Abstract
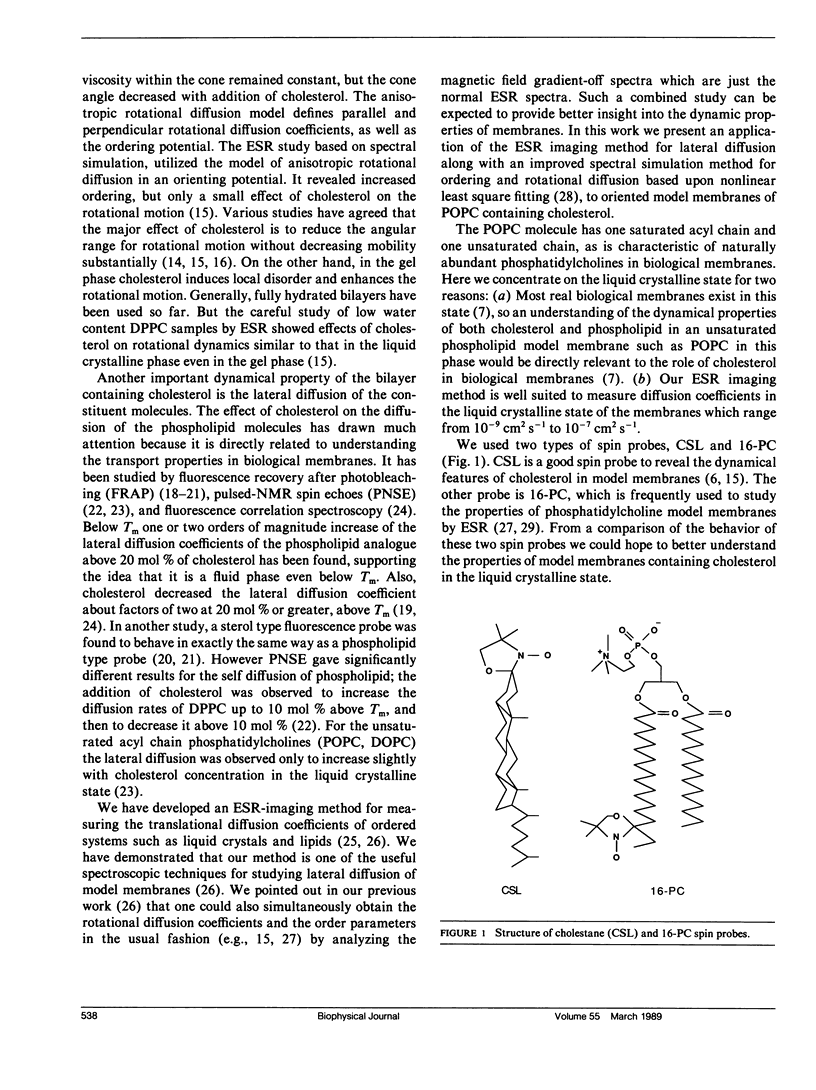
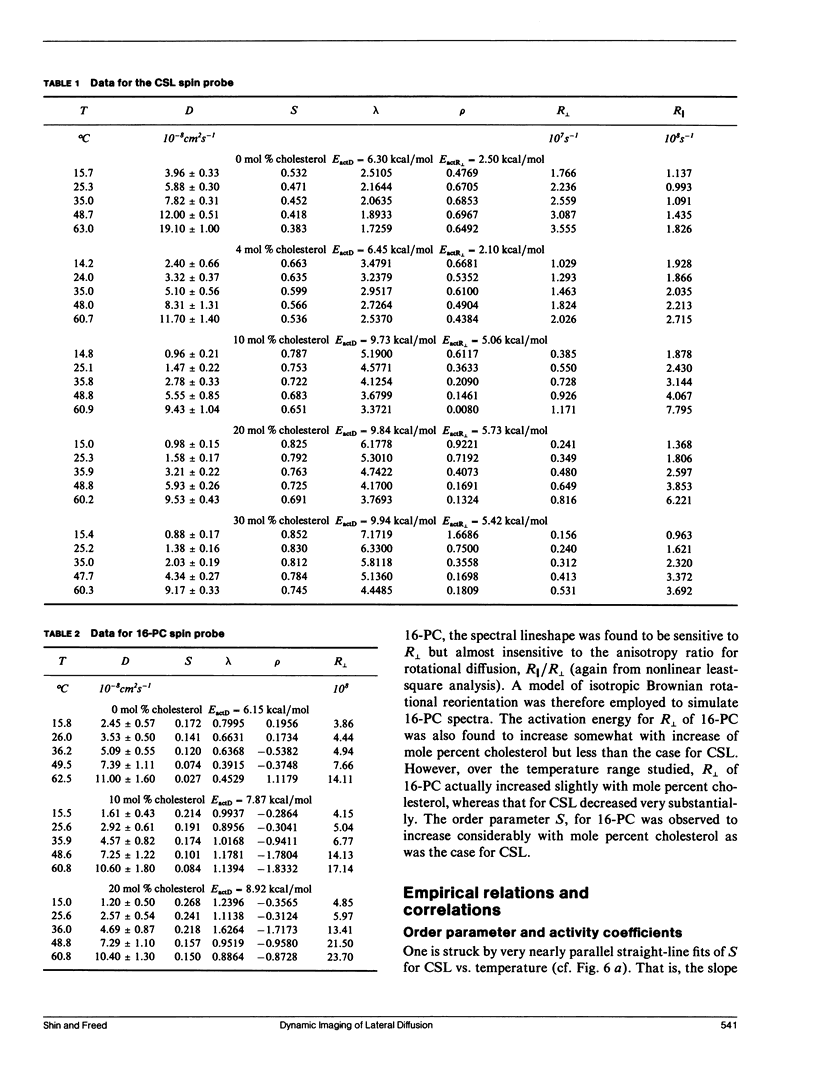
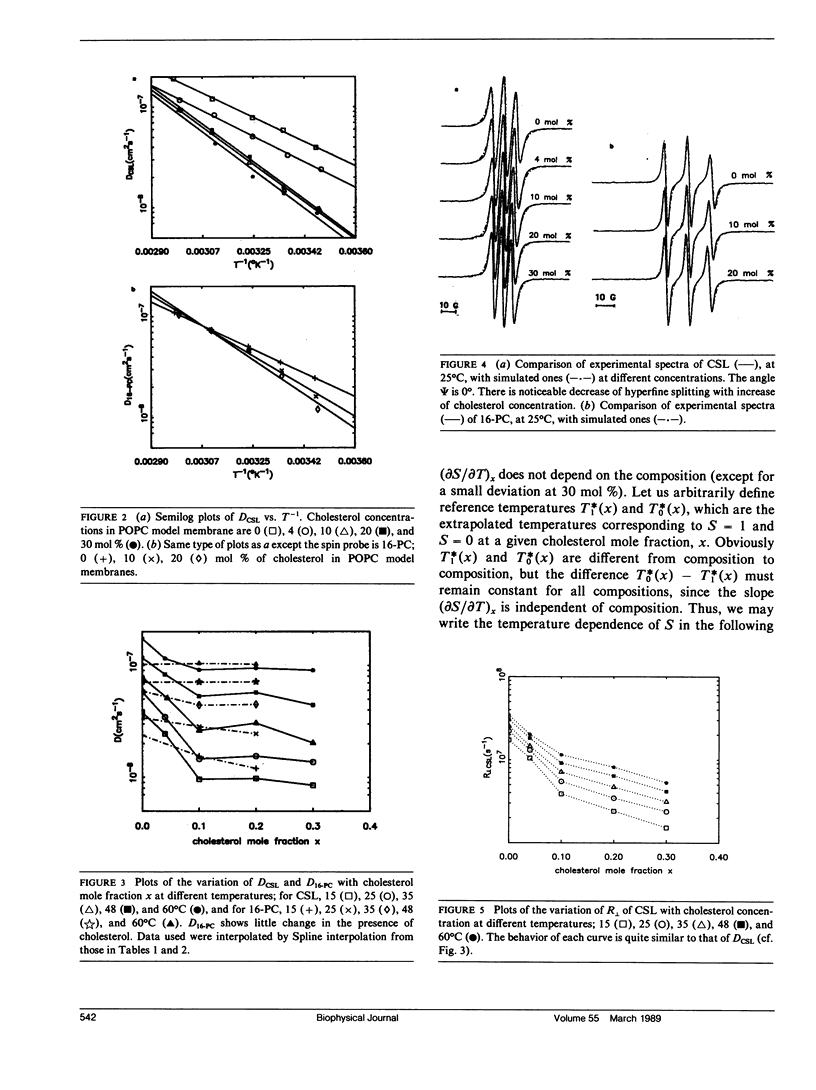
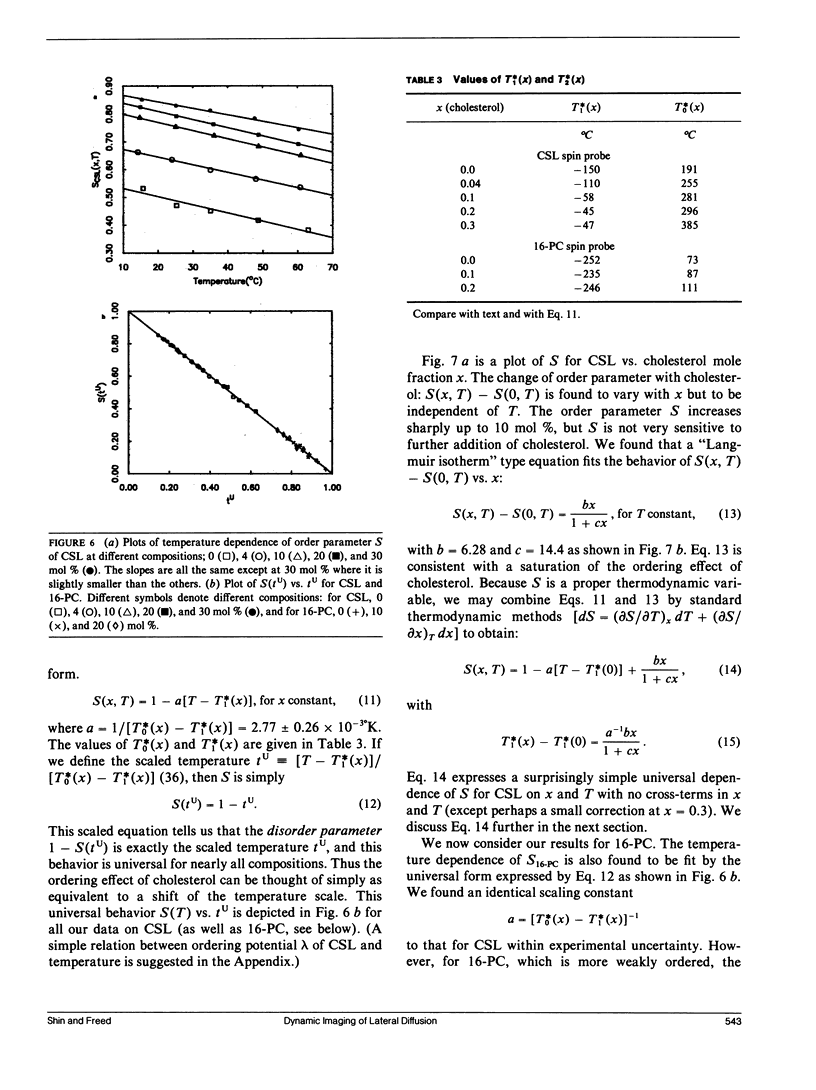
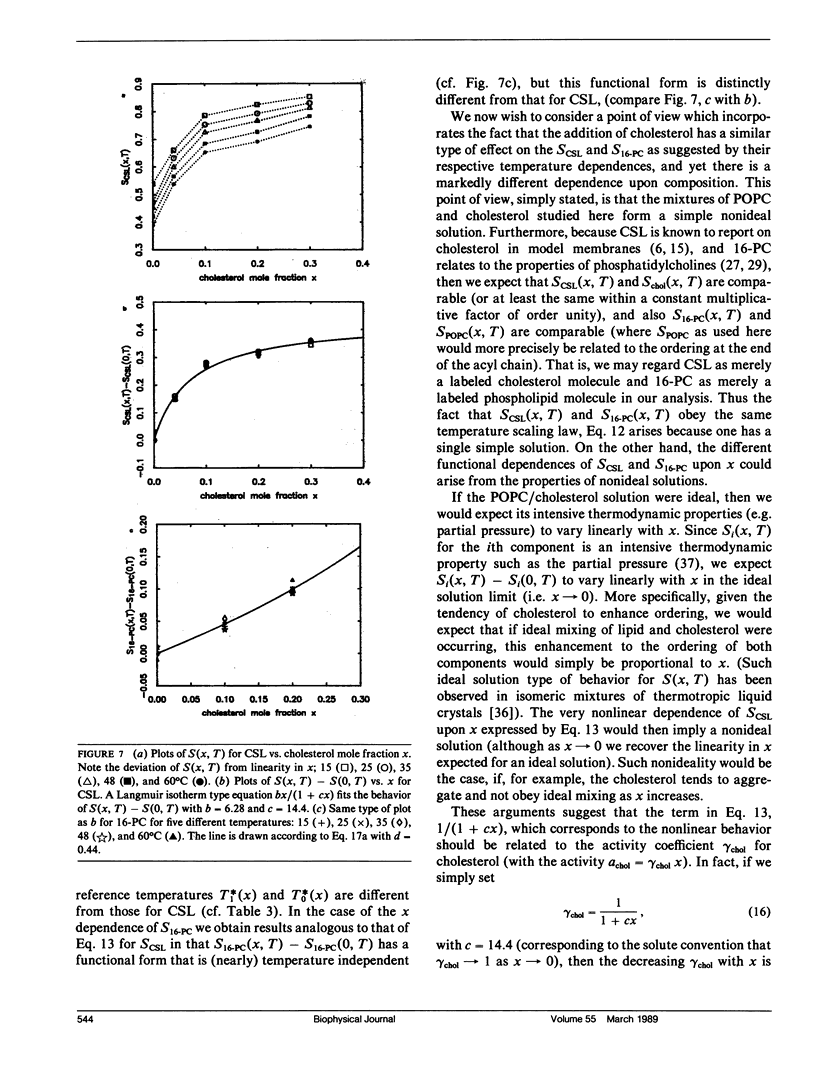
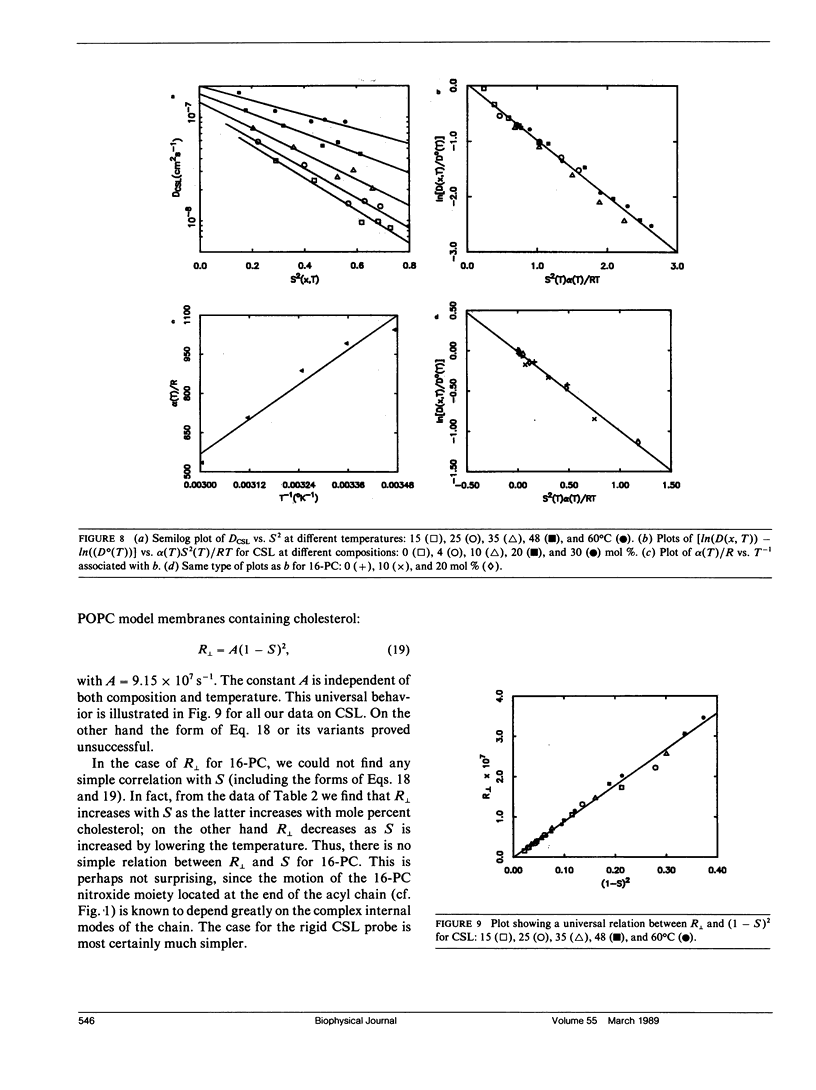
The effects of cholesterol on the dynamics and the structural properties of two different spin probes, the sterol type CSL and the phospholipid type 16-PC, in POPC/cholesterol oriented multilayer model membranes were examined. Our results are consistent with a nonideal solution containing cholesterol-rich clusters created by the self association of cholesterol in POPC model membranes. The lateral diffusion coefficient D of the spin probes was measured over the temperature range of 15 to 60 degrees C and over the concentration range of 0 to 30 mol% of cholesterol in the model membrane by the electron spin resonance (ESR) imaging method. The rotational diffusion coefficients (including R perpendicular) and the order parameter S were determined utilizing a nonlinear least square ESR spectral simulation method. D, R perpendicular and S of CSL deviate considerably from linear dependence on mole percent cholesterol. The D of CSL was decreased by a factor of four at 15 degrees C and a factor of two at 60 degrees C for concentrations of cholesterol over 10 mol %, whereas those of 16-PC were hardly affected. Cholesterol