Abstract
We investigated counteracting interactions between the lipopolysaccharides (LPS) from Escherichia coli (Ec-LPS) and Porphyromonas gingivalis (Pg-LPS), which induce cellular activation through Toll-like receptor 4 (TLR4) and TLR2, respectively. We found that Ec-LPS induced tolerance in THP-1 cells to subsequent tumor necrosis factor alpha (TNF-α) and interleukin 1 beta (IL-1β) induction by Pg-LPS, though the reverse was not true, and looked for explanatory differential effects on the signal transduction pathway. Cells exposed to Pg-LPS, but not to Ec-LPS, displayed persisting expression of IL-1 receptor-associated kinase without apparent degradation, presumably allowing prolonged relay of downstream signals. Accordingly, cells pretreated with Pg-LPS, but not with Ec-LPS, were effectively activated in response to subsequent exposure to either LPS molecule, as evidenced by assessing nuclear factor (NF)-κB activity. In fact, Pg-LPS primed THP-1 cells for enhanced NF-κB activation and TNF-α release upon restimulation with the same LPS. This was a dose-dependent effect and correlated with upregulation of surface TLR2 expression. Furthermore, we observed inhibition of NF-κB-dependent transcription in a reporter cell line pretreated with Ec-LPS and restimulated with Pg-LPS (compared to cells pretreated with medium only and restimulated with Pg-LPS), but not when the reverse treatment was made. Although Pg-LPS could not make cells tolerant to subsequent activation by Ec-LPS, Pg-LPS inhibited Ec-LPS-induced TNF-α and IL-6 release when the two molecules were added simultaneously into THP-1 cell cultures. Pg-LPS also suppressed P. gingivalis FimA protein-induced NF-κB-dependent transcription in the 3E10/huTLR4 reporter cell line, which does not express TLR2. This rules out competition for common signaling intermediates, suggesting that Pg-LPS may block component(s) of the TLR4 receptor complex. Interactions between TLR2 and TLR4 agonists may be important in the regulation of inflammatory reactions.
The recent discovery of the evolutionarily conserved Toll-like receptors (TLR) as important signal transducers for induction of innate immunity (17, 25) has provided new insight into some previously puzzling questions regarding the biology of bacterial lipopolysaccharides (LPS) (6, 39). Several receptors are involved in the host response to LPS. Serum-derived LPS-binding protein facilitates the interaction of LPS with soluble or membrane-anchored CD14 (33), but CD14 does not transduce intracellular signals because it lacks a transmembrane domain. LPS-induced intracellular signaling reactions are mediated by TLRs, which possess appropriate transmembrane signaling components (1). Highly purified LPS from enterobacteria or oral bacteria, such as Porphyromonas gingivalis and Prevotella intermedia, were known to activate macrophages through similar mechanisms involving LPS-binding protein and CD14; however, only LPS from these oral species could stimulate macrophages from C3H/HeJ mice (14). This could be attributed to structural differences between enterobacterial and oral LPS molecules (28). Nevertheless, it was only after enterobacterial LPS hyporesponsiveness in C3H/HeJ mice was attributed to a defective TLR4 gene (31, 34) that these biological differences started to be understood at the molecular level of cellular activation. Subsequently it was shown that unlike enterobacterial LPS, P. gingivalis LPS (Pg-LPS) activates TLR2 but not TLR4 (2, 11). Therefore, Pg-LPS and Escherichia coli LPS (Ec-LPS) are quite distinct endotoxins that can be used for investigating the molecular cross talk between TLR2 and TLR4. P. gingivalis has been known as a major pathogen in adult periodontal disease (41). However, its recent implication in systemic diseases (9, 20) suggests possible interactions with TLR4-utilizing LPS from other pathogens implicated in systemic diseases.
The innate immune system not only has evolved to recognize pathogens and respond with induction of proinflammatory mediators but also has developed the ability to downregulate excessive inflammatory reactions that could potentially contribute to tissue destruction (42). For example, prior exposure of monocytic cells to enterobacterial LPS induces a transient state of reduced ability to elicit proinflammatory cytokines to subsequent LPS restimulation. This phenomenon is known as endotoxin tolerance and has been attributed to either downregulation of TLR4 surface expression (27) or reprogramming of the TLR4 signal transduction pathway (18, 24, 43). These are not mutually exclusive mechanisms, and it was recently shown that endotoxin tolerance can occur independently of TLR4 expression levels (23). Alterations in the LPS-tolerant state resulting in reduced NF-κB activation include reduced expression of interleukin 1 receptor (IL-1R)-associated kinase (IRAK) (18), suppressed degradation of the inhibitory I-κBα and I-κBβ proteins (24), and changes in the ratio of the p50 and p65 subunits of NF-κB (13).
Interestingly, Pg-LPS behaves quite differently from Ec-LPS with regard to endotoxin tolerance (21). Exposure of monocytic THP-1 cells to Ec-LPS almost eliminates their ability to induce IL-1β, IL-6, and TNF-α release upon secondary stimulation with the same stimulus (21). In parallel experiments, Pg-LPS pretreatment reduced subsequent induction by Pg-LPS of IL-1β but not of IL-6 and TNF-α (21). In fact, IL-6 and TNF-α induction was upregulated when primary and secondary stimulation was done with ⩾1 μg of Pg-LPS/ml (21). In cross-tolerance experiments involving LPS and the proinflammatory FimA protein from P. gingivalis, pretreatment of THP-1 cells with Ec-LPS abrogated subsequent induction of IL-1β and TNF-α by FimA. In contrast, only IL-1β release was downregulated when pretreatment was done with Pg-LPS followed by restimulation with FimA (8).
