Abstract
Candida albicans is the causative agent of acute and recurrent vulvovaginal candidiasis (VVC), a common mucosal infection affecting significant numbers of women in their reproductive years. While any murine host protective role for cell-mediated immunity (CMI), humoral immunity, and innate resistance by neutrophils against the vaginal infection appear negligible, significant in vitro growth inhibition of Candida species by vaginal and oral epithelial cell-enriched cells has been observed. Both oral and vaginal epithelial cell anti-Candida activity has a strict requirement for cell contact to C. albicans with no role for soluble factors, and oral epithelial cells inhibit C. albicans through a cell surface carbohydrate moiety. The present study further evaluated the inhibitory mechanisms by murine vaginal epithelial cells and the fate of C. albicans by oral and vaginal epithelial cells. Similar to human oral cells, anti-Candida activity produced by murine vaginal epithelial cells is unaffected by enzymatic cleavage of cell surface proteins and lipids but sensitive to periodic acid cleavage of surface carbohydrates. Analysis of specific membrane carbohydrate moieties, however, showed no role for sulfated polysaccharides, sialic acid residues, or glucose and mannose-containing carbohydrates, also similar to oral cells. Staining for live and dead Candida in the coculture with fluorescein diacetate (FDA) and propidium iodide (PI), respectively, showed a clear predominance of live organisms, suggesting a static rather than cidal action. Together, the results suggest that oral and vaginal epithelial cells retard or arrest the growth rather than kill C. albicans through an as-yet-unidentified carbohydrate moiety in a noninflammatory manner.
Candida albicans, the causative agent of vulvovaginal candidiasis (VVC) and oropharyngeal candidiasis (OPC), is a dimorphic fungal organism that is a commensal of the gastrointestinal and reproductive tracts as well as an opportunistic pathogen of these same mucosal tissues (36). OPC is a significant problem in immunocompromised individuals and is extremely common during human immunodeficiency virus (HIV) infection, especially when CD4+ T cells are reduced (24, 26). In contrast, VVC is equally common in immunocompetent and immunocompromised individuals and affects approximately 75% of otherwise healthy women at least once during their child-bearing years (36, 37).
Understanding the complete repertoire of host defense mechanisms against mucosal candidiasis has been a challenge. Cell-mediated immunity (CMI) by Th1-type CD4+ T cells is considered the predominant host defense mechanism against mucosal C. albicans infections (7, 8). However, while local and systemic CMI appears to play a role in protection against OPC, both clinical and many experimental studies have found no role for systemic or local CMI in protection against VVC (16, 17, 19, 20, 44). Similarly, women suffering from recurrent VVC (RVVC) sustain normal levels of Candida-specific systemic Th1-type CMI, suggesting that any dysfunction or deficiency exists exclusively at the local level (17). Humoral immunity in protection against candidiasis is also uncertain. While clinical studies suggest that antibodies provide little to no protection against either OPC or VVC, certain Candida-specific antibodies have been shown to be protective in rat and mouse models of vaginal candidiasis (6, 11, 15, 40).
Uncertainty also currently surrounds any role for innate resistance against vaginal Candida infections. Despite the presence of polymorphonuclear leukocytes in vaginal lavage fluids of infected animals, there is no correlation to reduced vaginal fungal burden (16, 33). There is also no role for natural killer (NK) cells (2, 4, 5). Other innate mechanisms (i.e., macrophages and dendritic cells) against Candida at the oral and vaginal mucosa level are unknown. Recently, epithelial cells from the oral mucosa of humans and from the vaginal mucosa of humans, nonhuman primates, and mice have been shown to inhibit the growth of C. albicans in vitro (14, 38, 40, 41), suggesting a potential innate defense mechanism not previously considered. Interestingly, HIV-infected persons with OPC and women with RVVC have been shown to have reduced epithelial cell anti-Candida activity (3, 38), suggesting a possible contributing factor in susceptibility to either infection and providing an all-important clinical cause and effect relationship.
Compared to murine vaginal epithelial cells, oral epithelial cells have significantly greater activity at lower effector-to-target cell (E:T) ratios (38, 40). Regarding the properties of the epithelial cell anti-Candida activity, both oral and vaginal epithelial cells have a strict requirement for viable cell contact with Candida, with no demonstrable role for soluble factors (38, 40). However, inhibition does not involve phagocytosis or oxidative mechanisms or nonoxidative mechanisms through defensins or calprotectins (39). While the complete mechanism by which the epithelial cells function to inhibit Candida remains to be elucidated, enzymatic cleavage of carbohydrates, but not proteins or phospholipids, from oral epithelial cells eliminates the anti-Candida activity, suggesting a putative role for a carbohydrate moiety (39). The purpose of this study was to further define the requirements for the inhibitory properties by murine vaginal epithelial cells, as well as to determine whether oral and vaginal epithelial cells function to kill or simply retard or arrest the growth of Candida.
MATERIALS AND METHODS
Mice.
Female CBA/J (H-2K) mice (6 to 8 weeks old), purchased from the National Cancer Institute, Frederick, Md., were used throughout these studies. All animals were housed and handled according to institutionally recommended guidelines.
Human subjects.
Saliva was collected from healthy volunteers following informed consent and in accordance with the Institutional Review Board at Louisiana State University Health Sciences Center.
Vaginal epithelial cell isolation.
Vaginae from 10 sacrificed mice were excised and placed into a sterile glass petri dish containing 10 ml of dispase I-neutral protease (0.25 mg/ml; Roche Diagnostics, Mannheim, Germany) and incubated on a shaking platform (speed, 110 rpm) at 4°C for 8 h. The epithelial sheets were then easily removed from the intact vaginal tissue with forceps, minced with a sterile scalpel, and placed in a 15-ml sterile centrifuge tube. The remaining dispase solution was added to the cellular suspension and centrifuged at 800 × g for 5 min. The pellet was resuspended in 2 ml of 10× Trypsin-EDTA (Sigma, St. Louis, Mo.), and incubated at 37°C for 10 min. The suspension was then sheared using an 18-gauge needle (5 to 10 times), followed by a similar procedure using a 21-gauge needle. The cells were finally washed twice with sterile phosphate-buffered saline (PBS) and enumerated by trypan blue dye exclusion.