decreased R perpendicular by a factor of 10 at 30 mol % of cholesterol, but it increased slightly that of 16-PC. A significant increase of S for CSL due to the presence of cholesterol was observed. It is shown how the difference in variation of S for CSL vs. 16-PC with composition may be interpreted in terms of their respective activity coefficients, and how a single universal linear relation is obtained for the S of both probes in terms of a scaled temperature. Simple but general correlations of D and of R perpendicular with S were also found, which aid in the interpretation of these diffusion coefficients.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alecio M. R., Golan D. E., Veatch W. R., Rando R. R. Use of a fluorescent cholesterol derivative to measure lateral mobility of cholesterol in membranes. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5171–5174. doi: 10.1073/pnas.79.17.5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birrell G. B., Griffith O. H. Angle of tilt and domain structure in dipalmitoyl phosphatidylcholine multilayers. Arch Biochem Biophys. 1976 Feb;172(2):455–462. doi: 10.1016/0003-9861(76)90098-9. [DOI] [PubMed] [Google Scholar]
- Copeland B. R., McConnel H. M. The rippled structure in bilayer membranes of phosphatidylcholine and binary mixtures of phosphatidylcholine and cholesterol. Biochim Biophys Acta. 1980 Jun 20;599(1):95–109. doi: 10.1016/0005-2736(80)90059-0. [DOI] [PubMed] [Google Scholar]
