Abstract
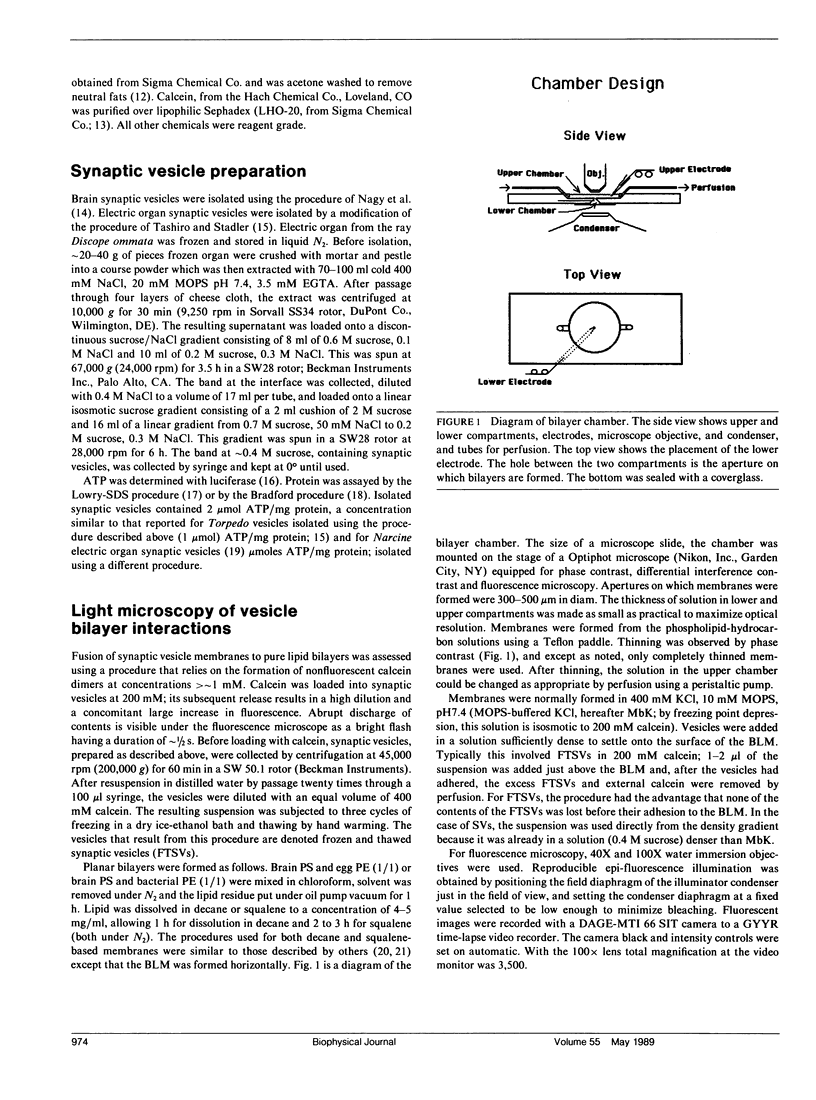
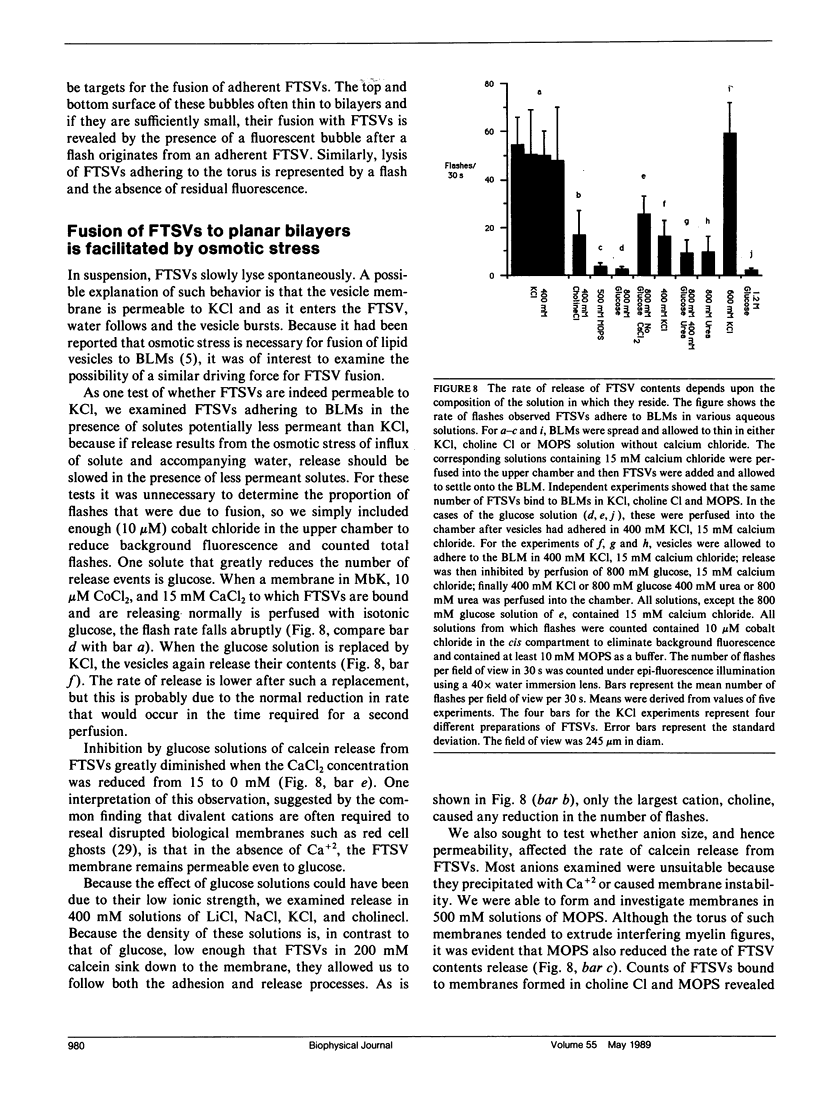
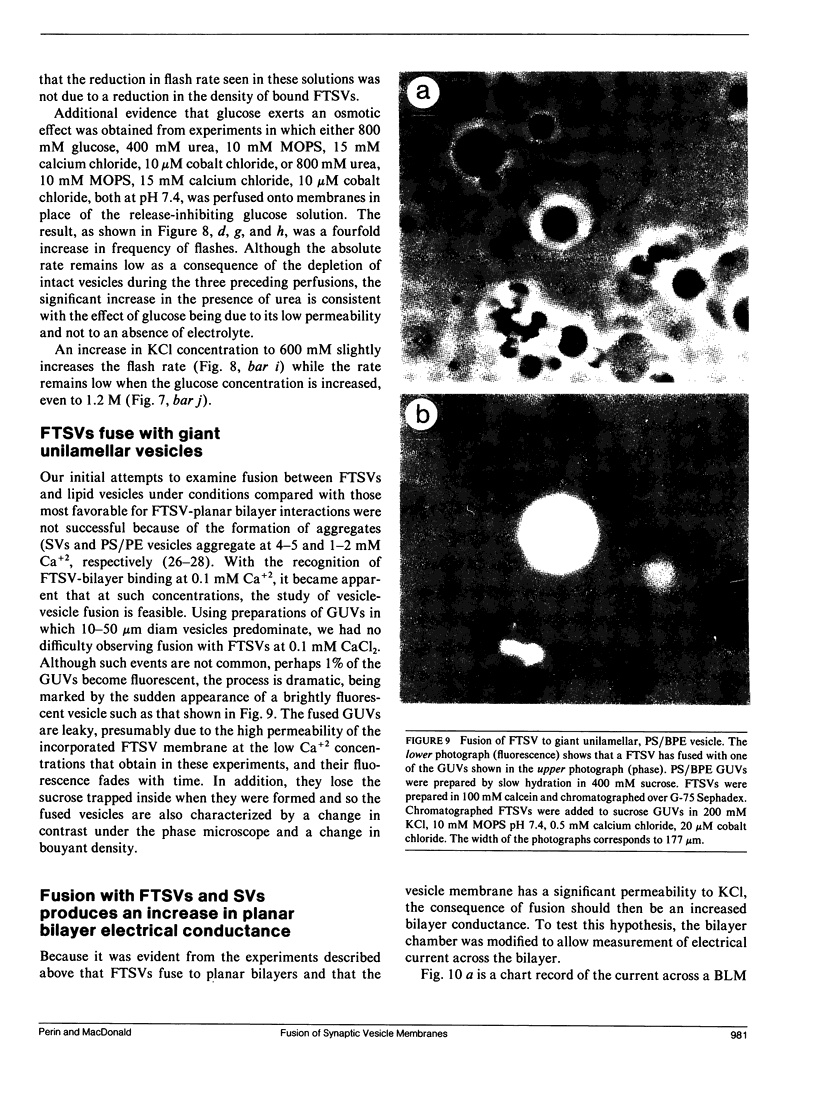
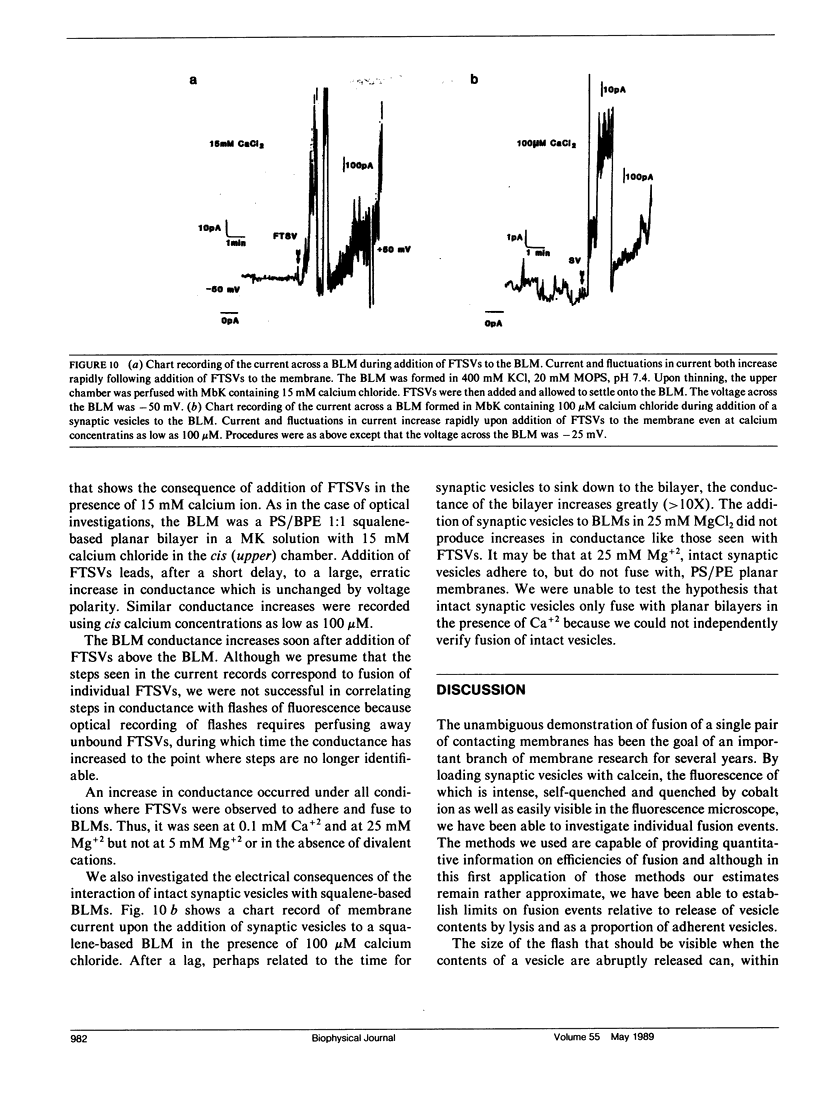
The interaction of synaptic vesicles with horizontal bilayer lipid membranes (BLMs) was investigated as a model system for neurotransmitter release. High concentrations (200 mM) of the fluorescent dye, calcein, were trapped within synaptic vesicles by freezing and thawing. In the presence of divalent ions (usually 15 mM CaCl2), these frozen and thawed synaptic vesicles (FTSVs) adhere to squalene-based phosphatidylserine-phosphatidylethanolamine BLMs whereupon they spontaneously release their contents which is visible by fluorescence microscopy as bright flashes. The highest rate of release was obtained in KCl solutions. Release was virtually eliminated in isotonic glucose, but could be elicited by perfusion with KCl or by addition of urea. The fusion and lysis of adhering FTSVs appears to be the consequence of stress resulting from entry of permeable external solute (KCl, urea) and accompanying water. An analysis of flash diameters in experiments where Co+2, which quenches calcein fluorescence, was present on one or both sides of the BLM, indicates that more than half of the flashes represent fusion events, i.e., release of vesicle contents on the trans side of the BLM. A population of small, barely visible FTSVs bind to BLMs at calcium ion concentrations of 100 microM. Although fusion of these small FTSVs to BLMs could not be demonstrated, fusion with giant lipid vesicles was obvious and dramatic, albeit infrequent. Addition of FTSVs or synaptic vesicles to BLMs in the presence of 100 microM-15 mM Ca2+ produced large increases in BLM conductance. The results presented demonstrate that synaptic vesicles are capable of fusing with model lipid membranes in the presence of Ca+2 ion which, at the lower limit, may begin to approach physiological concentrations.