Oral epithelial cell isolation.
Human oral epithelial cells were isolated as previously described (38, 39). The expectorated saliva was centrifuged at 800 × g for 5 min. The cell pellet was washed with sterile PBS, resuspended in Hanks' balanced salt solution (Life Technologies, Gaithersburg, Md.), and passed over a 20 μm sterile nylon membrane (Small Parts Inc., Miami Lakes, Fla.). The epithelial cells retained on the membrane were collected and washed with Hanks' balanced salt solution, and the pellet was stored at −70°C in 1 ml of cryopreservative solution (50% fetal bovine serum [FBS], 25% RPMI 1640 tissue culture medium, 15% dimethyl sulfoxide) until use. At the time of use the cells were thawed, washed twice in PBS, and enumerated by trypan blue dye exclusion. Viability was consistently 80 to 85% before and after freezing.
Target cells.
C. albicans 3153A from the National Collection of Pathogenic Fungi (London, United Kingdom) was grown on Sabouraud dextrose agar (Becton Dickinson, Cockeysville, Md.) at 34°C. One colony was used to inoculate 10 ml of phytone-peptone (PP) broth (Becton Dickinson) supplemented with 0.1% glucose for 18 h at 25°C in a shaking water bath. The stationary-phase blastoconidia were collected, washed twice with PBS, and enumerated on a hemacytometer using trypan blue dye exclusion.
Growth inhibition assay. (i) [3H]glucose uptake.
As previously described (38-40), stationary-phase blastoconidia were added to individual wells of a 96-well microtiter plate (Costar, Cambridge, Mass.) at 105 cells/ml in a volume of 100 μl (PP broth) supplemented with 10% FBS, 1% penicillin (100 U/ml), and streptomycin (100 μg/ml). Epithelial (effector) cells at various E:T ratios were added to triplicate wells in a volume of 100 μl of PP broth. Controls included effector cells and target cells cultured separately. [3H]glucose (1 μCi/well; ICN, Costa Mesa, Calif.) was added to each well, and the culture was incubated for 9 h at 37°C, 5% CO2. Following this incubation, the wells were treated with 100 μl of sodium hypochlorite solution (bleach) for 5 min, and the cells were harvested onto glass fiber filter paper using a PHD cell harvester (Cambridge Technologies, Watertown, Mass.). The incorporated [3H]glucose in the cell lysates was measured by liquid scintillation counting. Candida characteristically incorporated 15,000 to 25,000 cpm, while epithelial cells incorporated 500 to 2,000 cpm. Percent growth inhibition was calculated as follows: % growth inhibition = [1 − (mean cpm for experimental cells − mean cpm for effector cells)/mean cpm Candida cells ] × 100.
(ii) Quantitative plate count.
Experiments involving periodic acid require a quantitative plate count method to measure the growth inhibition of C. albicans (38-40). Briefly, effector and target cell cocultures were prepared as described above in the absence of [3H]glucose. Following the incubation period, 100 μl of 0.3% Triton X-100 was added to each well, and adherent Candida cells were scraped from the surface while aspirating with a pipette tip. The contents of each well were then collected, serially diluted (1:10), and plated on Sabouraud dextrose agar plates. The numbers of CFU were determined after incubation for 48 h at 34°C. Wells containing C. albicans alone acted as controls. Percent growth inhibition was calculated as follows: % growth inhibition = (1 − CFU for experimental cells/CFU for Candida cells) × 100.
Epithelial cellular treatments.
Specific cell surface moieties on vaginal epithelial cells were examined in a manner similar to human oral epithelial cells (39). For examination of existing membrane-associated protein moieties, murine vaginal epithelial cells were pretreated with proteinase K for 10 min at 37°C (25 μg/ml; Roche Corp, Mannheim, Germany) in a volume of 1 ml of PBS (28, 39). The control included epithelial cells incubated in PBS alone. To confirm the action of proteinase K, supernatants from treated and control cells were assayed for total protein by the bicinchoninic acid protein assay (Pierce, Rockford, Ill.) with bovine serum albumin (Sigma) as the standard (39). Absorbance at 595 nm using an automated plate reader (Labsystems Multiskan Ascent, Helsinki, Finland) was used to determine the released protein concentration (in milligrams per milliliter).
For examination of lipid moieties, murine vaginal epithelial cells were pretreated with phospholipase A2 (PLA2) (50 U/ml; Sigma) for 30 min at 37°C in a volume of 1 ml of PBS (9, 31, 39). To confirm PLA2 enzymatic activity, PLA2 was incubated with autoclaved [14C]oleic acid-labeled Escherichia coli for 1 h at 37°C as previously described (13, 39). The hydrolysis products released into the supernatant were trapped by the addition of 0.5% bovine serum albumin (Sigma). The samples were then centrifuged to sediment undegraded lipids, and the trapped products of hydrolysis (free [14C]oleic acid) in the supernatants were quantified by liquid scintillation counting (39).