On the basis of the above considerations, it becomes important to investigate host regulatory interactions between Ec-LPS and Pg-LPS. Specifically, we investigated such interactions when these two endotoxin molecules are added either sequentially (cross-tolerance) or concurrently to THP-1 cells. In principle, cross-tolerance could be induced either by downregulation of cellular receptors or by alterations in signal transduction pathways. However, the reported effects of these endotoxins on cellular receptor regulation are unlikely to play a role in induction of cross-tolerance. In Ec-LPS-treated monocytes/macrophages, TLR2 expression is either upregulated (5, 22) or not affected (21), whereas CD14 expression is not significantly influenced (21, 27). Ec-LPS would not therefore downregulate receptors utilized by Pg-LPS. The reverse is also true, since Pg-LPS upregulates CD14 (21, 35) without affecting TLR4 expression (21). We thus investigated possible alterations in the intracellular TLR2 and TLR4 signal transduction pathways.
MATERIALS AND METHODS
Reagents.
LPS from P. gingivalis 381 was highly purified by phenol-water extraction and subsequent treatment with DNase I, RNase A, and proteinase K, followed by chromatographic purification using a column of Sephacryl S-400 HR (2.5 by 40 cm; Pharmacia, Fine Chemicals, Piscataway, N.J.) (32). The purity of the preparation was confirmed by immunodiffusion analysis and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with silver staining. Highly purified LPS from E. coli K235 was prepared according to the phenol reextraction method (10). Induction of TNF-α and IL-1β by these Pg-LPS and Ec-LPS preparations was found to be inhibited by anti-TLR2 and anti-TLR4 monoclonal antibodies (MAbs), respectively, in accordance with previous reports on their being TLR2 and TLR4 agonists, respectively (11, 21). Recombinant FimA (rFimA) was expressed and purified from Escherichia coli BL21(DE3) (Novagen, Madison, Wis.) transformed with the fimA gene of P. gingivalis 381, as previously described (8). The rFimA preparation was considered negative for LPS or other contaminating substances as judged by SDS-PAGE visualized with silver staining. As a further precaution, all assays involving rFimA were performed in the presence of the LPS inhibitor, polymyxin B sulfate (10 μg/ml; Sigma, St. Louis, Mo.).
Fluorescein isothiocyanate (FITC)-conjugated mouse MAb to human CD25 (clone 3G10, immunoglobulin G1 [IgG1]), FITC-conjugated goat anti-mouse IgG, and mouse IgG1 as an isotype control were purchased from Caltag (Burlingame, Calif.). Mouse MAb to human TLR2 (clone 2392, IgG1) was obtained from Genentech (South San Francisco, Calif.). Polyclonal rabbit IgG antibodies to human IRAK and myeloid differentiation factor 88 (MyD88) were from Santa Cruz Biotechnology (Santa Cruz, Calif.). Goat anti-rabbit IgG conjugated to horseradish peroxidase was from Southern Biotechnology (Birmingham, Ala.).
Cytokine assays.
THP-1 cells (ATCC TIB-202) were differentiated with 10 ng of phorbol myristate acetate/ml for 3 days in 96-well polystyrene culture plates at 37°C in a humidified atmosphere containing 5% CO2. The culture medium consisted of RPMI 1640 (Life Technologies, Gaithersburg, Md.) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 2 mM l-glutamine, 10 mM HEPES, 100 U of penicillin G/ml, 100 μg of streptomycin/ml, and 0.05 mM 2-mercaptoethanol. Differentiated THP-1 cells (106/ml) were used for cytokine induction assays in a total volume of 200 μl per well in the absence or presence of microbial stimulants. The doses used were chosen on the basis of results from previous publications by the authors (8, 21). Culture supernatants were collected after overnight incubation (about 14 h) and stored at −70°C until assayed. None of the stimulants tested was found to affect the viability of the cells. Release of TNF-α, IL-1β, or IL-6 into the culture medium was quantitated using CLB Pelikine enzyme-linked immunosorbent assay (ELISA) kits (obtained through Caltag) according to protocols recommended by the manufacturer. In tolerance induction experiments, the cells were pretreated for 24 h with or without microbial stimulants. Following removal of the culture supernatants and three washes with warm culture medium, the cells were exposed to a secondary stimulation.
Fluorescence-activated cell sorter (FACS) analysis.
Differentiated THP-1 cells were treated with increasing concentrations of Pg-LPS (0.01 to 10 μg/ml) for 24 h. They were then washed in ice-cold phosphate-buffered saline containing 3% FBS and 0.05% sodium azide (FACS staining buffer). Surface expression of TLR2 was detected by mouse anti-TLR2 IgG1 monoclonal antibody (1 μg per 106 cells) followed by goat anti-mouse IgG-FITC in two 45-min incubation steps on ice with in-between washings with FACS buffer. After the final wash, the cells were analyzed by means of a FACScan flow cytometer (Becton Dickinson).
Analysis of NF-κB subunit composition.
Activation of the p50 and p65 subunits of NF-κB in differentiated THP-1 cells was determined by means of NF-κB p50/p65 ELISA-based transcription factor assay kits (Active Motif, Carlsbad, Calif.). The detecting antibodies recognize epitopes on either p50 or p65 that are accessible only when NF-κB is activated and bound to its target DNA (containing the NF-κB consensus binding site, 5′-GGGACTTTCC-3′) attached to 96-well plates. The specificity of the assay was verified by including an excess of soluble oligonucleotides containing a wild-type or mutated NF-κB consensus binding site. For tolerance induction experiments, cells were pretreated with medium or bacterial components for 20 h, washed with Hanks' balanced salt solution, allowed to rest for 2 h in fresh medium, and were restimulated for 1 h. Extract preparation and NF-κB ELISA were carried out according to protocols supplied by the manufacturer. The optimal time of restimulation and amount of total protein (5 μg) used in the NF-κB ELISA were determined in preliminary experiments.
Reporter assay for NF-κB-dependent transcription.