- Fahey P. F., Koppel D. E., Barak L. S., Wolf D. E., Elson E. L., Webb W. W. Lateral diffusion in planar lipid bilayers. Science. 1977 Jan 21;195(4275):305–306. doi: 10.1126/science.831279. [DOI] [PubMed] [Google Scholar]
- Gershfeld N. L. Equilibrium studies of lecithin-cholesterol interactions I. Stoichiometry of lecithin-cholesterol complexes in bulk systems. Biophys J. 1978 Jun;22(3):469–488. doi: 10.1016/S0006-3495(78)85500-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golan D. E., Alecio M. R., Veatch W. R., Rando R. R. Lateral mobility of phospholipid and cholesterol in the human erythrocyte membrane: effects of protein-lipid interactions. Biochemistry. 1984 Jan 17;23(2):332–339. doi: 10.1021/bi00297a024. [DOI] [PubMed] [Google Scholar]
- Hinz H. J., Sturtevant J. M. Calorimetric investigation of the influence of cholesterol on the transition properties of bilayers formed from synthetic L- -lecithins in aqueous suspension. J Biol Chem. 1972 Jun 10;247(11):3697–3700. [PubMed] [Google Scholar]
- Hubbell W. L., McConnell H. M. Molecular motion in spin-labeled phospholipids and membranes. J Am Chem Soc. 1971 Jan 27;93(2):314–326. doi: 10.1021/ja00731a005. [DOI] [PubMed] [Google Scholar]