For examination of carbohydrate moieties, epithelial cells were pretreated with periodic acid (5 mM, 10 min at 37°C) (22, 30, 39, 43) or a combination (cocktail) of specific carbohydrate-removing enzymes. The combination included heparinase, heparitinase, chondroitinase (each at 10 U/ml; all from Sigma) (60 min at 37°C) (23, 39), alpha-glucosidase (150 U/ml; Roche Corp., Mannheim, Germany) (10 min at 37°C) (32, 39), mannosidase (30 min at 37°C) (50 U/ml; Sigma) (34, 39), and neuraminidase (60 min at 37°C) (2.5 U/ml; Sigma) (29). Also tested were N-glycosidase F (PNGase F) at 0.2 U/ml, for 30 min at 37°C (23, 47) and endoglycosidase H at 0.1 U/ml, for 30 min at 37°C (23, 39, 47). Controls included epithelial cells incubated in PBS alone. To confirm carbohydrate release, the supernatants from treated and control cells were assayed for total carbohydrate content (12, 39). For this, 25 μl of 80% phenol and 2.5 ml of concentrated sulfuric acid (both from Sigma) were added to undiluted supernatants and standards (250-μg/ml mannose serially diluted 1:2) for 20 min at room temperature. The absorbance values at 490 nm, read using a Ceres 900 automated microplate reader (Bio-Tek, Winooski, Vt.), were used to determine released carbohydrate (in micrograms per milliliter) (39). Following all treatments, epithelial cell viability was assessed by trypan blue dye exclusion and the cells were assessed for growth inhibition, as described above, at E:T ratios of 80:1, 40:1, and 20:1.
Adherence assay.
An adherence assay was performed as previously described (35, 39, 45). Immediately following pretreatment with periodic acid, the epithelial cells (105 cells/ml) were added to C. albicans (107 cells/ml) (1:100 ratio) for 1 h at 37°C. Following the incubation, the coculture was collected and centrifuged at 300 × g for 10 s, and the supernatant was discarded. The epithelial cell pellet was resuspended in 1 ml of PBS and passed over a sterile 10-μm-pore-size nylon membrane. The epithelial cells retained on the membrane were collected and centrifuged at 300 × g for 10 s, and aliquots of the epithelial cell pellet were viewed microscopically. A parallel [3H]glucose 9-h growth inhibition assay was also run to confirm inhibition by the cells used in the adherence assay.
Vital staining of Candida in the coculture.
The epithelial cell-Candida coculture (E:T ratio of oral cells, 5:1; E:T ratio of vaginal cells, 80:1) was performed in the absence of [3H]glucose, in a volume of 2 ml in a 24-well plate (Costar). Following the 9-h incubation, the cocultures were collected and washed twice. FDA (50 μg/ml) (which stains living cells) and PI (1 μg/ml) (which stains dead cells) (Sigma) (10, 21) were added simultaneously to the cell pellets and placed in the dark for 30 min at room temperature. Preliminary studies were conducted to determine optimal concentrations of each dye. Following the incubation, the cocultures were washed with PBS followed by 20% FBS and then were washed again with PBS. The pellets were resuspended in 100 μl of PBS, and 5 μl of each coculture was added to a slide and examined under dual-filter fluorescent microscopy (Nikon Instruments, Melville, N.Y.). Controls for vital staining included living and dead (heat-killed) Candida in the presence and absence of oral and vaginal epithelial cells. A parallel [3H]glucose 9-h growth inhibition assay was conducted to confirm inhibition by the cells.
Statistical analysis.
The unpaired Student's t test was used to analyze data. Significant differences were defined at a confidence level where P was <0.05. All statistics were evaluated using Prism Software (Graph Pad, San Diego, Calif.)
RESULTS
Vaginal epithelial cell surface moiety important for growth inhibition activity.
Based on the fact that oral epithelial cells mediate their inhibitory activity against Candida through a carbohydrate moiety without any role for proteins or phospholipids (39), we sought to determine if vaginal epithelial cells function similarly. Accordingly, murine vaginal epithelial cells were treated with proteinase K (which cleaves a wide range of proteins) (28), PLA2 (which cleaves a wide range of lipids) (9, 31), and periodic acid (which cleaves a wide range of carbohydrates) prior to the coculture with Candida (22, 30, 43).
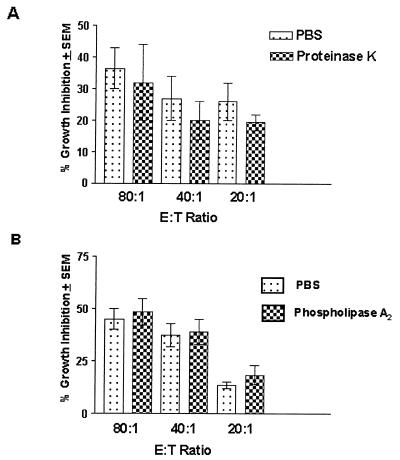
The results in Fig. 1A show that despite a significant amount of protein cleaved from the epithelial cells (250 ± 11 mg/ml for PBS-treated cells versus 1,100 ± 72 mg/ml for proteinase K-treated cells, reported as means ± standard errors of the means) (P < 0.0001), growth inhibition was similar in proteinase K and PBS treated cells. Figure 1B shows that the growth inhibition of epithelial cells treated with PLA2 was also similar to that of PBS-treated cells.
FIG. 1.
Effects of membrane protein or lipid moieties on murine vaginal epithelial cell anti-Candida activity. Vaginae from 10 mice were excised, and the epithelial cells were isolated and collected. Enriched epithelial cells were pretreated with proteinase K (25 μg/ml) (A), PLA2 (50 U/ml) (B), and PBS as a control. Thereafter, the cells were washed and examined for in vitro growth inhibition of C. albicans by [3H]glucose uptake at various E:T ratios. The figure shows the cumulative results of three separate experiments. SEM, standard error of the mean (error bars).
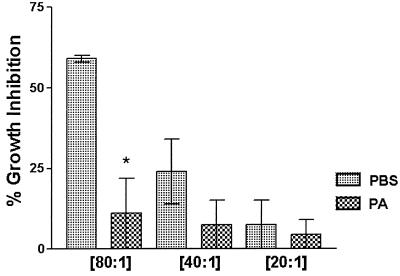
Figure 2 shows that treatment of the cells with periodic acid significantly reduced the epithelial cell activity compared to cells treated with PBS alone (P = 0.009). Periodic acid treatment resulted in a carbohydrate release of 42 ± 17 mg/ml compared to 14 ± 2.5 mg/ml for PBS-treated cells (P = 0.04). Periodic acid had no effect on epithelial cell viability.