Chinese hamster ovary (CHO) fibroblasts used for FACS analysis were cultured in 24-well plates at 37°C in a humidified atmosphere containing 5% CO2. The culture medium consisted of Ham's F-12 nutrient mixture (Life Technologies) supplemented with 2 mM l-glutamine, 10% heat-inactivated FBS, 100 U of penicillin/ml, 100 μg of streptomycin/ml, 400 U of hygromycin B (Calbiochem, San Diego, Calif.)/ml, and 1 mg of G418 (Life Technologies)/ml. The clones used were the stably transfected CD14-positive CHO reporter cell lines 3E10/huTLR2 and 3E10/huTLR4, which express inducible cell surface human CD25 under the transcriptional control of an NF-κB-dependent E-selectin promoter, in response to TLR2 and TLR4 agonists, respectively (19). After overnight (20 h) incubation in the presence or absence of bacterial stimuli, the cells were detached from the wells by means of gentle pipetting with Hanks' solution-based cell-dissociation buffer (Life Technologies) and then washed in ice-cold FACS staining buffer (see above). Approximately 2 × 105 cells were incubated for 45 min on ice with 0.2 μg of FITC-conjugated monoclonal antibody to CD25 in a total volume of 100 μl of staining buffer. FACS analysis of surface CD25 expression was performed as above. Tolerance experiments were performed according to a model involving pretreatment of these cell lines for 5 h with medium or bacterial stimuli, followed by washing and 18-h restimulation (23). Induction of tolerance is manifested as diminished CD25 surface expression, which reflects reduced NF-κB activation upon restimulation.
Western blot analysis of signaling intermediates.
Cellular extracts from activated THP-1 cells were prepared by resuspending washed cells for 10 min in ice-cold lysis buffer (100 μl of buffer per 106 cells) comprising 50 mM HEPES (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1% Nonidet P-40, 0.1% SDS, 0.5% deoxycholate, 1 mM sodium orthovanadate, 1 mM sodium fluoride, 1 mM phenylmethylsulfonyl fluoride, and 1-mg/ml aprotinin. Clarified soluble extracts were prepared, and 20 μg of total cellular protein was subjected to SDS-PAGE and subsequently blotted onto nitrocellulose membranes as previously described (21). The membranes were probed for MyD88 and IRAK by means of specific rabbit polyclonal IgG antibodies, and bands were visualized using enhanced chemiluminescence according to a protocol supplied by the manufacturer (Santa Cruz Biotechnology).
Statistical analysis.
Data were evaluated by analysis of variance and the Tukey multiple-comparison test using the InStat program (GraphPad Software, San Diego, Calif.). Statistical differences were considered significant at the level of P values of <0.05. All reported experiments were performed at least twice for verification.
RESULTS
Cross-tolerance at the cytokine induction level.
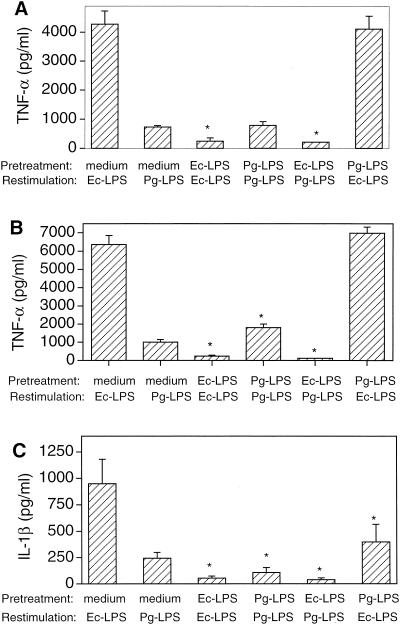
Pretreatment of THP-1 cells with Ec-LPS resulted in significantly (P < 0.05) diminished TNF-α and IL-1β responses upon restimulation with either Ec-LPS or Pg-LPS (Fig. 1A to C). However, when the cells were pretreated with Pg-LPS, cytokine hyporesponsiveness upon secondary stimulation with either Pg-LPS or Ec-LPS was observed only for IL-1β (Fig. 1C). TNF-α responses were not affected when cells were exposed to 0.1 μg of Pg-LPS/ml prior to restimulation (Fig. 1A) but were significantly enhanced (P < 0.05) when 1 μg of Pg-LPS/ml was used to pretreat the cells followed by restimulation with the same stimulus (Fig. 1B). These data show that Ec-LPS makes cells tolerant to subsequent cytokine induction by itself or Pg-LPS, although the reverse depended on the cytokine measured and the Pg-LPS dose used.
FIG. 1.
Cross-tolerance between Ec-LPS and Pg-LPS at the cytokine induction level. THP-1 cells were pretreated for 24 h with either medium only or LPS molecules. Following removal of the culture supernatants and washing, the cells were restimulated as indicated in the figures. Ec-LPS or Pg-LPS was used at either 0.1 (A and C) or 1 (B) μg/ml. Culture supernatants were collected after overnight incubation and assayed for TNF-α (A and B) or IL-1β (C) responses. Results are presented as means ± standard deviations of triplicate determinations. Groups pretreated with medium and similarly restimulated with medium had undetectable cytokine levels at the concentration tested (1/10). Asterisks indicate groups the values of which are significantly different (P < 0.05) from those of corresponding medium only-pretreated THP-1 groups.
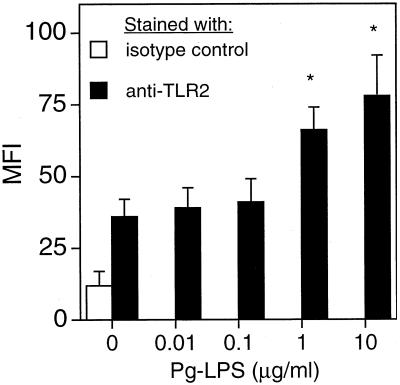
Pg-LPS dose-dependently upregulates surface TLR2 expression.
The property of Pg-LPS-pretreated cells of responding with enhanced TNF-α release upon restimulation with Pg-LPS (Fig. 1B) suggested that this effect was unlikely to involve Pg-LPS-induced alterations of signal intermediates that are common between the TLR2 and TLR4 pathways. The reason is that such an enhancing effect was not observed when restimulation was done with Ec-LPS. Moreover, the lack of enhanced secondary TNF-α responses when cells were pretreated with only 0.1 μg of Pg-LPS/ml (Fig. 1A) suggested that this is a dose-response effect. We thus determined the effect of increasing concentrations of Pg-LPS on the expression of TLR2 by flow-cytometric analysis. TLR2 surface expression was significantly (P < 0.05) upregulated by Pg-LPS at ⩾1 μg/ml but was unaffected at ⩽0.1 μg/ml (Fig. 2), which correlated with the results of TNF-α release (Fig. 1A and B). Therefore, upregulation of TLR2 expression during primary exposure of cells to Pg-LPS may account for enhanced TNF-α induction upon restimulation with Pg-LPS.
