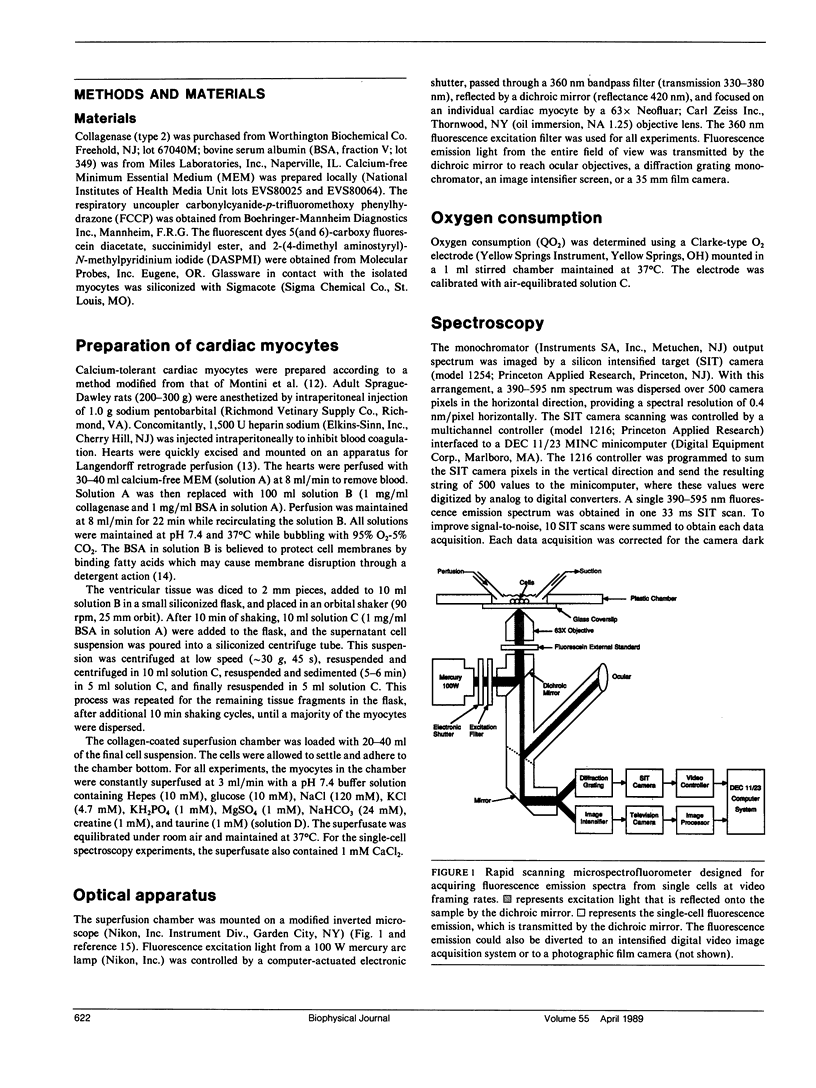
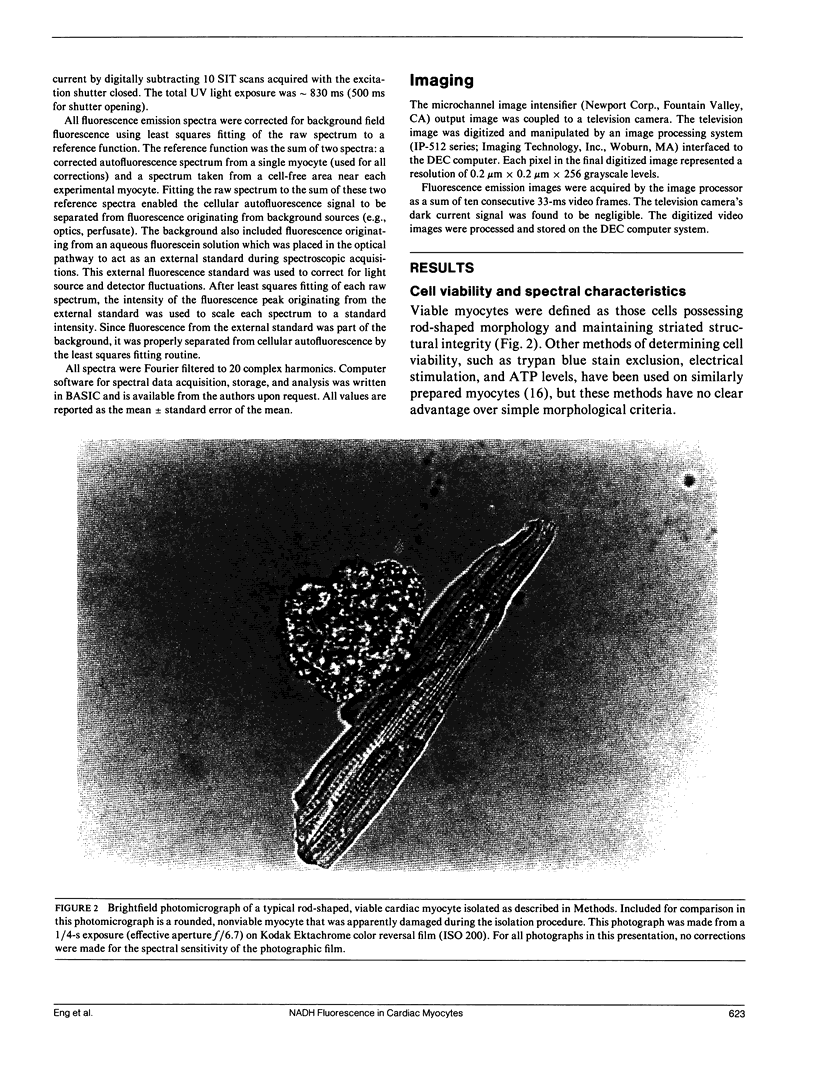
Abstract
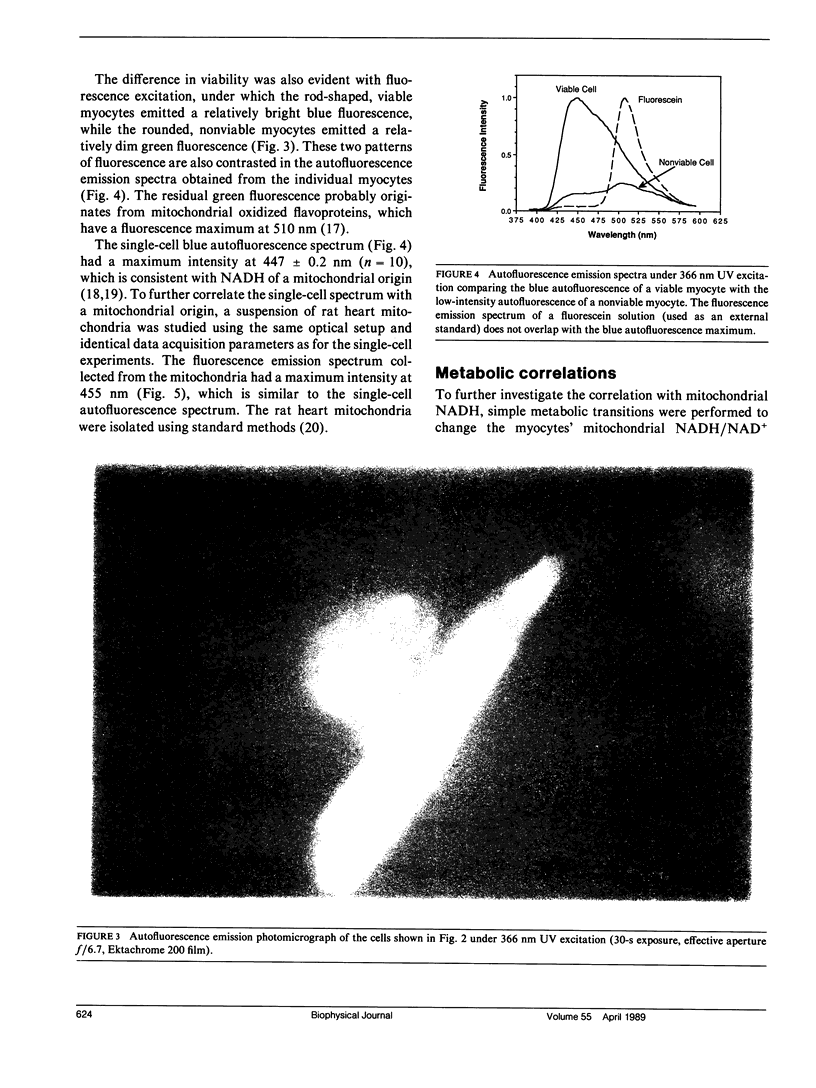
Nicotinamide adenine dinucleotide (NADH) plays a critical role in oxidative phosphorylation as the primary source of reducing equivalents to the respiratory chain. Using a modified fluorescence microscope, we have obtained spectra and images of the blue autofluorescence from single rat cardiac myocytes. The optical setup permitted rapid acquisition of fluorescence emission spectra (390-595 nm) or intensified digital video images of individual myocytes. The spectra showed a broad fluorescence centered at 447 +/- 0.2 nm, consistent with mitochondrial NADH. Addition of cyanide resulted in a 100 +/- 10% increase in fluorescence, while the uncoupler FCCP resulted in a 82 +/- 4% decrease. These two transitions were consistent with mitochondrial NADH and implied that the myocytes were 44 +/- 6% reduced under the resting control conditions. Intracellular fluorescent structures were observed that correlated with the distribution of a mitochondrial selective fluorescent probe (DASPMI), the mitochondrial distribution seen in published electron micrographs, and a metabolic digital subtraction image of the cyanide fluorescence transition. These data are consistent with the notion that the blue autofluorescence of rat cardiac myocytes originates from mitochondrial NADH.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balaban R. S., Mandel L. J. Metabolic substrate utilization by rabbit proximal tubule. An NADH fluorescence study. Am J Physiol. 1988 Mar;254(3 Pt 2):F407–F416. doi: 10.1152/ajprenal.1988.254.3.F407. [DOI] [PubMed] [Google Scholar]