- Hui S. W., He N. B. Molecular organization in cholesterol-lecithin bilayers by X-ray and electron diffraction measurements. Biochemistry. 1983 Mar 1;22(5):1159–1164. doi: 10.1021/bi00274a026. [DOI] [PubMed] [Google Scholar]
- Ipsen J. H., Karlström G., Mouritsen O. G., Wennerström H., Zuckermann M. J. Phase equilibria in the phosphatidylcholine-cholesterol system. Biochim Biophys Acta. 1987 Nov 27;905(1):162–172. doi: 10.1016/0005-2736(87)90020-4. [DOI] [PubMed] [Google Scholar]
- Jost P. C., Griffith O. H. The molecular reorganization of lipid bilayers by osmium tetroxide. A spin-label study of orientation and restricted y-axis anisotropic motion in model membrane systems. Arch Biochem Biophys. 1973 Nov;159(1):70–81. doi: 10.1016/0003-9861(73)90430-x. [DOI] [PubMed] [Google Scholar]
- Kar L., Ney-Igner E., Freed J. H. Electron spin resonance and electron-spin-echo study of oriented multilayers of L alpha-dipalmitoylphosphatidylcholine water systems. Biophys J. 1985 Oct;48(4):569–595. doi: 10.1016/S0006-3495(85)83814-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawato S., Kinosita K., Jr, Ikegami A. Effect of cholesterol on the molecular motion in the hydrocarbon region of lecithin bilayers studied by nanosecond fluorescence techniques. Biochemistry. 1978 Nov 14;17(23):5026–5031. doi: 10.1021/bi00616a026. [DOI] [PubMed] [Google Scholar]