FIG. 2.
Dose-dependent effect of Pg-LPS on TLR2 surface expression. THP-1 cells were treated with increasing concentrations of Pg-LPS for 24 h and then were stained with FITC by means of anti-TLR2 MAb or isotype-matched control IgG1. Receptor expression was analyzed by flow cytometry. Data are shown as mean fluorescent intensity (MFI) values ± standard deviations of triplicate determinations. Values that are significantly different (P < 0.05) from that of the unstimulated and TLR2-stained group are indicated by an asterisk.
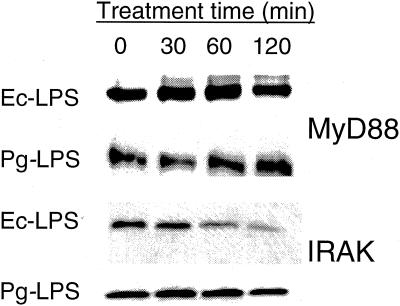
Pg-LPS does not diminish IRAK levels.
Upon TLR activation, IRAK is recruited by MyD88 to the receptor complex and subsequently relays the signal downstream (1). Since IRAK levels are diminished upon Ec-LPS activation and remain low even after restimulation (18), it is believed that this represents a major tolerance induction mechanism. In the absence of reported information on the effect of Pg-LPS on IRAK expression, we monitored IRAK levels by Western blot analysis in THP-1 cells exposed to 1 μg of Pg-LPS or Ec-LPS/ml. In contrast to Ec-LPS-treated cells, where IRAK expression was substantially reduced after 120 min of activation, Pg-LPS-treated cells maintained constant IRAK levels (Fig. 3). IRAK expression levels remained steady even after 24 h of primary stimulation with Pg-LPS or upon restimulation (data not shown). MyD88 levels were monitored in parallel but were not affected by either LPS molecule (Fig. 3). Pg-LPS, therefore, behaves differently from Ec-LPS with regard to regulation of IRAK levels, and this may at least partially account for their differential effects on cross-tolerance induction.
FIG. 3.
IRAK levels are not reduced in Pg-LPS-treated THP-1 cells. THP-1 cells were exposed to 1 μg of either Ec-LPS or Pg-LPS/ml for the indicated amount of time. At each time point cellular lysates were prepared and subsequently analyzed by Western blotting for MyD88 and IRAK expression using specific antibodies.
Cross-tolerance at the NF-κB activation level.
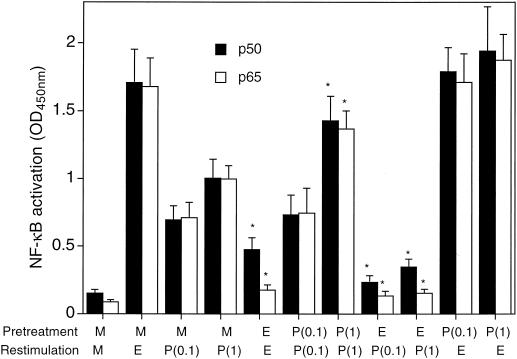
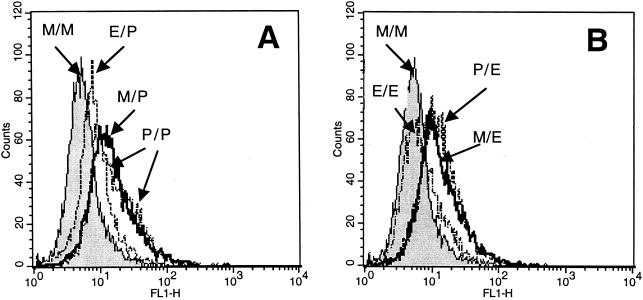
Stimulation of medium only-pretreated THP-1 cells with either Ec-LPS or Pg-LPS resulted in activation of both the p50 and p65 subunits of NF-κB (Fig. 4). However, restimulation of Ec-LPS-pretreated cells with either Ec-LPS or Pg-LPS resulted in significantly (P < 0.05) reduced activation of NF-κB and especially of its p65 subunit (Fig. 4). In contrast, NF-κB activation was not inhibited in Pg-LPS-pretreated cells when restimulated with Pg-LPS itself or Ec-LPS (Fig. 4), which is consistent with the finding that IRAK levels are maintained in Pg-LPS-treated cells (Fig. 3). Moreover, both p50 and p65 subunits were upregulated upon restimulation with Pg-LPS of Pg-LPS-pretreated cells when concentrations of 1 μg/ml, but not 0.1 μg/ml, were used (Fig. 4); this correlated with the findings of dose-dependent upregulation of TLR2 expression (Fig. 2) and of TNF-α release (Fig. 1A and B). To determine whether reduced p50 and p65 NF-κB subunit activation (observed in cross-tolerance experiments involving Ec-LPS-pretreated cells) results in reduced NF-κB-dependent transcription, we employed the 3E10/huTLR2 reporter cell line. This clone overexpresses transfected human TLR2 but also contains endogenous hamster TLR4 and is thus responsive to Ec-LPS (19). Pg-LPS upregulated NF-κB-dependent expression of CD25 in medium only-pretreated cells and in Pg-LPS-pretreated cells, but this Pg-LPS activity was inhibited in Ec-LPS-pretreated cells (Fig. 5A). However, the observed cross-tolerance was one-sided, since Pg-LPS did not make the cells tolerant to subsequent NF-κB activation by Ec-LPS (Fig. 5B). In contrast, in the same experiment, Ec-LPS was capable of inducing tolerance in the cells to a secondary stimulation with the same LPS molecule (Fig. 5B).
FIG. 4.