- Bereiter-Hahn J. Dimethylaminostyrylmethylpyridiniumiodine (daspmi) as a fluorescent probe for mitochondria in situ. Biochim Biophys Acta. 1976 Jan 15;423(1):1–14. doi: 10.1016/0005-2728(76)90096-7. [DOI] [PubMed] [Google Scholar]
- Bihler I., Jeanrenaud B. ATP content of isolated fat cells. Effects of insulin, ouabain, and lipolytic agents. Biochim Biophys Acta. 1970 May 5;202(3):496–506. doi: 10.1016/0005-2760(70)90120-7. [DOI] [PubMed] [Google Scholar]
- CHANCE B., BALTSCHEFFSKY H. Respiratory enzymes in oxidative phosphorylation. VII. Binding of intramitochondrial reduced pyridine nucleotide. J Biol Chem. 1958 Sep;233(3):736–739. [PubMed] [Google Scholar]
- CHANCE B., COHEN P., JOBSIS F., SCHOENER B. Intracellular oxidation-reduction states in vivo. Science. 1962 Aug 17;137(3529):499–508. doi: 10.1126/science.137.3529.499. [DOI] [PubMed] [Google Scholar]
- CHANCE B., THORELL B. Localization and kinetics of reduced pyridine nucleotide in living cells by microfluorometry. J Biol Chem. 1959 Nov;234:3044–3050. [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- Chance B., Schoener B., Oshino R., Itshak F., Nakase Y. Oxidation-reduction ratio studies of mitochondria in freeze-trapped samples. NADH and flavoprotein fluorescence signals. J Biol Chem. 1979 Jun 10;254(11):4764–4771. [PubMed] [Google Scholar]