Full text
PDF
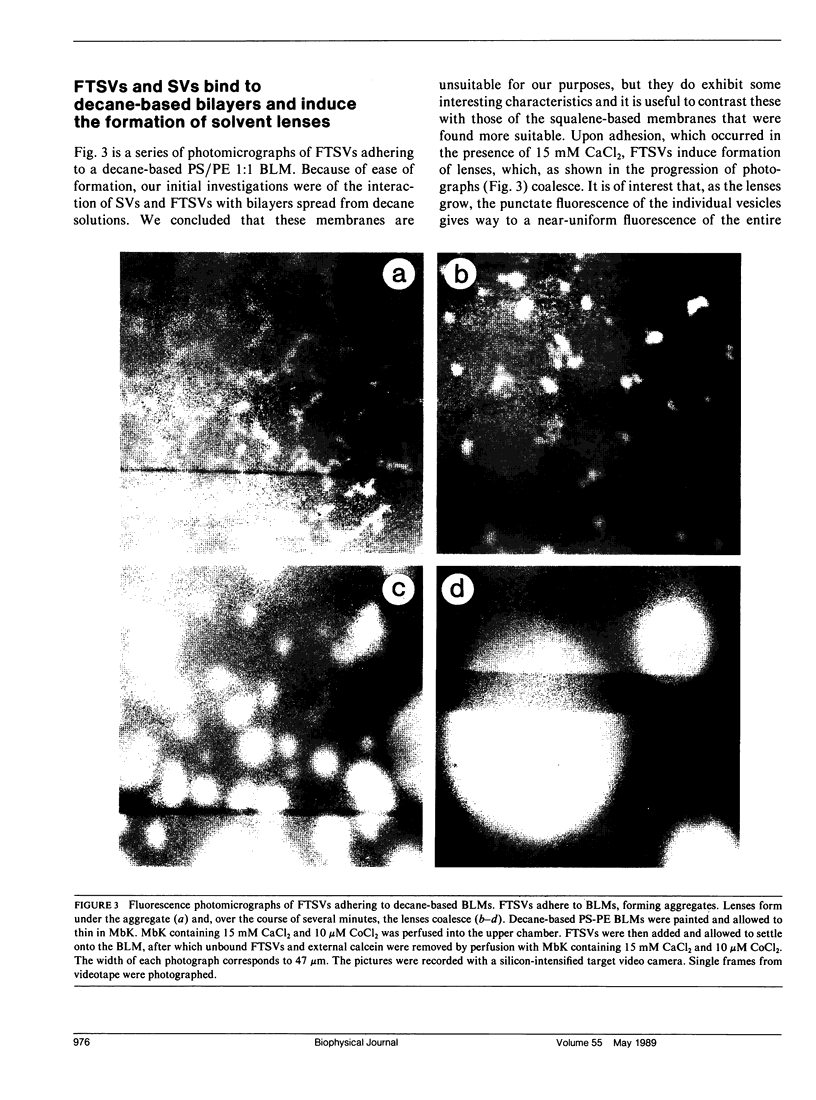
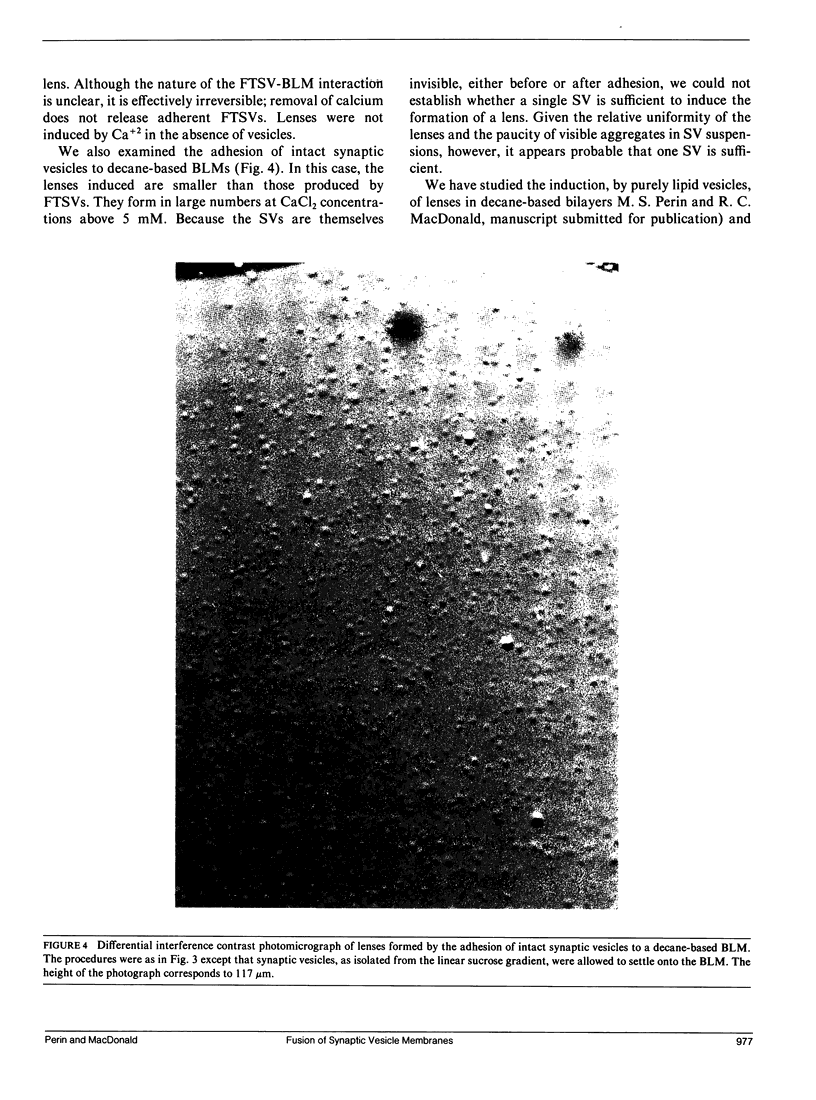
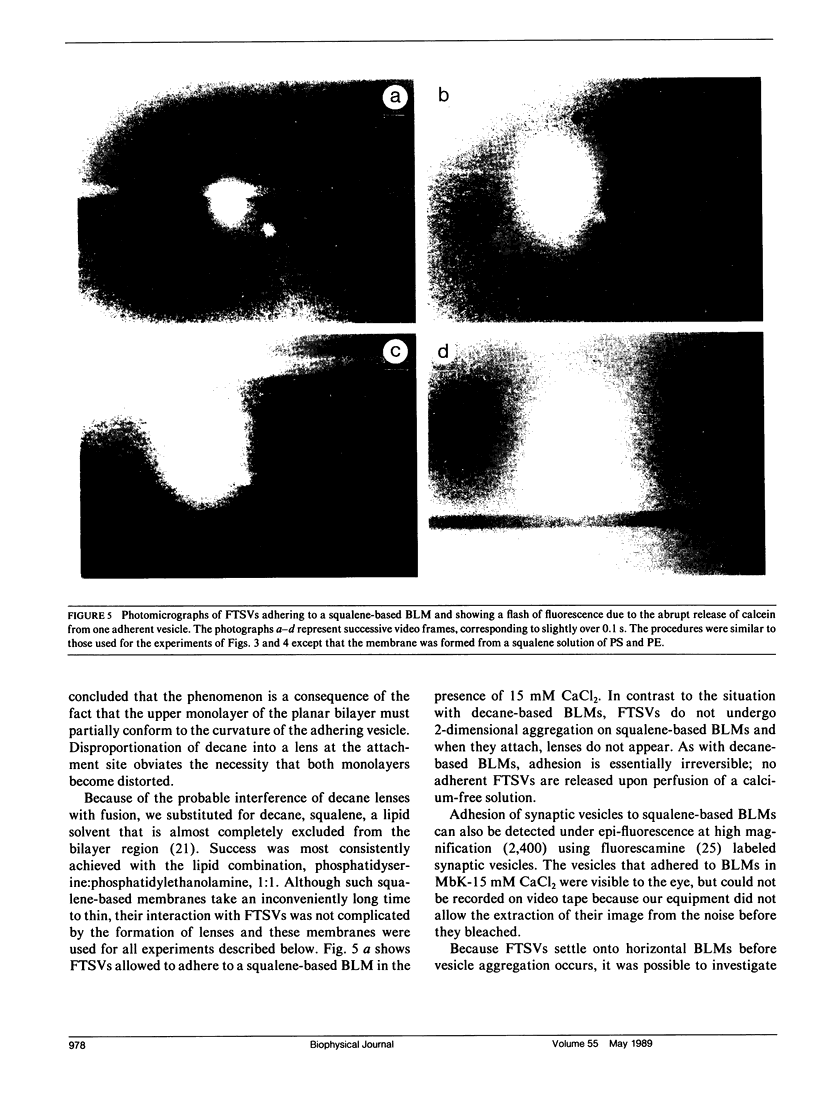
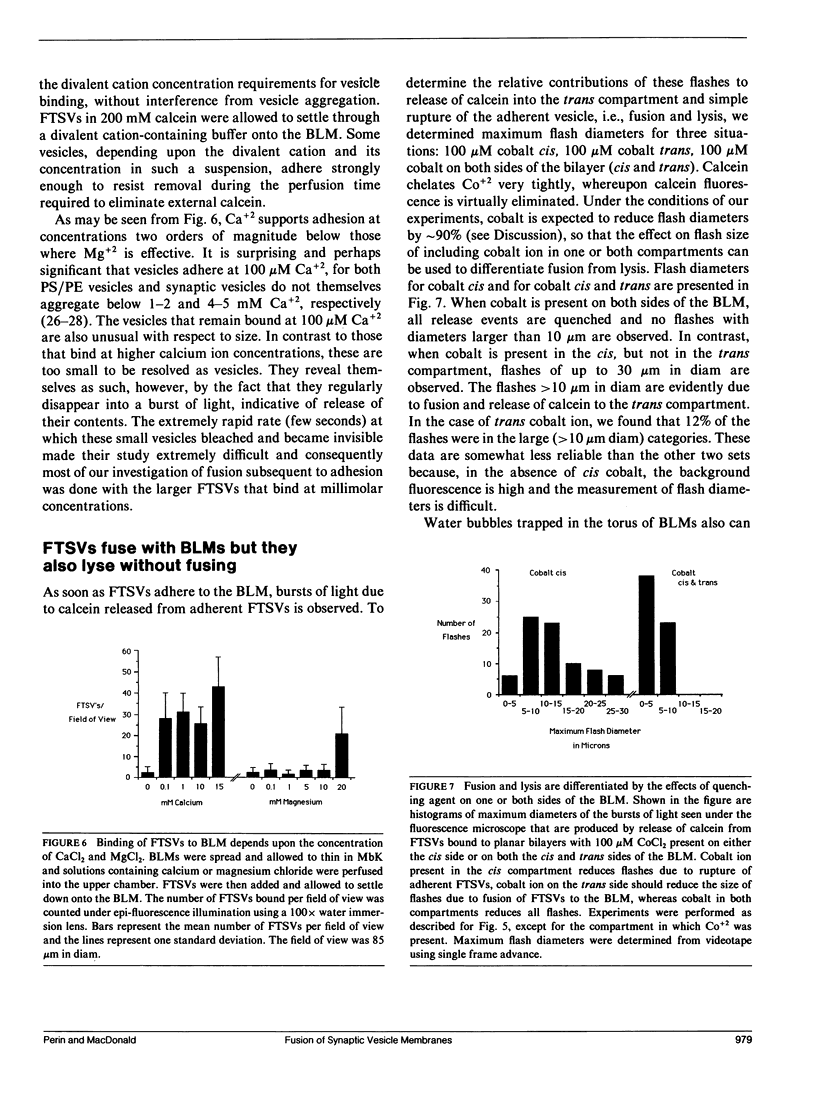




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akabas M. H., Cohen F. S., Finkelstein A. Separation of the osmotically driven fusion event from vesicle-planar membrane attachment in a model system for exocytosis. J Cell Biol. 1984 Mar;98(3):1063–1071. doi: 10.1083/jcb.98.3.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen T. M., Cleland L. G. Serum-induced leakage of liposome contents. Biochim Biophys Acta. 1980 Apr 10;597(2):418–426. doi: 10.1016/0005-2736(80)90118-2. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carlson S. S., Wagner J. A., Kelly R. B. Purification of synaptic vesicles from elasmobranch electric organ and the use of biophysical criteria to demonstrate purity. Biochemistry. 1978 Apr 4;17(7):1188–1199. doi: 10.1021/bi00600a009. [DOI] [PubMed] [Google Scholar]
- Cohen F. S., Akabas M. H., Finkelstein A. Osmotic swelling of phospholipid vesicles causes them to fuse with a planar phospholipid bilayer membrane. Science. 1982 Jul 30;217(4558):458–460. doi: 10.1126/science.6283637. [DOI] [PubMed] [Google Scholar]