NF-κB p50 and p65 activation in Ec-LPS and Pg-LPS cross-tolerance. THP-1 cells were pretreated for 20 h with medium only (M), Ec-LPS at 0.1 μg/ml (E), or Pg-LPS at 0.1 [P(0.1)] or 1.0 [P(1)] μg/ml, followed by restimulation for 1 h as indicated. NF-κB activation in cellular extracts was analyzed using an NF-κB p50/p65 ELISA-based assay kit, and results are shown as means ± standard deviations of triplicate determinations. Asterisks indicate LPS-restimulated groups the values of which are significantly different (P < 0.05) from those of corresponding medium only-pretreated groups.
FIG. 5.
Cross-tolerance at the NF-κB-dependent transcription level. 3E10/huTLR2 cells were pretreated for 5 h with medium (M) or LPS. Ec-LPS (E) was used at 0.1 μg/ml (A), and Pg-LPS (P) was used at 1 μg/ml (B). The cells were subsequently restimulated for 20 h with the same concentrations of Pg-LPS (A) or Ec-LPS (B). NF-κB-dependent surface expression of CD25 was evaluated by flow cytometry. The mean fluorescent intensity values of a typical experiment shown above were as follows. (A) M/M, 6.4; E/P, 9.9; M/P, 18.5; P/P, 20.8; (B) M/M, 6.4; P/E, 15. 9; M/E, 15.8; and E/E, 8.5.
Inhibitory effects of Pg-LPS on cellular activation by Ec-LPS or rFimA.
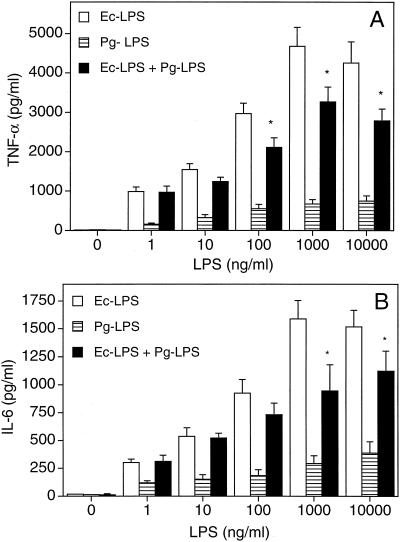
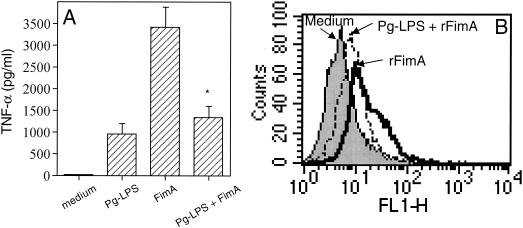
The concurrent addition of increasing concentrations of Pg-LPS and Ec-LPS in THP-1 cultures did not result in additive or synergistic effects on TNF-α (Fig. 6A) or IL-6 release (Fig. 6B). The relatively high levels of cytokine release induced by Ec-LPS alone tended to be downregulated in the presence of Pg-LPS, and the differences reached statistical significance for LPS concentrations of ⩾100 and ⩾1,000 ng/ml for TNF-α (Fig. 6A) and IL-6 (Fig. 6B) responses, respectively. To determine whether this inhibitory effect of Pg-LPS was not restricted against LPS molecules, we incubated THP-1 cells or 3E10/huTLR4 cells with Pg-LPS (10 μg/ml) and the proinflammatory FimA protein (1 μg/ml) from P. gingivalis. Because of the presence of polymyxin B in the cell cultures exposed to rFimA, we chose a dose of Pg-LPS which is completely resistant to inhibition by high concentrations of polymyxin B (14; also our observations). Pg-LPS was found to suppress rFimA-induced TNF-α release in THP-1 cells (Fig. 7A) and NK-κB-dependent expression of CD25 in 3E10/huTLR4 (Fig. 7B).
FIG. 6.
Inhibitory effect of Pg-LPS on Ec-LPS-induced cytokine release. THP-1 cells were incubated overnight with increasing concentrations of Ec-LPS, Pg-LPS, or both. Culture supernatants were subsequently collected and assayed for TNF-α (A) or IL-6 (B) release. Results are presented as means ± standard deviations of triplicate determinations. Asterisks indicate statistically significant (P < 0.05) inhibition in comparison to Ec-LPS treatment only.
FIG. 7.
Pg-LPS inhibits rFimA-induced cellular activation. (A) THP-1 cells were exposed to Pg-LPS (10 μg/ml), rFimA (1 μg/ml), or both. After overnight incubation, culture supernatants were collected and assayed for TNF-α release. Results are presented as means ± standard deviations of triplicate determinations. The asterisk indicates statistically significant (P < 0.05) inhibition in comparison to rFimA treatment only. (B) 3E10/huTLR4 cells were incubated overnight with medium, rFimA, or rFimA plus Pg-LPS using the same concentrations as above. NF-κB-dependent surface expression of CD25 was evaluated by flow cytometry. The mean fluorescent intensity values of a typical experiment shown above were as follows. Medium, 6.7; rFimA, 20.8; Pg-LPS plus rFimA, 10.5.
DISCUSSION
The results of the present study show that cellular activation by Ec-LPS or Pg-LPS can be cross-regulated, although different mechanisms are used by each LPS molecule. Ec-LPS can effectively make cells tolerant against subsequent activation by Pg-LPS, whereas Pg-LPS antagonizes the effects of Ec-LPS when cells are exposed simultaneously to both LPS molecules. Such anti-inflammatory mechanisms during mixed infections appear to have a survival value both for the pathogens and, in cases of extreme inflammatory reactions, for the host.