FIG. 2.
Effects of membrane carbohydrate moieties on murine vaginal cell anti-Candida activity. Vaginae from 10 mice were excised, and the epithelial cells were isolated and collected. Enriched epithelial cells were pretreated with 5 mM periodic acid or PBS as a control. Thereafter, the cells were washed and examined for in vitro growth inhibition of C. albicans by quantitative plate count at various E:T ratios. The figure shows the cumulative results of three separate experiments. Error bars, standard error of the mean. ∗, significant difference (P < 0.05).
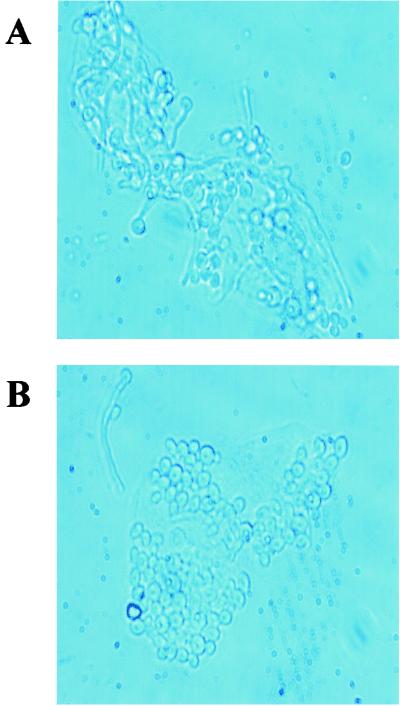
To examine whether the elimination of epithelial cell anti-Candida activity by periodic acid was due to a loss of adherence, epithelial cells treated with periodic acid or PBS were cultured for 1 h with Candida and examined microscopically. The results in Fig. 3 show similar levels of adherence for both sets of treated epithelial cells.
FIG. 3.
Periodic acid treatment of murine vaginal epithelial cells does not affect adherence to C. albicans. Vaginae from 10 mice were excised, and the epithelial cells were isolated and collected. Enriched epithelial cells were pretreated with 5 mM periodic acid and thereafter were washed and examined for adherence to vaginal epithelial cells after a 1 h coculture by a standard adherence assay. The figure shows a representative illustration of PBS-treated cells (A) and periodic acid-treated cells (B) from three repeats viewed under phase contrast microscopy. Magnification, ×360.
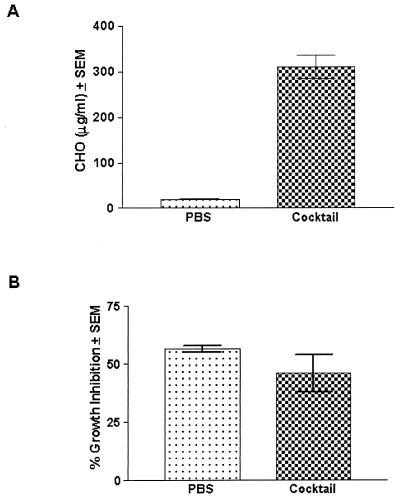
To examine the role of specific carbohydrate moieties, epithelial cells were treated with a cocktail of enzymes that were known to result in release of carbohydrates from vaginal epithelial cells. This included heparinase, heparitinase, and chondroitinase for the removal of sulfated polysaccharide, neuraminidase for the removal of sialic acid residues, and alpha-glucosidase and mannosidase for the removal of glucose- and mannose-containing carbohydrates. Control cells were treated with PBS. The results in Fig. 4 show that despite significant carbohydrate release by cells treated with the enzyme cocktail versus PBS-treated cells (Fig. 4A) (P < 0.0001), the levels of epithelial cell-mediated anti-Candida activity were similar (Fig. 4B). PNGase F and endoglycosidase H were also considered; however, since they did not result in release of CHO from the cells, they were not evaluated in the cellular assay.
FIG. 4.
Effects of sulfated polysaccharides, sialic acid residues, or glucose- and mannose-containing carbohydrates in the vaginal epithelial cell anti-Candida activity. Vaginae from 10 mice were excised, and the epithelial cells were isolated and collected. Enriched epithelial cells were pretreated with a cocktail of carbohydrate-removing enzymes at concentrations used in published reports. The carbohydrate release was measured for both PBS- and carbohydrate enzyme-treated cells (A). Following the treatment, the cells were washed and examined for in vitro growth inhibition of C. albicans by [3H]glucose (B). The figure shows cumulative results of three separate experiments. CHO, carbohydrate; SEM, standard error of the mean (error bars).
Static or cidal activity by vaginal and oral epithelial cells.