- Cheung J. Y., Leaf A., Bonventre J. V. Determination of isolated myocyte viability: staining methods and functional criteria. Basic Res Cardiol. 1985;80 (Suppl 1):23–29. doi: 10.1007/978-3-662-11041-6_4. [DOI] [PubMed] [Google Scholar]
- ESTABROOK R. W. Fluorometric measurement of reduced pyridine nucleotide in cellular and subcellular particles. Anal Biochem. 1962 Sep;4:231–245. doi: 10.1016/0003-2697(62)90006-4. [DOI] [PubMed] [Google Scholar]
- Frank J. S., Brady A. J., Farnsworth S., Mottino G. Ultrastructure and function of isolated myocytes after calcium depletion and repletion. Am J Physiol. 1986 Feb;250(2 Pt 2):H265–H275. doi: 10.1152/ajpheart.1986.250.2.H265. [DOI] [PubMed] [Google Scholar]
- Hansford R. G. Relation between mitochondrial calcium transport and control of energy metabolism. Rev Physiol Biochem Pharmacol. 1985;102:1–72. doi: 10.1007/BFb0034084. [DOI] [PubMed] [Google Scholar]
- KOHEN E. PYRIDINE NUCLEOTIDE COMPARTMENTALIZATION IN GLASS-GROWN ASCITES CELLS. Exp Cell Res. 1964 Jul;35:303–316. doi: 10.1016/0014-4827(64)90097-7. [DOI] [PubMed] [Google Scholar]
- Katz L. A., Koretsky A. P., Balaban R. S. Respiratory control in the glucose perfused heart. A 31P NMR and NADH fluorescence study. FEBS Lett. 1987 Sep 14;221(2):270–276. doi: 10.1016/0014-5793(87)80939-0. [DOI] [PubMed] [Google Scholar]
- Koretsky A. P., Katz L. A., Balaban R. S. Determination of pyridine nucleotide fluorescence from the perfused heart using an internal standard. Am J Physiol. 1987 Oct;253(4 Pt 2):H856–H862. doi: 10.1152/ajpheart.1987.253.4.H856. [DOI] [PubMed] [Google Scholar]