It was rather puzzling that induction of IL-1β, but not of TNF-α, was downregulated in cells previously exposed to Pg-LPS and restimulated with either Pg-LPS itself or Ec-LPS. Pg-LPS-pretreated cells maintain stable IRAK levels and effectively activate NF-κB upon restimulation, including its transactivating p65 subunit. Therefore, the reduced levels of IL-1β observed in secondary stimulation may not be attributable to toleragenic mechanisms involving NF-κB. Although a weak stimulus for proinflammatory cytokines, Pg-LPS is a potent inducer of IL-1R antagonist (IL-1RA) (29), the expression of which is persistently elevated in the endotoxin-tolerant state (15). High levels of IL-1RA during subsequent stimulation of Pg-LPS-pretreated cells may downregulate secondary IL-1β responses by blocking the binding of IL-1β to IL-1R and thereby inhibiting the positive feedback loop for IL-1β production (7).
Decreased levels of IRAK in cells exposed to Ec-LPS correlated with downregulation of the DNA binding activity of the p50 subunit and especially of the p65 subunit of NF-κB upon restimulation with Ec-LPS itself or Pg-LPS. Predominance of p50-p50 homodimers over p50-p65 heterodimers has been observed in the tolerant state (43). Since p50 possesses promoter-binding activity but, unlike p65, lacks a transactivation domain, p50-p50 homodimers can block access of transactivating p50-p65 complexes to the promoter, resulting in transcriptional repression (13). In contrast to cells exposed to Ec-LPS, cells treated with Pg-LPS maintained stable levels of IRAK that would presumably allow efficient relay of signals leading to degradation of Iκβ proteins and NF-κB translocation into the nucleus for transcription of target genes upon restimulation (12). Indeed, IRAK has been shown to be involved in Pg-LPS-induced signaling. Treatment of human gingival fibroblasts with Pg-LPS results in activation of several intracellular proteins, including IRAK (38). Moreover, stimulation of MyD88-deficient mouse macrophages with Pg-LPS led to significantly reduced activation of NF-κB compared to wild-type mouse macrophages (M. Martin, unpublished observation). Since the MyD88-dependent pathway is required for IRAK activation (1), this finding implies that IRAK is an essential component in P. gingivalis-induced signaling which leads to NF-κB activation.
The priming of NF-κB activation and TNF-α induction in cells pretreated and reactivated with 1 μg of Pg-LPS/ml may be due to the observed upregulation of TLR2 expression at this concentration. In this regard, pretreatment of cells with Pg-LPS primes TNF-α responses induced by Bacteroides forsythus protein A, a TLR2 agonist, but not by P. gingivalis FimA, a TLR4 agonist (8; also unpublished observations). NF-κB is involved in positive regulation of the TLR2 gene, the promoter region of which contains two NF-κB binding sites (26). Thus, the ability of Pg-LPS-treated cells to maintain NF-κB activity even after restimulation may result in continuous expression of high levels of surface TLR2, which in turn may lead to further activation of NF-κB, leading to cytokine induction. It appears that for relatively weak inducers of inflammation (such as Pg-LPS), which are unlikely to cause excessive inflammation on their own, the innate immune system might be programmed to keep sensing their presence and maintaining cytokine responses.
Other TLR2 agonists were shown to behave differently from Pg-LPS regarding interactions with enterobacterial LPS (16, 23, 36). For example, the mycoplasmal lipopeptide MALP-2 induces tolerance against subsequent enterobacterial LPS-mediated cellular activation, but simultaneous addition of MALP-2 and LPS in the cell cultures results in synergistic activation (36). However, these differences may not be surprising considering that TLR2 can have functional associations with other TLRs (30), thereby diversifying the possible outcomes of TLR2 activation. It has also been observed that different TLR4 agonists induce differential cell activation (37), and it is possible that the use of different coreceptors or of different TLR interfaces involved in pathogen recognition may influence the intensity or quality of the induced signals.
The antagonistic effect of Pg-LPS on Ec-LPS-induced cytokine release could be explained by competition for the use of common receptors (CD14 and TLRs) or signal intermediates. However, the finding that Pg-LPS inhibited rFimA-mediated activation of 3E10/huTLR4, which is not responsive to Pg-LPS (2; also our unpublished data) due to lack of TLR2 expression (19), suggests that sequestration of common signaling intermediates is not a likely mechanism. Although Pg-LPS and Ec-LPS display different binding interactions with CD14 (3), competition for binding to CD14 cannot be theoretically ruled out, since steric hindrance is always possible when relatively large molecules are involved. However, it is not very likely that CD14 may become a limiting factor in our test system, especially since antagonistic effects were observed when Ec-LPS and Pg-LPS were each used at 100 ng/ml (Fig. 6A) and cytokine induction by Ec-LPS alone was enhanced with increasing concentrations of up to 1 μg/ml (Fig. 6A). Rhodobacter sphaeroides lipid A has the unusual property of acting as an antagonist for human and mouse TLR4 but as an agonist for hamster TLR4 (19). Similarly, Pg-LPS does not activate TLR4 in the mouse (11) or human (8, 21) system but appears to be a hamster TLR4 agonist (40). It is thus possible that Pg-LPS may antagonize TLR4-transduced signals in a species-dependent way like R. sphaeroides lipid A. Indeed, recent reports indicate that Pg-LPS antagonizes certain LPS molecules that are TLR4 agonists (4, 40). Pg-LPS might, therefore, be able to bind to but not activate human TLR4 and block access of TLR4 agonists, including Ec-LPS and P. gingivalis FimA, to the receptor complex.
Research in the past 5 years has established TLRs as mediators of communication between innate host defense and microbial virulence molecules. The availability of a variety of TLRs with distinct recognition properties, yet with (at least partially) shared signal transduction pathways that allow cross talk, may help coordinate the host response against the combined action of pathogen-associated molecular patterns with differential TLR agonist activity.
Acknowledgments
We thank D. T. Golenbock for providing the 3E10 cell lines.
This work was supported by Public Health Service Grant no. DE07034.