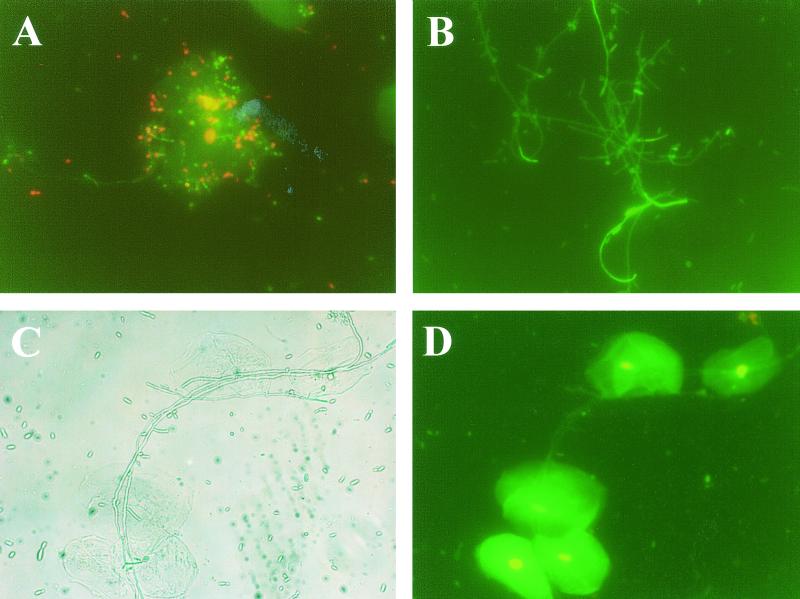
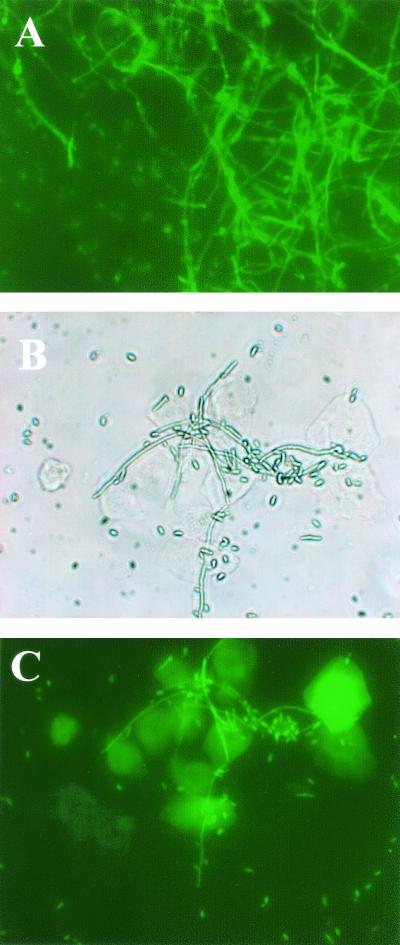
To examine whether human oral and murine vaginal epithelial cells killed or simply retarded the growth of C. albicans, following the 9-h coculture, FDA (which stains living cells green) and PI (which stains dead cells red) were added to cells from the coculture. Controls included Candida cultured alone. Figure 5A shows confirmation of correct staining of live and killed Candida in the presence of oral epithelial cells. Figure 5B shows live Candida in the control wells following the 9-h culture without human oral epithelial cells. Figure 5C and D show phase-contrast bright-field and fluorescence images, respectively, for human oral epithelial cells and Candida following the 9-h coculture that revealed predominantly live Candida. A parallel standard oral epithelial cell coculture evaluated by [3H]glucose uptake showed 51% growth inhibition. Figure 6 illustrates the results for murine vaginal epithelial cells. Figure 6A shows live Candida in the control wells following the 9-h culture without vaginal epithelial cells. Figure 6B and C show phase-contrast bright-field and fluorescent images, respectively, for the vaginal epithelial cell-Candida coculture at the conclusion of the 9 h. Results revealed, once again, the presence of predominantly live Candida. A parallel standard coculture evaluated by [3H]glucose uptake showed 30% inhibition.
FIG. 5.
Oral epithelial cells inhibit but do not kill Candida. Whole unstimulated saliva was collected from healthy human volunteers, and epithelial cell-enriched populations were isolated by nylon membrane retention and placed into the culture with Candida at a 5:1 E:T ratio. Following the 9-h coculture, oral epithelial cells and Candida were stained with FDA and PI. Controls included staining of Candida cultured alone for 9 h and live and dead Candida added to oral epithelial cells for confirmation of proper staining. The figure shows a representative illustration of four repeats using saliva from separate volunteers. (A) Live and dead Candida with oral epithelial cells; (B) Candida cultured alone for 9 h, (C) phase-contrast bright-field image of epithelial cells cultured with Candida for 9 h; (D) fluorescent image of the phase-contrast image. Magnification, ×320.
FIG. 6.
Murine vaginal epithelial cells inhibit but do not kill Candida. Vaginae from 10 mice were excised, and the epithelial cells were isolated, collected, and placed into the culture with Candida at an 80:1 E:T ratio. Following the 9-h coculture, murine vaginal epithelial cells and Candida were stained with FDA and PI. Candida cultured alone for 9 h served as a control. The figure shows a representative illustration of four repeats. (A) Candida cultured alone for 9 h; (B) phase-contrast bright-field image of vaginal epithelial cells cultured with Candida for 9 h; (C) fluorescent image of the phase-contrast image. Magnification, ×300.
DISCUSSION
With the recent discovery that oral and vaginal epithelial cells have the ability to inhibit the growth of C. albicans in vitro (14, 38, 40, 41), possibly representing an important innate host defense mechanism, efforts have focused on the properties and mechanism of this inhibitory action. To date, oral epithelial cell activity has been studied in humans alone (38, 39), while vaginal epithelial cell activity has been documented and studied in humans, nonhuman primates, and mice (14, 40, 41). Despite the verification of the vaginal epithelial activity in several species, the majority of the studies regarding the properties and mechanism have been restricted to oral epithelial cells due to the stronger anti-Candida activity. To date, vaginal and oral epithelial cell anti-Candida activity requires cell contact by viable epithelial cells with no role for soluble factors (14, 39, 40). In addition, oral epithelial cell activity does not involve phagocytosis, oxidative killing, or nonoxidative mechanisms via calprotectin or defensins (39). Studies evaluating specific moieties on oral epithelial cells revealed that while there is no role for surface proteins or lipids in the anti-Candida activity, there is a role for a carbohydrate moiety. This had been evidenced by the elimination of the activity following treatment of the epithelial cells with periodic acid (39). However, attempts to identify the specific carbohydrate moiety(ies) through a variety of carbohydrate-specific enzymes have failed to identify the moiety(ies) involved (39). Nevertheless, it was critical to follow up with vaginal epithelial cells to determine if the mechanism of action is similar, taking into account differences in the cells at the anatomic sites (i.e., columnar versus squamous) and differential strength of the activity. Based on the fact that yields of murine vaginal epithelial cells were higher than those from human vaginal lavage, and could be pooled from several individual mice, the vaginal studies were conducted exclusively using murine cells, recognizing that all other properties studied to date have been similar for human, macaque, and murine vaginal cells (38, 40, 41).