- Kurtz I., Balaban R. S. Fluorescence emission spectroscopy of 1,4-dihydroxyphthalonitrile. A method for determining intracellular pH in cultured cells. Biophys J. 1985 Sep;48(3):499–508. doi: 10.1016/S0006-3495(85)83805-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montini J., Bagby G. J., Burns A. H., Spitzer J. J. Exogenous substrate utilization in Ca2+-tolerant myocytes from adult rat hearts. Am J Physiol. 1981 Apr;240(4):H659–H663. doi: 10.1152/ajpheart.1981.240.4.H659. [DOI] [PubMed] [Google Scholar]
- Neely J. R., Rovetto M. J. Techniques for perfusing isolated rat hearts. Methods Enzymol. 1975;39:43–60. doi: 10.1016/s0076-6879(75)39008-3. [DOI] [PubMed] [Google Scholar]
- Nuutinen E. M., Hiltunen J. K., Hassinen I. E. The glutamate dehydrogenase system and the redox state of mitochondrial free nicotinamide adenine dinucleotide in myocardium. FEBS Lett. 1981 Jun 15;128(2):356–360. doi: 10.1016/0014-5793(81)80116-0. [DOI] [PubMed] [Google Scholar]
- Nuutinen E. M. Subcellular origin of the surface fluorescence of reduced nicotinamide nucleotides in the isolated perfused rat heart. Basic Res Cardiol. 1984 Jan-Feb;79(1):49–58. doi: 10.1007/BF01935806. [DOI] [PubMed] [Google Scholar]
- Vercesi A., Reynafarje B., Lehninger A. L. Stoichiometry of H+ ejection and Ca2+ uptake coupled to electron transport in rat heart mitochondria. J Biol Chem. 1978 Sep 25;253(18):6379–6385. [PubMed] [Google Scholar]