- Cohen F. S., Akabas M. H., Zimmerberg J., Finkelstein A. Parameters affecting the fusion of unilamellar phospholipid vesicles with planar bilayer membranes. J Cell Biol. 1984 Mar;98(3):1054–1062. doi: 10.1083/jcb.98.3.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen F. S., Zimmerberg J., Finkelstein A. Fusion of phospholipid vesicles with planar phospholipid bilayer membranes. II. Incorporation of a vesicular membrane marker into the planar membrane. J Gen Physiol. 1980 Mar;75(3):251–270. doi: 10.1085/jgp.75.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronado R., Latorre R. Phospholipid bilayers made from monolayers on patch-clamp pipettes. Biophys J. 1983 Aug;43(2):231–236. doi: 10.1016/S0006-3495(83)84343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanspal M., Ralston G. B. Purification of a trypsin-insensitive fragment of spectrin from human erythrocyte membranes. Biochim Biophys Acta. 1981 Jul 28;669(2):133–139. doi: 10.1016/0005-2795(81)90234-8. [DOI] [PubMed] [Google Scholar]
- Haynes D. H., Lansman J., Cahill A. L., Morris S. J. Kinetics of cation-induced aggregation of Torpedo electric organ synaptic vesicles. Biochim Biophys Acta. 1979 Nov 2;557(2):340–353. doi: 10.1016/0005-2736(79)90332-8. [DOI] [PubMed] [Google Scholar]
- Kendall D. A., MacDonald R. C. A fluorescence assay to monitor vesicle fusion and lysis. J Biol Chem. 1982 Dec 10;257(23):13892–13895. [PubMed] [Google Scholar]
- Knight D. E., Baker P. F. Calcium-dependence of catecholamine release from bovine adrenal medullary cells after exposure to intense electric fields. J Membr Biol. 1982;68(2):107–140. doi: 10.1007/BF01872259. [DOI] [PubMed] [Google Scholar]
