] [Google Scholar]
- 46.Snapper, C. M., T. M. McIntyre, R. Mandler, L. M. Pecanha, F. D. Finkelman, A. Lees, and J. J. Mond. 1992. Induction of IgG3 secretion by interferon gamma: a model for T cell-independent class switching in response to T cell-independent type 2 antigens. J. Exp. Med. 175:1367-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stoute, J. A., K. E. Kester, U. Krzych, B. T. Wellde, T. Hall, K. White, G. Glenn, C. F. Ockenhouse, N. Garcon, R. Schwenk, D. E. Lanar, P. Sun, P. Momin, R. A. Wirtz, C. Golenda, M. Slaoui, G. Wortmann, C. Holland, M. Dowler, J. Cohen, and W. R. Ballou. 1998. Long-term efficacy and immune responses following immunization with the RTS,S malaria vaccine. J. Infect. Dis. 178:1139-1144. [DOI] [PubMed] [Google Scholar]
- 48.Stoute, J. A., M. Slaoui, D. G. Heppner, P. Momin, K. E. Kester, P. Desmons, B. T. Wellde, N. Garcon, U. Krzych, M. Marchand, W. R. Ballou, and J. D. Cohen. 1997. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. N. Engl. J. Med. 336:86-91. [DOI] [PubMed] [Google Scholar]
- 49.Toledo, H., A. Baly, O. Castro, S. Resik, J. Laferte, F. Rolo, L. Navea, L. Lobaina, O. Cruz, J. Miguez, T. Serrano, B. Sierra, L. Perez, M. E. Ricardo, M. Dubed, A. L. Lubian, M. Blanco, J. C. Millan, A. Ortega, E. Iglesias, E. Penton, Z. Martin, J. Perez, M. Diaz, and C. A. Duarte. 2001. A phase I clinical trial of a multi-epitope polypeptide TAB9 combined with Montanide ISA 720 adjuvant in non-HIV-1 infected human volunteers. Vaccine 19:4328-4336. [DOI] [PubMed] [Google Scholar]
- 50.Ulrich, R., M. Nassal, H. Meisel, and D. H. Kruger. 1998. Core particles of hepatitis B virus as carrier for foreign epitopes. Adv. Virus Res. 50:141-182. [DOI] [PubMed] [Google Scholar]
- 51.Wang, R., Y. Charoenvit, G. Corradin, P. De La Vega, E. D. Franke, and S. L. Hoffman. 1996. Protection against malaria by Plasmodium yoelii sporozoite surface protein 2 linear peptide induction of CD4+ T cell- and IFN-gamma-dependent elimination of infected hepatocytes. J. Immunol. 157:4061-4067. [PubMed] [Google Scholar]
- 52.Zavala, F., A. H. Cochrane, E. H. Nardin, R. S. Nussenzweig, and V. Nussenzweig. 1983. Circumsporozoite proteins of malaria parasites contain a single immunodominant region with two or more identical epitopes. J. Exp. Med. 157:1947-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]