Results showed that the vaginal cell anti-Candida activity was sensitive to periodic acid but not proteinase K or PLA2, suggesting that, like the oral epithelial cells, the activity is mediated by a carbohydrate moiety. While the effective cleavage of each of these broad-based enzymes was confirmed, we cannot rule out the possibility that the activity of either proteinase K or PLA2 was not optimal by concentration, time of treatment, or temperature of treatment despite the use of conditions optimized for the oral epithelial cells. Accordingly, attempts were made to evaluate proteinase K at higher temperatures in lieu of changing the incubation time or concentrations since each was tested thoroughly with the oral cells (39). Unfortunately, however, higher temperatures could not be evaluated, as they affected the viability of the epithelial cells. On the other hand, testing higher temperatures using proteinase K with oral cells, where viability was not affected, had no effect on the epithelial cell activity (data no shown). Thus, all available data suggest that proteins, glycoproteins, or phospholipids are not involved with carbohydrates in the anti-Candida activity.
Taking into account the role for a carbohydrate moiety, it was important to address whether or not adherence to Candida was affected by the treatment with periodic acid that would indirectly affect the growth inhibition based on the strict requirement for cell contact (39). Results showed that, similar to what was observed in oral epithelial cells (39), adherence was unaffected by periodic acid treatment of the vaginal cells, suggesting that the carbohydrate moiety was directly involved in the anti-Candida activity rather than a secondary effect of reduced adherence. Unfortunately, attempts to identify the specific carbohydrate involved were unsuccessful, as no evidence could be found for a role of sulfated polysaccharides, sialic acid residues, or glucose- and mannose-containing carbohydrates in the anti-Candida activity. This represents yet another identical property of vaginal and oral epithelial cell activity. In fact, several other enzymes that were ineffective in removing carbohydrates from oral epithelial cells (i.e., PNGase and endoglycosidase H) were also ineffective at releasing carbohydrates from the murine vaginal epithelial cells. Based on these results, studies to evaluate any forms of oxidative mechanisms or nonoxidative factors (i.e., defensins and calprotectins) previously evaluated for the oral cells, and found to be negative (39), were deemed unnecessary in the case of the vaginal cells.
A second objective of the study was to determine whether the epithelial cell anti-Candida activity functions by killing the Candida or by simply inhibiting its growth. This was difficult to infer from either the quantitative plate counts or [3H]glucose uptake, because both an arrest of growth and cidal effects would result in decreased growth demonstrable by reduced [3H]glucose uptake or the presence of fewer CFU. Even longer incubation periods (>96 h) of the plates showing no new colony growth (data not shown) could not be interpreted as cidal if the cells were still arrested. We thus chose to evaluate the fate of Candida by using distinct fluorescent dyes that differentially stain live (FDA) or dead (PI) microorganisms. Results of these experiments showed a predominance of live Candida following the 9-h coculture with murine vaginal and human oral epithelial cells, suggesting that both oral and vaginal cells retard or arrest growth rather than kill Candida. Taken together, oral and vaginal epithelial cells appear to function identically against Candida, with the only differences being the strength of the inhibitory action.
Given the uncertainty and/or controversy surrounding roles for adaptive immunity by Candida-specific T cells and antibodies (18, 27), as well as the limited roles for polymorphonuclear leukocytes and NK cells (1, 42) against C. albicans in the vagina, epithelial cells may fill an important void in the mucosal host defense against vaginal C. albicans infection, albeit weaker and with the requirement for high E:T ratios. While innate and adaptive inflammatory responses are critical to host defense in the periphery and at mucosal sites, inflammatory responses are not preferable at a reproductive site, especially in the case of a commensal organism. Accordingly, immunoregulation has been postulated as a means to reduce or eliminate inflammatory responses against infection (46). Epithelial cells, on the other hand, appear to function in a noninflammatory manner and thus may play an important role in protection against infection. Furthermore, the static rather than cidal activity would be important for maintaining Candida as a commensal. At the oral mucosa, a stronger activity through the same mechanism of action may be required, given the fact that the levels of asymptomatic yeast colonization in the oral cavity are higher than those in the vagina (25). In either case, while epithelial cells may represent a novel antifungal host defense mechanism, this mechanism is defined as innate and as such can be overcome, resulting in infection. Along these lines, the low and high frequency of oral and vaginal Candida infections, respectively, in healthy individuals may be a function of the relative levels of epithelial cell-mediated anti-Candida activity at each site. We recognize, however, that the anti-Candida activity that formed the basis of these hypotheses comes from in vitro data alone. On the other hand, an argument for some cause and effect in vivo can be taken from the reduced oral epithelial cell anti-Candida activity in HIV-infected persons with OPC (39, 40) and a similar reduction by vaginal epithelial cells in women with RVVC (3). Furthermore, the anti-Candida activity appears to function against several Candida species (40). Thus, a case can be made for protection against infection by a broad range of commensal Candida species. Studies that identify the carbohydrate and elucidate the complete mechanism of anti-Candida activity (e.g., intracellular signals) by the vaginal and oral epithelial cells, as well as those evaluating the activity longitudinally in specific populations susceptible or at risk for infection, will undoubtedly continue to shed light on these hypotheses.
Acknowledgments
This work was supported by National Institutes of Health Public Health service grants DE 12178 from the National Institute of Dental and Craniofacial Research and AI 32556 from the National Institute of Allergy and Infectious Diseases.
