Abstract
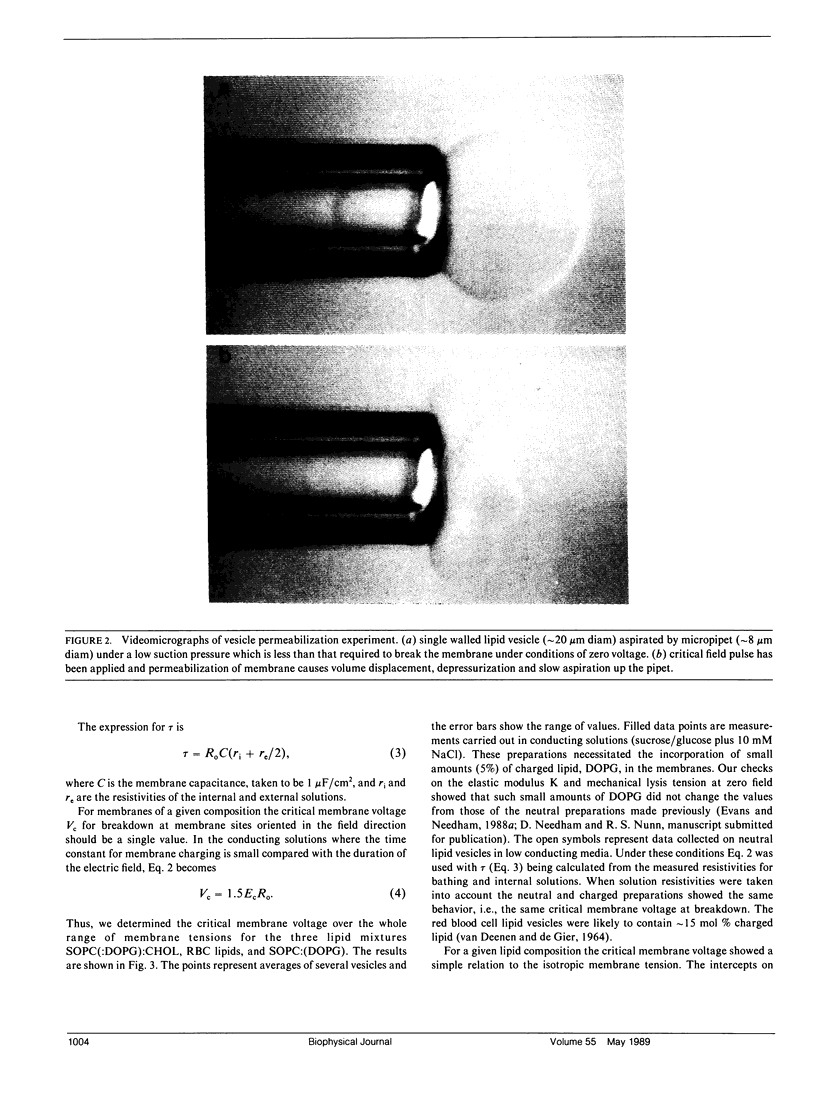
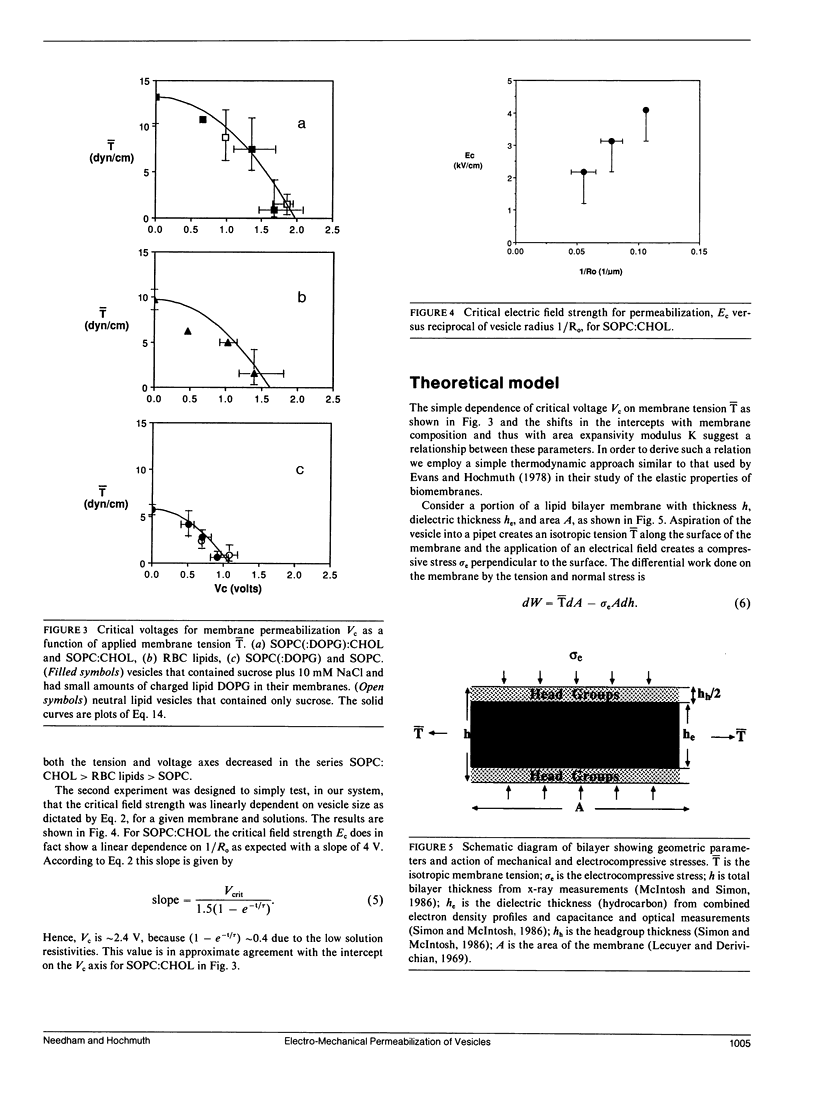
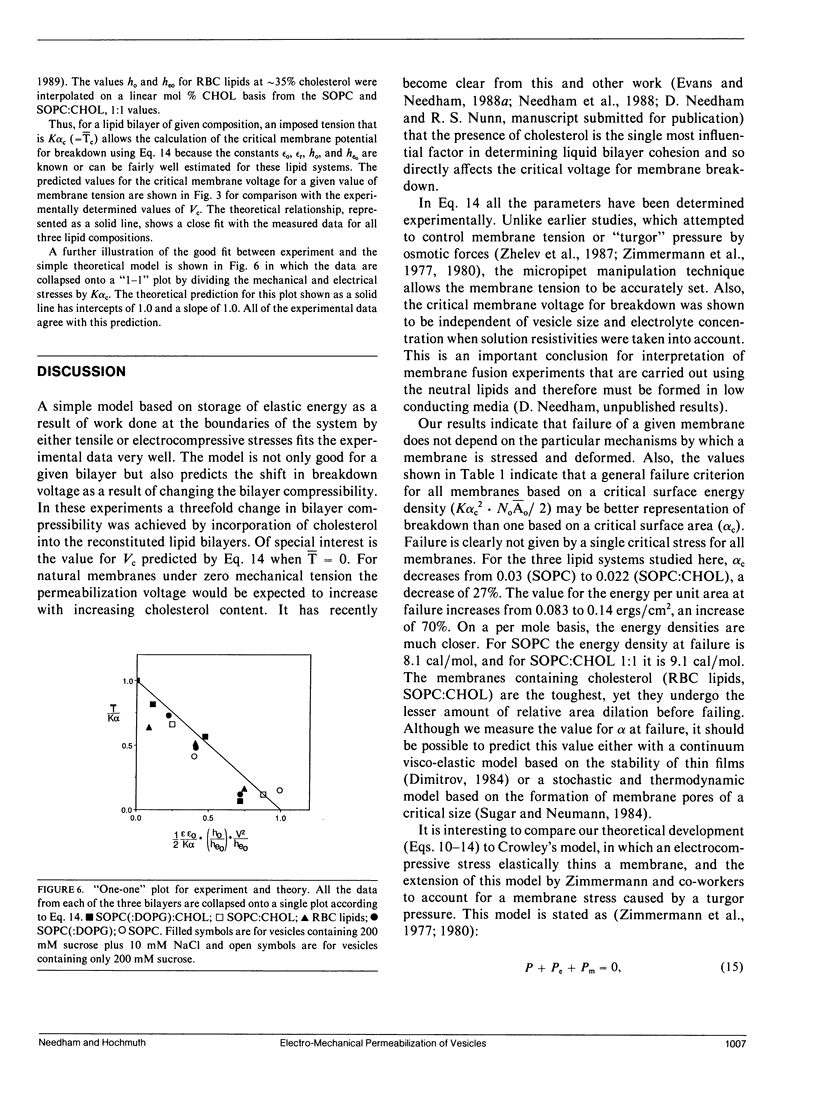
A simple micropipet technique was used to determine the critical electric field strength for membrane breakdown as a function of the applied membrane tension for three different reconstituted membranes: stearoyloleoylphosphatidylcholine (SOPC), red blood cell (RBC) lipid extract, and SOPC cholesterol (CHOL), 1:1. For these membranes the elastic area expansivity modulus increases from approximately 200 to 600 dyn/cm, and the tension at lysis increases from 5.7 to 13.2 dyn/cm, i.e., the membranes become more cohesive with increasing cholesterol content. The critical membrane voltage, Vc, required for breakdown was also found to increase with increasing cholesterol from 1.1 to 1.8 V at zero membrane tension. We have modeled the behavior in terms of the bilayer expansivity. Membrane area can be increased by either tensile or electrocompressive stresses. Both can store elastic energy in the membrane and eventually cause breakdown at a critical area dilation or critical energy. The model predicts a relation between tension and voltage at breakdown and this relation is verified experimentally for the three reconstituted membrane systems studied here.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crowley J. M. Electrical breakdown of bimolecular lipid membranes as an electromechanical instability. Biophys J. 1973 Jul;13(7):711–724. doi: 10.1016/S0006-3495(73)86017-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov D. S. Electric field-induced breakdown of lipid bilayers and cell membranes: a thin viscoelastic film model. J Membr Biol. 1984;78(1):53–60. doi: 10.1007/BF01872532. [DOI] [PubMed] [Google Scholar]