- Manery J. F. Effects of Ca ions on membranes. Fed Proc. 1966 Nov-Dec;25(6):1804–1810. [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Miller C., Racker E. Ca++-induced fusion of fragmented sarcoplasmic reticulum with artificial planar bilayers. J Membr Biol. 1976;30(3):283–300. doi: 10.1007/BF01869673. [DOI] [PubMed] [Google Scholar]
- Morris S. J., Gibson C. C., Smith P. D., Greif P. C., Stirk C. W., Bradley D., Haynes D. H., Blumenthal R. Rapid kinetics of Ca2+-induced fusion of phosphatidylserine/phosphatidylethanolamine vesicles. The effect of bilayer curvature on leakage. J Biol Chem. 1985 Apr 10;260(7):4122–4127. [PubMed] [Google Scholar]
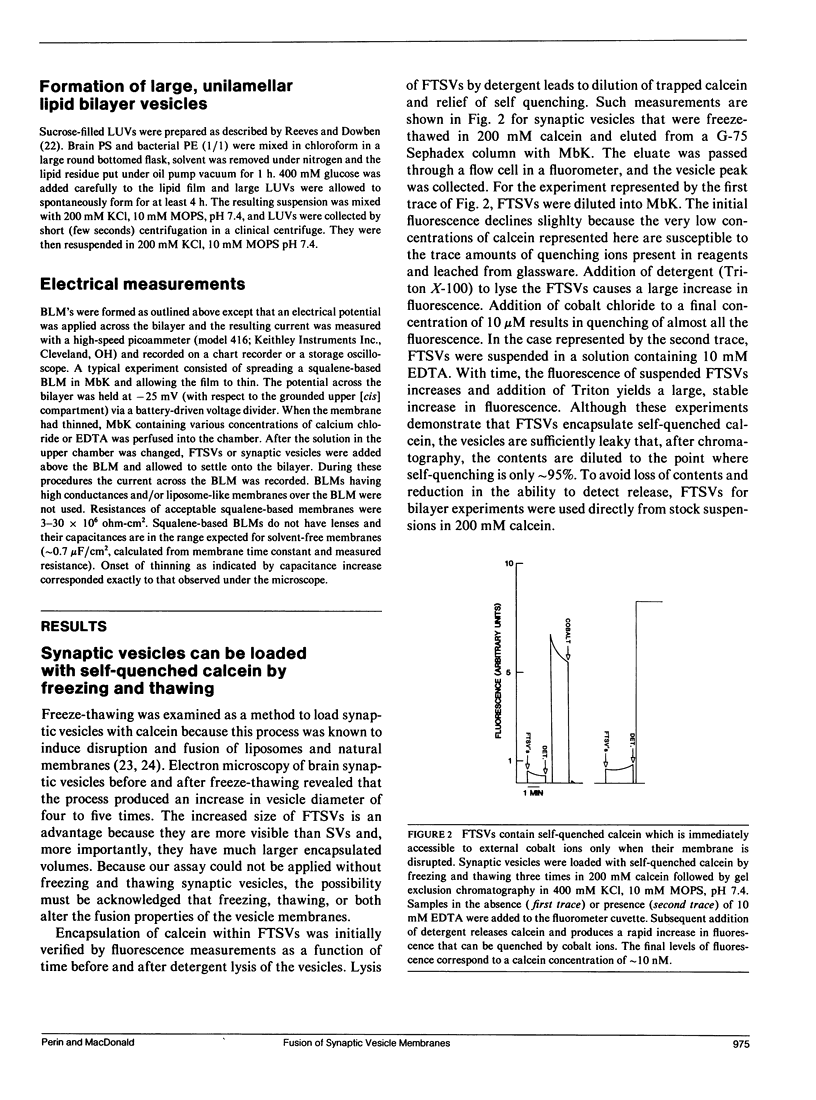
- Nagy A., Baker R. R., Morris S. J., Whittaker V. P. The preparation and characterization of synaptic vesicles of high purity. Brain Res. 1976 Jun 11;109(2):285–309. doi: 10.1016/0006-8993(76)90531-x. [DOI] [PubMed] [Google Scholar]
- Niles W. D., Cohen F. S. Video fluorescence microscopy studies of phospholipid vesicle fusion with a planar phospholipid membrane. Nature of membrane-membrane interactions and detection of release of contents. J Gen Physiol. 1987 Nov;90(5):703–735. doi: 10.1085/jgp.90.