Editor: T. R. Kozel
REFERENCES
- 1.Ahrens, J. C., M. R. Price, L. Daneo-Moore, and H. R. Buckley. 1983. Effects of culture density on the kinetics of germ tube formation in Candida albicans. J. Gen. Microbiol. 129:3001-3006. [DOI] [PubMed] [Google Scholar]
- 2.Baccarini, M., F. Bistoni, P. Puccetti, and E. Garaci. 1983. Natural cell-mediated cytotoxicity against Candida albicans induced by cyclophosphamide: nature of the in vitro cytotoxic effector. Infect. Immun. 42:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barousse, M. M., C. Steele, K. Dunlap, T. Espinosa, D. Boikov, J. D. Sobel, and P. L. Fidel, Jr. 2001. Growth inhibition of Candida albicans by human vaginal epithelial cells. J. Infect. Dis. 184:1489-1493. [DOI] [PubMed] [Google Scholar]
- 4.Beno, D. W. A., and H. L. Mathews. 1990. Growth inhibition of Candida albicans by interleukin-2-induced lymph node cells. Cell. Immunol. 128:89-100. [DOI] [PubMed] [Google Scholar]
- 5.Beno, D. W. A., A. G. Stover, and H. L. Mathews. 1995. Growth inhibition of Candida albicans hyphae by CD8+ lymphocytes. J. Immunol. 154:5273-5281. [PubMed] [Google Scholar]
- 6.Cassone, A., M. Boccanera, D. A. Adriani, G. Santoni, and F. De Bernardis. 1995. Rats clearing a vaginal infection by Candida albicans aquire specific, antibody-mediated resistance to vaginal infection. Infect. Immun. 63:2619-2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cenci, E., A. Mencacci, R. Spaccapelo, L. Tonnetti, P. Mosci, K. H. Enssle, P. Puccetti, L. Romani, and F. Bistoni. 1995. T helper cell type 1 (Th1)- and Th2-like responses are present in mice with gastric candidiasis but protective immunity is associated with Th1 development. J. Infect. Dis. 171:1279-1288. [DOI] [PubMed] [Google Scholar]
- 8.Cenci, E., L. Romani, A. Vecchiarelli, P. Puccetti, and F. Bistoni. 1990. T cell subsets and INF-gamma production in resistance to systemic candidosis in immunized mice. J. Immunol. 144:4333-4339. [PubMed] [Google Scholar]
- 9.Cottrell, R. C. 1981. Phospholipase A2 from bee venom. Methods Enzymol. 71:698-702. [DOI] [PubMed] [Google Scholar]
- 10.Darzynkiewicz, Z., and X. Li. 1996. Measurements of cell death by flow cytometry, p. 71-106. In T. G. Cotter and S. J. Martin (ed.), Techniques in apoptosis: a user's guide to cytometry. Portland Press, London, United Kingdom.
- 11.De Bernardis, F., M. Boccanera, D. Adriani, E. Spreghini, G. Santoni, and A. Cassone. 1997. Protective role of antimannan and anti-aspartyl proteinase antibodies in an experimental model of Candida albicans vaginitis in rats. Infect. Immun. 65:3399-3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubois, M., K. A. Gilles, J. K. Hamilton, P. A. Rebers, and F. Smith. 1956. Colorimetric method for determination of sugars and related substances. Anal. Chem. 28:350-356. [Google Scholar]
- 13.Fichorova, R. N., and D. J. Anderson. 1999. Differential expression of immunobiological mediators by immortalized human cervical and vaginal epithelial cells. Biol. Reprod. 60:508-514. [DOI] [PubMed] [Google Scholar]
- 14.Fidel, P. L., Jr., J. L. Cutright, and C. Steele. 2000. Effects of Reproductive hormones on experimental vaginal candidiasis. Infect. Immun. 68:651-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fidel, P. L., Jr., K. A. Ginsburg, J. L. Cutright, N. A. Wolf, D. Leaman, K. Dunlap, and J. D. Sobel. 1997. Vaginal-associated immunity in women with recurrent vulvovaginal candidiasis: evidence for vaginal Th1-type responses following intravaginal challenge with Candida antigen. J. Infect. Dis. 176:728-739. [DOI] [PubMed] [Google Scholar]
- 16.Fidel, P. L., Jr., W. Luo, C. Steele, J. Chabain, M. Baker, and F. L. Wormley. 1999. Analysis of vaginal cell populations during experimental vaginal candidiasis. Infect. Immun. 67:3135-3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fidel, P. L., Jr., M. E. Lynch, V. Redondo-Lopez, J. D. Sobel, and R. Robinson. 1993. Systemic cell-mediated immune reactivity in women with recurrent vulvovaginal candidiasis (RVVC). J. Infect. Dis. 168:1458-1465. [DOI] [PubMed] [Google Scholar]
- 18.Fidel, P. L., Jr., M. E. Lynch, and J. D. Sobel. 1993. Candida-specific cell-mediated immunity is demonstrable in mice with experimental vaginal candidiasis. Infect. Immun. 61:1990-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fidel, P. L., Jr., M. E. Lynch, and J. D. Sobel. 1994. Effects of preinduced Candida-specific systemic cell-mediated immunity on experimental vaginal candidiasis. Infect. Immun. 62:1032-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fidel, P. L., Jr., M. E. Lynch, and J. D. Sobel. 1995. Circulating CD4 and CD8 T cells have little impact on host defense against experimental vaginal candidiasis. Infect. Immun. 63:2403-2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guilbault, G. G., and D. N. Kramer. 1964. Fluorometric determination of lipase, acylase, alpha and gamma chymotrypsin, and inhibitors of these enzymes. Anal. Chem. 36:409-412. [Google Scholar]
- 22.Herlander, A., G. C. Hansson, and A. M. Svennerholm. 1997. Binding of enterotoxigenic Escherichia coli to isolated enterocytes and intestinal mucus. Microb. Pathog. 23:335-346. [DOI] [PubMed] [Google Scholar]