- Dimitrov D. S., Jain R. K. Membrane stability. Biochim Biophys Acta. 1984 Dec 4;779(4):437–468. doi: 10.1016/0304-4157(84)90020-0. [DOI] [PubMed] [Google Scholar]
- Evans E. A., Waugh R., Melnik L. Elastic area compressibility modulus of red cell membrane. Biophys J. 1976 Jun;16(6):585–595. doi: 10.1016/S0006-3495(76)85713-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. A., Waugh R. Osmotic correction to elastic area compressibility measurements on red cell membrane. Biophys J. 1977 Dec;20(3):307–313. doi: 10.1016/S0006-3495(77)85551-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinosita K., Jr, Ashikawa I., Saita N., Yoshimura H., Itoh H., Nagayama K., Ikegami A. Electroporation of cell membrane visualized under a pulsed-laser fluorescence microscope. Biophys J. 1988 Jun;53(6):1015–1019. doi: 10.1016/S0006-3495(88)83181-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson J. C., Yee D. Electroporation: parameters affecting transfer of DNA into mammalian cells. Anal Biochem. 1987 Jul;164(1):44–52. doi: 10.1016/0003-2697(87)90365-4. [DOI] [PubMed] [Google Scholar]
- Kwok R., Evans E. Thermoelasticity of large lecithin bilayer vesicles. Biophys J. 1981 Sep;35(3):637–652. doi: 10.1016/S0006-3495(81)84817-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecuyer H., Dervichian D. G. Structure of aqueous mixtures of lecithin and cholesterol. J Mol Biol. 1969 Oct 14;45(1):39–57. doi: 10.1016/0022-2836(69)90208-3. [DOI] [PubMed] [Google Scholar]