Editor: B. B. Finlay
REFERENCES
- 1.Akira, S. 2001. Toll-like receptors and innate immunity. Adv. Immunol. 78:1-56. [DOI] [PubMed] [Google Scholar]
- 2.Bainbridge, B. W., and R. P. Darveau. 2001. Porphyromonas gingivalis lipopolysaccharide: an unusual pattern recognition receptor ligand for the innate host defense system. Acta Odontol. Scand. 59:131-138. [DOI] [PubMed] [Google Scholar]
- 3.Cunningham, M. D., R. A. Shapiro, C. Seachord, K. Ratcliff, L. Cassiano, and R. P. Darveau. 2000. CD14 employs hydrophilic regions to “capture” lipopolysaccharides. J. Immunol. 164:3255-3263. [DOI] [PubMed] [Google Scholar]
- 4.Darveau, R. P., S. Arbabi, I. Garcia, B. Bainbridge, and R. V. Maier. 2002. Porphyromonas gingivalis lipopolysaccharide is both agonist and antagonist for p38 mitogen-activated protein kinase activation. Infect. Immun. 70:1867-1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flo, T. H., O. Halaas, S. Torp, L. Ryan, E. Lien, B. Dybdahl, A. Sundan, and T. Espevik. 2001. Differential expression of Toll-like receptor 2 in human cells. J. Leukoc. Biol. 69:474-481. [PubMed] [Google Scholar]
- 6.Golenbock, D. T., and M. J. Fenton. 2001. Extolling the diversity of bacterial endotoxins. Nat. Immunol. 2:286-288. [DOI] [PubMed] [Google Scholar]
- 7.Granowitz, E. V., E. Vannier, D. D. Poutsiaka, and C. A. Dinarello. 1992. Effect of interleukin-1 (IL-1) blockade on cytokine synthesis. II. IL-1 receptor antagonist inhibits lipopolysaccharide-induced cytokine synthesis by human monocytes. Blood 79:2364-2369. [PubMed] [Google Scholar]
- 8.Hajishengallis, G., M. Martin, H. T. Sojar, A. Sharma, R. E. Schifferle, E. DeNardin, M. W. Russell, and R. J. Genco. 2002. Dependence of bacterial protein adhesins on Toll-Like receptors for proinflammatory cytokine induction. Clin. Diagn. Lab. Immunol. 9:403-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haraszthy, V. I., J. J. Zambon, M. Trevisan, M. Zeid, and R. J. Genco. 2000. Identification of periodontal pathogens in atheromatous plaques. J. Periodontol. 71:1554-1560. [DOI] [PubMed] [Google Scholar]
- 10.Hirschfeld, M., Y. Ma, J. H. Weis, S. N. Vogel, and J. J. Weis. 2000. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine Toll-like receptor 2. J. Immunol. 165:618-622. [DOI] [PubMed] [Google Scholar]
- 11.Hirschfeld, M., J. J. Weis, V. Toshchakov, C. A. Salkowski, M. J. Cody, D. C. Ward, N. Qureshi, S. M. Michalek, and S. N. Vogel. 2000. Signaling by Toll-like receptor 2 and 4 agonists results in differential gene expression in murine macrophages. Infect. Immun. 69:1477-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karin, M., and M. Delhase. 2000. The IκB kinase (IKK) and NF-κB: key elements of proinflammatory signalling. Semin. Immunol. 12:85-98. [DOI] [PubMed] [Google Scholar]
- 13.Kastenbauer, S., and H. W. Zeigler-Heitbrock. 1999. NF-κB1 (p50) is upregulated in lipopolysaccharide tolerance and can block tumor necrosis factor gene expression. Infect. Immun. 67:1553-1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirikae, T., T. Nitta, F. Kirikae, Y. Suda, S. Kusumoto, N. Qureshi, and M. Nakano. 1999. Lipopolysaccharides (LPS) of oral black-pigmented bacteria induce tumor necrosis factor production by LPS-refractory C3H/HeJ macrophages in a way different from that of Salmonella LPS. Infect. Immun. 67:1736-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Learn, C. A., S. B. Mizel, and C. E. McCall. 2000. mRNA and protein stability regulate the differential expression of pro- and anti-inflammatory genes in endotoxin-tolerant THP-1 cells. J. Biol. Chem. 275:12185-12193. [DOI] [PubMed] [Google Scholar]
- 16.Lehner, M. D., S. Morath, K. S. Michelsen, R. R. Schumann, and T. Hartung. 2001. Induction of cross-tolerance by lipopolysaccharide and highly purified lipoteichoic acid via different Toll-like receptors independent of paracrine mediators. J. Immunol. 166:5161-5167. [DOI] [PubMed] [Google Scholar]
- 17.Lemaitre, B., E. Nicolas, L. Michaut, J. M. Reichhart, and J. A. Hoffmann. 1996. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 86:973-983. [DOI] [PubMed] [Google Scholar]
- 18.Li, L., S. Cousart, J. Hu, and C. E. McCall. 2000. Characterization of interleukin-1 receptor-associated kinase in normal and endotoxin-tolerant cells. J. Biol. Chem. 275:23340-23345. [DOI] [PubMed] [Google Scholar]
- 19.Lien, E., T. K. Means, H. Heine, A. Yoshimura, S. Kusumoto, K. Fukase, M. J. Fenton, M. Oikawa, N. Qureshi, B. Monks, R. W. Finberg, R. R. Ingalls, and D. T. Golenbock. 2000. Toll-like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide. J. Clin. Investig. 105:497-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loesche, W. J. 1999. Anaerobic periodontal infections as risk factors for medical diseases. Curr. Infect. Dis. Rep. 1:33-38. [DOI] [PubMed] [Google Scholar]
- 21.Martin, M., J. Katz, S. N. Vogel, and S. M. Michalek. 2001. Differential induction of endotoxin tolerance by lipopolysaccharides derived from Porphyromonas gingivalis and Escherichia coli. J. Immunol. 167:5278-5285. [DOI] [PubMed] [Google Scholar]
- 22.Matsuguchi, T., T. Musikacharoen, T. Ogawa, and Y. Yoshikai. 2000. Gene expressions of Toll-like receptor 2, but not Toll-like receptor 4, is induced by LPS and inflammatory cytokines in mouse macrophages. J. Immunol. 165:5767-5772. [DOI] [PubMed] [Google Scholar]