- 23.Isaacs, R. D. 1994. Borrelia burgdorferi bind to epithelial cell proteoglycans. J. Clin. Investig. 93:809-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klein, R. S., C. A. Harris, C. B. Small, B. Moll, M. Lesser, and G. H. Friedland. 1984. Oral candidiasis in high-risk patients as the initial manifestation of the acquired immunodeficiency syndrome. N. Engl. J. Med. 311:354-357. [DOI] [PubMed] [Google Scholar]
- 25.Leigh, J. E., M. Barousse, R. K. Swoboda, T. Myers, S. Hager, N. A. Wolf, J. L. Cutright, J. Thompson, J. D. Sobel, and P. L. Fidel, Jr. 2001. Candida-specific systemic cell-mediated immune reactivities in HIV-infected persons with and without mucosal candidiaisis. J. Infect. Dis 183:277-285. [DOI] [PubMed] [Google Scholar]
- 26.Macher, A. M. 1988. The pathology of AIDS. Public Health Rep. 103:246-254. [PMC free article] [PubMed] [Google Scholar]
- 27.Mathur, S., G. Virella, J. Koistinen, E. O. Horger, T. A. Mahvi, and H. H. Fudenberg. 1977. Humoral immunity in vaginal candidiasis. Infect. Immun. 15:287-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyata, R., K. Iwabuchi, S. Watanabe, N. Sato, and I. Nagaoka. 1999. Exposure of intestinal epithelial cell HT29 to bile acids and ammonia enhances Mac-1-mediated neutrophil adhesion. Inflamm. Res. 48:265-273. [DOI] [PubMed] [Google Scholar]
- 29.Nohara, K., M. Kunimoto, and H. Fujimaki. 1998. Antibody against ganglioside GD1c containing NeuGcalpha2-8NeuGc cooperates with CD3 and CD4 in rat T cell activation. J. Biochem. 124:194-199. [DOI] [PubMed] [Google Scholar]
- 30.Okuma, K., Y. Matsuura, H. Tatsuo, Y. Inagaki, M. Nakamura, N. Yamamot, and Y. Yanagi. 2001. Analysis of molecules involved in human T-cell leukaemia virus type 1 entry by a vesicular stomatitis virus pseudotype bearing its envelope glycoproteins. J. Gen. Virol. 82:821-830. [DOI] [PubMed] [Google Scholar]
- 31.Pikula, S., L. Epstein, and A. Martonosi. 1994. The relationship between phospholipid content and Ca2+-ATPase activity in the sarcoplasmic reticulum. Biochim. Biophys. Acta 1196:1-13. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez Acosta, F. A., B. Galindo, and I. M. Cesari. 1983. Chemical nature of the interaction between macrophage fusion factor and macrophage membranes. Scand. J. Immunol. 18:407-410. [DOI] [PubMed] [Google Scholar]
- 33.Saavedra, M., B. Taylor, N. W. Lukacs, and P. L. Fidel, Jr. 1999. Local production of chemokines during experimental vaginal candidiasis. Infect. Immun. 67:5820-5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Savage, S. M., L. A. Donaldson, and M. L. Sopori. 1993. T cell-B cell interaction: autoreactive T cells recognize B cells through a terminal mannose-containing superantigen-like glycoprotein. Cell. Immunol. 146:11-27. [DOI] [PubMed] [Google Scholar]
- 35.Schwab, U., D. Milatovic, and I. Braveny. 1997. Increased adherence of Candida albicans to buccal epithelial cells from patients with AIDS. Eur. J. Clin. Microbiol. Infect. Dis. 16:848-851. [DOI] [PubMed] [Google Scholar]
- 36.Sobel, J. D. 1988. Pathogenesis and epidemiology of vulvovaginal candidiasis. Ann. N. Y. Acad. Sci. 544:547-557. [DOI] [PubMed] [Google Scholar]
- 37.Sobel, J. D. 1992. Pathogenesis and treatment of recurrent vulvovaginal candidiasis. Clin. Infect. Dis. 14:S148-S153. [DOI] [PubMed] [Google Scholar]
- 38.Steele, C., J. E. Leigh, R. K. Swoboda, and P. L. Fidel, Jr. 2000. Growth inhibition of Candida by human oral epithelial cells. J. Infect. Dis. 182:1479-1485. [DOI] [PubMed] [Google Scholar]
- 39.Steele, C., J. E. Leigh, R. K. Swoboda, H. Ozenci, and P. L. Fidel, Jr. 2001. Potential role for a carbohydrate moiety in anti-Candida activity of human oral epithelial cells. Infect. Immun. 69:7091-7099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steele, C., H. Ozenci, W. Luo, M. Scott, and P. L. Fidel, Jr. 1999. Growth inhibition of Candida albicans by vaginal cells from naive mice. Med. Mycol. 37:251-260. [PubMed] [Google Scholar]
- 41.Steele, C., M. Ratterree, and P. L. Fidel, Jr. 1999. Differential susceptibility to experimental vaginal candidiasis in macaques. J. Infect. Dis. 180:802-810. [DOI] [PubMed] [Google Scholar]
- 42.Strockbine, N. A., M. T. Largen, S. M. Zweibel, and H. R. Buckley. 1984. Identification and molecular weight characterization of antigens from Candida albicans that are recognized by human sera. Infect. Immun. 43:715-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sussich, F., and A. Cesaro. 2000. The kinetics of periodate oxidation of carbohydrates: a calorimetric approach. Carbohydr. Res. 239:87-95. [DOI] [PubMed] [Google Scholar]
- 44.Taylor, B. N., M. Saavedra, and P. L. Fidel, Jr. 2000. Local Th1/Th2 cytokine production during experimental vaginal candidiasis. J. Med. Mycol. 38:419-431. [DOI] [PubMed] [Google Scholar]
- 45.Williams, D. W., R. Walker, M. A. Lewis, R. T. Allison, and A. J. Potts. 1999. Adherence of Candida albicans to oral epithelial cells differentiated by Papanicolaou staining. J. Clin. Pathol. 52:529-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wormley, F. L., Jr., J. Chaiban, and P. L. Fidel, Jr. 2001. Cell adhesion molecule and lymphocyte activation marker expression during experimental vaginal candidiasis. Infect. Immun. 69:5072-5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang, J. P., and R. S. Stephens. 1992. Mechanism of C. trachomatis attachment to eukaryotic host cells. Cell 69:861-869. [DOI] [PubMed] [Google Scholar]