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oku N., MacDonald R. C. Differential effects of alkali metal chlorides on formation of giant liposomes by freezing and thawing and dialysis. Biochemistry. 1983 Feb 15;22(4):855–863. doi: 10.1021/bi00273a023. [DOI] [PubMed] [Google Scholar]
- Reeves J. P., Dowben R. M. Formation and properties of thin-walled phospholipid vesicles. J Cell Physiol. 1969 Feb;73(1):49–60. doi: 10.1002/jcp.1040730108. [DOI] [PubMed] [Google Scholar]
- Simon S. M., Llinás R. R. Compartmentalization of the submembrane calcium activity during calcium influx and its significance in transmitter release. Biophys J. 1985 Sep;48(3):485–498. doi: 10.1016/S0006-3495(85)83804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro T., Stadler H. Chemical composition of cholinergic synaptic vesicles from Torpedo marmorata based on improved purification. Eur J Biochem. 1978 Oct 16;90(3):479–487. doi: 10.1111/j.1432-1033.1978.tb12627.x. [DOI] [PubMed] [Google Scholar]
- Udenfriend S., Stein S., Böhlen P., Dairman W., Leimgruber W., Weigele M. Fluorescamine: a reagent for assay of amino acids, peptides, proteins, and primary amines in the picomole range. Science. 1972 Nov 24;178(4063):871–872. doi: 10.1126/science.178.4063.871. [DOI] [PubMed] [Google Scholar]