- Knoll W., Schmidt G., Ibel K., Sackmann E. Small-angle neutron scattering study of lateral phase separation in dimyristoylphosphatidylcholine-cholesterol mixed membranes. Biochemistry. 1985 Sep 10;24(19):5240–5246. doi: 10.1021/bi00340a043. [DOI] [PubMed] [Google Scholar]
- Kuo A. L., Wade C. G. Lipid lateral diffusion by pulsed nuclear magnetic resonance. Biochemistry. 1979 May 29;18(11):2300–2308. doi: 10.1021/bi00578a026. [DOI] [PubMed] [Google Scholar]
- Lentz B. R., Barrow D. A., Hoechli M. Cholesterol-phosphatidylcholine interactions in multilamellar vesicles. Biochemistry. 1980 Apr 29;19(9):1943–1954. doi: 10.1021/bi00550a034. [DOI] [PubMed] [Google Scholar]
- Levin I. W., Keihn E., Harris W. C. A Raman spectroscopic study on the effect of cholesterol on lipid packing in diether phosphatidylcholine bilayer dispersions. Biochim Biophys Acta. 1985 Oct 24;820(1):40–47. doi: 10.1016/0005-2736(85)90213-5. [DOI] [PubMed] [Google Scholar]
- Lindblom G., Johansson L. B., Arvidson G. Effect of cholesterol in membranes. Pulsed nuclear magnetic resonance measurements of lipid lateral diffusion. Biochemistry. 1981 Apr 14;20(8):2204–2207. doi: 10.1021/bi00511a020. [DOI] [PubMed] [Google Scholar]
- Mabrey S., Mateo P. L., Sturtevant J. M. High-sensitivity scanning calorimetric study of mixtures of cholesterol with dimyristoyl- and dipalmitoylphosphatidylcholines. Biochemistry. 1978 Jun 13;17(12):2464–2468. doi: 10.1021/bi00605a034. [DOI] [PubMed] [Google Scholar]