- Letter: Lenses and the compression of black lipid membranes by an electric field. Biophys J. 1975 Jan;15(1):77–81. doi: 10.1016/S0006-3495(75)85793-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh T. J., Magid A. D., Simon S. A. Cholesterol modifies the short-range repulsive interactions between phosphatidylcholine membranes. Biochemistry. 1989 Jan 10;28(1):17–25. doi: 10.1021/bi00427a004. [DOI] [PubMed] [Google Scholar]
- Needham D., McIntosh T. J., Evans E. Thermomechanical and transition properties of dimyristoylphosphatidylcholine/cholesterol bilayers. Biochemistry. 1988 Jun 28;27(13):4668–4673. doi: 10.1021/bi00413a013. [DOI] [PubMed] [Google Scholar]
- Neumann E., Schaefer-Ridder M., Wang Y., Hofschneider P. H. Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J. 1982;1(7):841–845. doi: 10.1002/j.1460-2075.1982.tb01257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand R. P., Fuller N., Parsegian V. A., Rau D. C. Variation in hydration forces between neutral phospholipid bilayers: evidence for hydration attraction. Biochemistry. 1988 Oct 4;27(20):7711–7722. doi: 10.1021/bi00420a021. [DOI] [PubMed] [Google Scholar]
- Reeves J. P., Dowben R. M. Formation and properties of thin-walled phospholipid vesicles. J Cell Physiol. 1969 Feb;73(1):49–60. doi: 10.1002/jcp.1040730108. [DOI] [PubMed] [Google Scholar]
- Schwister K., Deuticke B. Formation and properties of aqueous leaks induced in human erythrocytes by electrical breakdown. Biochim Biophys Acta. 1985 Jun 27;816(2):332–348. doi: 10.1016/0005-2736(85)90501-2. [DOI] [PubMed] [Google Scholar]
- Simon S. A., McIntosh T. J. Depth of water penetration into lipid bilayers. Methods Enzymol. 1986;127:511–521. doi: 10.1016/0076-6879(86)27041-x. [DOI] [PubMed] [Google Scholar]