- White S. H. Formation of "solvent-free" black lipid bilayer membranes from glyceryl monooleate dispersed in squalene. Biophys J. 1978 Sep;23(3):337–347. doi: 10.1016/S0006-3495(78)85453-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilschut J., Düzgüneş N., Fraley R., Papahadjopoulos D. Studies on the mechanism of membrane fusion: kinetics of calcium ion induced fusion of phosphatidylserine vesicles followed by a new assay for mixing of aqueous vesicle contents. Biochemistry. 1980 Dec 23;19(26):6011–6021. doi: 10.1021/bi00567a011. [DOI] [PubMed] [Google Scholar]
- Woodbury D. J., Hall J. E. Role of channels in the fusion of vesicles with a planar bilayer. Biophys J. 1988 Dec;54(6):1053–1063. doi: 10.1016/S0006-3495(88)83042-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerberg J., Cohen F. S., Finkelstein A. Fusion of phospholipid vesicles with planar phospholipid bilayer membranes. I. Discharge of vesicular contents across the planar membrane. J Gen Physiol. 1980 Mar;75(3):241–250. doi: 10.1085/jgp.75.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerberg J., Cohen F. S., Finkelstein A. Micromolar Ca2+ stimulates fusion of lipid vesicles with planar bilayers containing a calcium-binding protein. Science. 1980 Nov 21;210(4472):906–908. doi: 10.1126/science.7434004. [DOI] [PubMed] [Google Scholar]
- Zimmermann H., Denston C. R. Separation of synaptic vesicles of different functional states from the cholinergic synapses of the Torpedo electric organ. Neuroscience. 1977;2(5):715–730. doi: 10.1016/0306-4522(77)90025-2. [DOI] [PubMed] [Google Scholar]