- Presti F. T., Chan S. I. Cholesterol-phospholipid interaction in membranes. 1. Cholestane spin-label studies of phase behavior of cholesterol-phospholipid liposomes. Biochemistry. 1982 Aug 3;21(16):3821–3830. doi: 10.1021/bi00259a016. [DOI] [PubMed] [Google Scholar]
- Recktenwald D. J., McConnell H. M. Phase equilibria in binary mixtures of phosphatidylcholine and cholesterol. Biochemistry. 1981 Jul 21;20(15):4505–4510. doi: 10.1021/bi00518a042. [DOI] [PubMed] [Google Scholar]
- Rubenstein J. L., Owicki J. C., McConnell H. M. Dynamic properties of binary mixtures of phosphatidylcholines and cholesterol. Biochemistry. 1980 Feb 5;19(3):569–573. doi: 10.1021/bi00544a027. [DOI] [PubMed] [Google Scholar]
- Rubenstein J. L., Smith B. A., McConnell H. M. Lateral diffusion in binary mixtures of cholesterol and phosphatidylcholines. Proc Natl Acad Sci U S A. 1979 Jan;76(1):15–18. doi: 10.1073/pnas.76.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimshick E. J., McConnell H. M. Lateral phase separation in phospholipid membranes. Biochemistry. 1973 Jun 5;12(12):2351–2360. doi: 10.1021/bi00736a026. [DOI] [PubMed] [Google Scholar]
- Smith L. M., Weis R. M., McConnell H. M. Measurement of rotational motion in membranes using fluorescence recovery after photobleaching. Biophys J. 1981 Oct;36(1):73–91. doi: 10.1016/S0006-3495(81)84717-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