- Simon S. A., McIntosh T. J., Latorre R. Influence of cholesterol on water penetration into bilayers. Science. 1982 Apr 2;216(4541):65–67. doi: 10.1126/science.7063872. [DOI] [PubMed] [Google Scholar]
- Sowers A. E. A long-lived fusogenic state is induced in erythrocyte ghosts by electric pulses. J Cell Biol. 1986 Apr;102(4):1358–1362. doi: 10.1083/jcb.102.4.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugar I. P., Neumann E. Stochastic model for electric field-induced membrane pores. Electroporation. Biophys Chem. 1984 May;19(3):211–225. doi: 10.1016/0301-4622(84)87003-9. [DOI] [PubMed] [Google Scholar]
- Teissie J., Tsong T. Y. Electric field induced transient pores in phospholipid bilayer vesicles. Biochemistry. 1981 Mar 17;20(6):1548–1554. doi: 10.1021/bi00509a022. [DOI] [PubMed] [Google Scholar]
- Zimmermann U., Beckers F., Coster H. G. The effect of pressure on the electrical breakdown in the membranes of Valonia utricularis. Biochim Biophys Acta. 1977 Jan 21;464(2):399–346. doi: 10.1016/0005-2736(77)90014-1. [DOI] [PubMed] [Google Scholar]
- Zimmermann U. Electric field-mediated fusion and related electrical phenomena. Biochim Biophys Acta. 1982 Nov 30;694(3):227–277. doi: 10.1016/0304-4157(82)90007-7. [DOI] [PubMed] [Google Scholar]
- Zimmermann U. Electrical breakdown, electropermeabilization and electrofusion. Rev Physiol Biochem Pharmacol. 1986;105:176–256. [PubMed] [Google Scholar]
- Zimmermann U., Pilwat G., Péqueux A., Gilles R. Electro-mechanical properties of human erythrocyte membranes: the pressure-dependence of potassium permeability. J Membr Biol. 1980 May 23;54(2):103–113. doi: 10.1007/BF01940564. [DOI] [PubMed] [Google Scholar]