- 23.Medvedev, A. E., P. Henneke, A. Schromm, E. Lien, R. Ingalls, M. J. Fenton, D. T. Golenbock, and S. N. Vogel. 2001. Induction of tolerance to lipopolysaccharide and mycobacterial components in Chinese hamster ovary/CD14 cells is not affected by overexpression of Toll-like receptors 2 or 4. J. Immunol. 167:2257-2267. [DOI] [PubMed] [Google Scholar]
- 24.Medvedev, A. E., K. M. Kopydlowski, and S. N. Vogel. 2000. Inhibition of lipopolysaccharide-induced signal transduction in endotoxin-tolerized mouse macrophages: dysregulation of cytokine, chemokine, and toll-like receptor 2 and 4 gene expression. J. Immunol. 164:5564-5574. [DOI] [PubMed] [Google Scholar]
- 25.Medzhitov, R., P. Preston-Hurlburt, and C. A. Janeway. 1997. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388:394-397. [DOI] [PubMed] [Google Scholar]
- 26.Musikacharoen, T., T. Matsuguchi, T. Kikuchi, and Y. Yoshikai. 2001. NF-κB and STAT5 play important roles in the regulation of mouse Toll-like receptor 2 gene expression. J. Immunol. 166:4516-4524. [DOI] [PubMed] [Google Scholar]
- 27.Nomura, F., S. Akashi, Y. Sakao, S. Sato, T. Kawai, M. Matsumoto, K. Nakanishi, M. Kimoto, K. Miyake, K. Takeda, and S. Akira. 2000. Endotoxin tolerance in mouse macrophages correlates with down-regulation of surface Toll-like receptor 4 expression. J. Immunol. 164:3476-3479. [DOI] [PubMed] [Google Scholar]
- 28.Ogawa, T. 1994. Immunobiological properties of chemically defined lipid A from lipopolysaccharide of Porphyromonas (Bacteroides) gingivalis. Eur. J. Biochem. 219:737-742. [DOI] [PubMed] [Google Scholar]
- 29.Ogawa, T., H. Uchida, and K. Amino. 1994. Immunobiological activities of chemically defined lipid A from lipopolysaccharides of Porphyromonas gingivalis. Microbiology 140:1209-1216. [DOI] [PubMed] [Google Scholar]
- 30.Ozinsky, A., D. M. Underhill, J. D. Fontenot, A. M. Hajjar, K. D. Smith, C. B. Wilson, L. Schroeder, and A. Aderem. 2000. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc. Natl. Acad. Sci. USA 97:13766-13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. V. Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 32.Preshaw, P. M., R. E. Schifferle, and J. D. Walters. 1999. Porphyromonas gingivalis lipopolysaccharide delays human polymorphonuclear leukocyte apoptosis in vitro. J. Periodont. Res. 34:197-202. [DOI] [PubMed] [Google Scholar]
- 33.Pugin, J., C. C. Schurer-Maly, D. Leturcq, A. Moriarty, R. J. Ulevitch, and P. S. Tobias. 1993. Lipopolysaccharide activation of human endothelial and epithelial cells is mediated by lipopolysaccharide-binding protein and soluble CD14. Proc. Natl. Acad. Sci. USA 90:2744-2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qureshi, S. T., L. Lariviere, G. Leveque, S. Clermont, K. J. Moore, P. Gros, and D. Malo. 1999. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4). J. Exp. Med. 189:615-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roberts, F. A., G. Richardson, and S. M. Michalek. 1997. Effects of Porphyromonas gingivalis and Escherichia coli lipopolysaccharides on mononuclear phagocytes. Infect. Immun. 65:3248-3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sato, S., F. Nomura, T. Kawai, O. Takeuchi, P. F. Mühlradt, K. Takeda, and S. Akira. 2000. Synergy and cross-tolerance between Toll-like receptor (TLR) 2- and TLR4-mediated signaling pathways. J. Immunol. 165:7096-7101. [DOI] [PubMed] [Google Scholar]
- 37.Shoham, S., C. Huang, J. M. Chen, D. T. Golenbock, and S. M. Levitz. 2001. Toll-like receptor 4 mediates intracellular signalling without TNF-α release in response to Cryptococcus neoformans polysaccharide capsule. J. Immunol. 166:4620-4626. [DOI] [PubMed] [Google Scholar]
- 38.Wang, P.-L., and K. Ohura. 2002. Porphyromonas gingivalis lipopolysaccharide signaling in gingival fibroblasts—CD14 and Toll-like receptors. Crit. Rev. Oral Biol. Med. 13:132-142. [DOI] [PubMed] [Google Scholar]
- 39.Wright, S. D. 1999. Toll, a new piece in the puzzle of innate immunity. J. Exp. Med. 189:605-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshimura, A., T. Kaneko, Y. Kato, D. T. Golenbock, and Y. Hara. 2002. Lipopolysaccharides from periodontopathic bacteria Porphyromonas gingivalis and Capnocytophaga ochracea are antagonists for human toll-like receptor 4. Infect. Immun. 70:218-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zambon, J. J., S. Grossi, R. Dunford, V. I. Harazsthy, H. Preus, and R. J. Genco. 1994. Epidemiology of subgingival bacterial pathogens in periodontal diseases, p. 3-12. In R. J. Genco, S. Hamada, J. R. Lehrer, J. R. McGhee, and S. Mergenhangen (ed.), Molecular pathogenesis of periodontal disease. American Society for Microbiology, Washington, D.C.
- 42.Ziegler-Heitbrock, H. W. 1995. Molecular mechanism in tolerance to lipopolysaccharide. J. Inflamm. 45:13-26. [PubMed] [Google Scholar]
- 43.Ziegler-Heitbrock, H. W. L., A. Wedel, W. Schraut, M. Ströbel, P. Wendelgass, T. Sternsdorf, P. A. Bäuerle, J. G. Haas, and G. Riethmüller. 1994. Tolerance to lipopolysaccharide involves mobilization of nuclear factor κB with predominance of p50 homodimers. J. Biol. Chem. 269:17001-17004. [PubMed] [Google Scholar]