Abstract
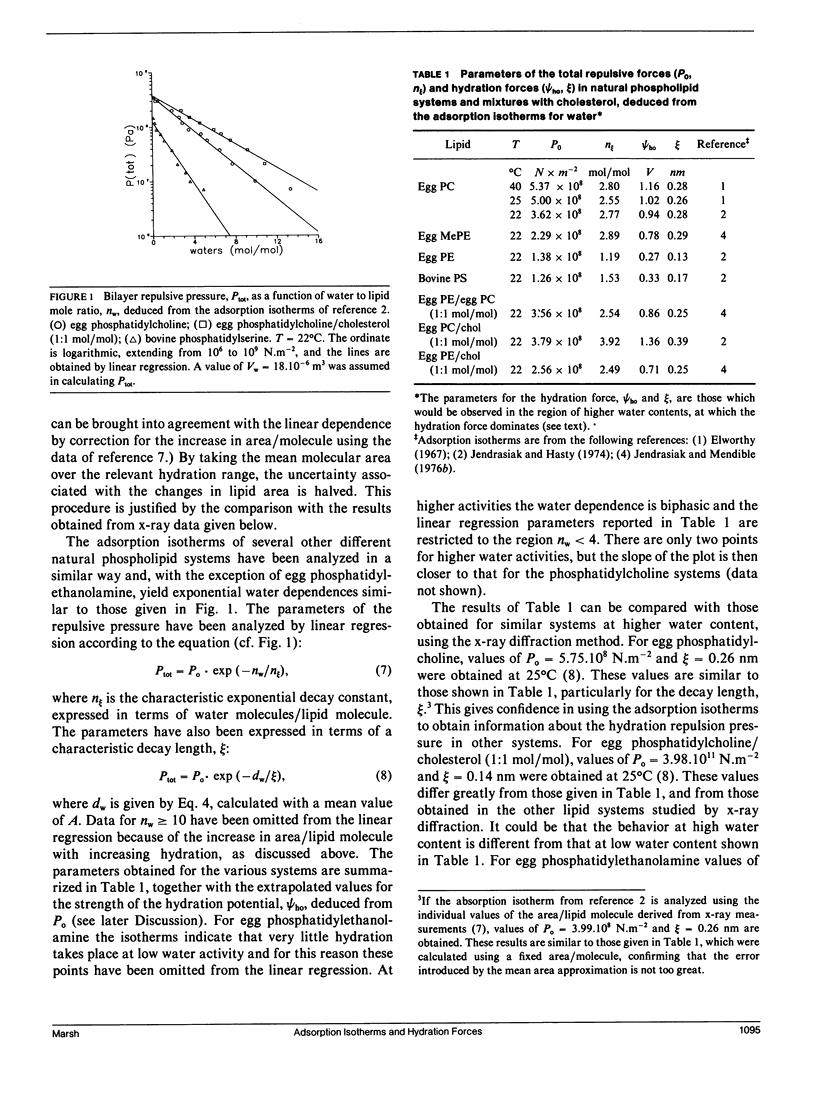
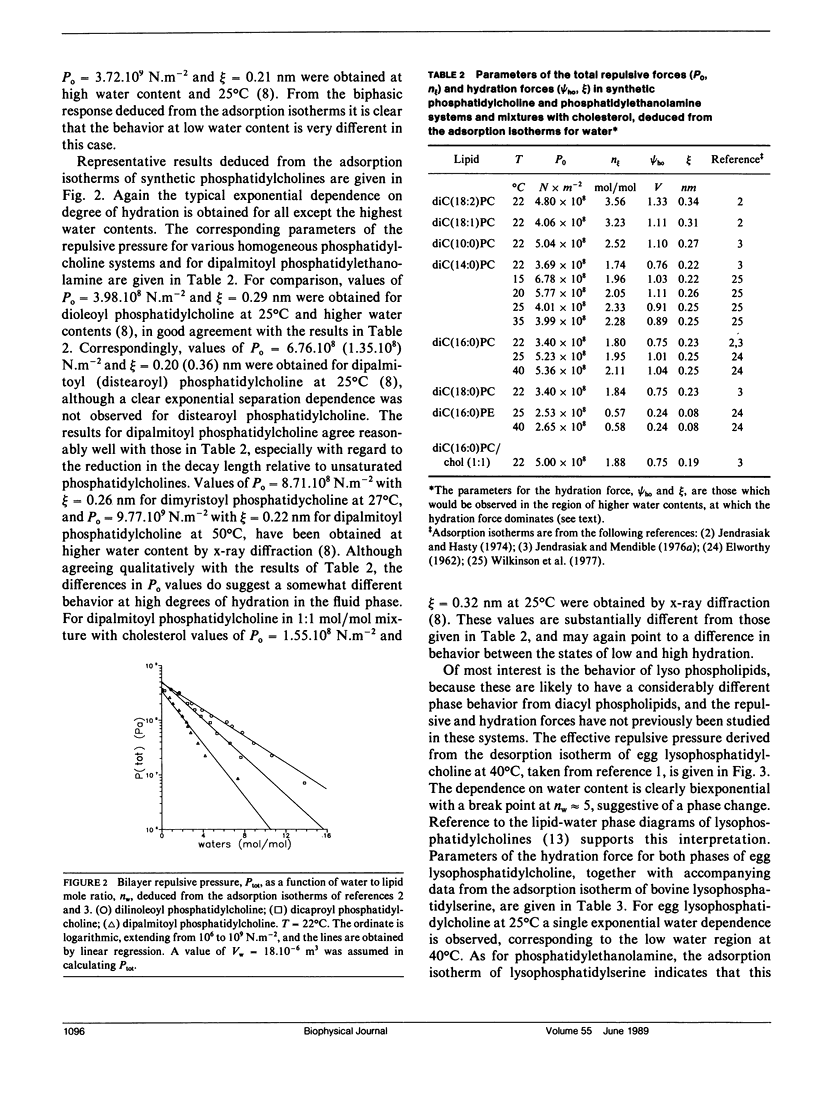
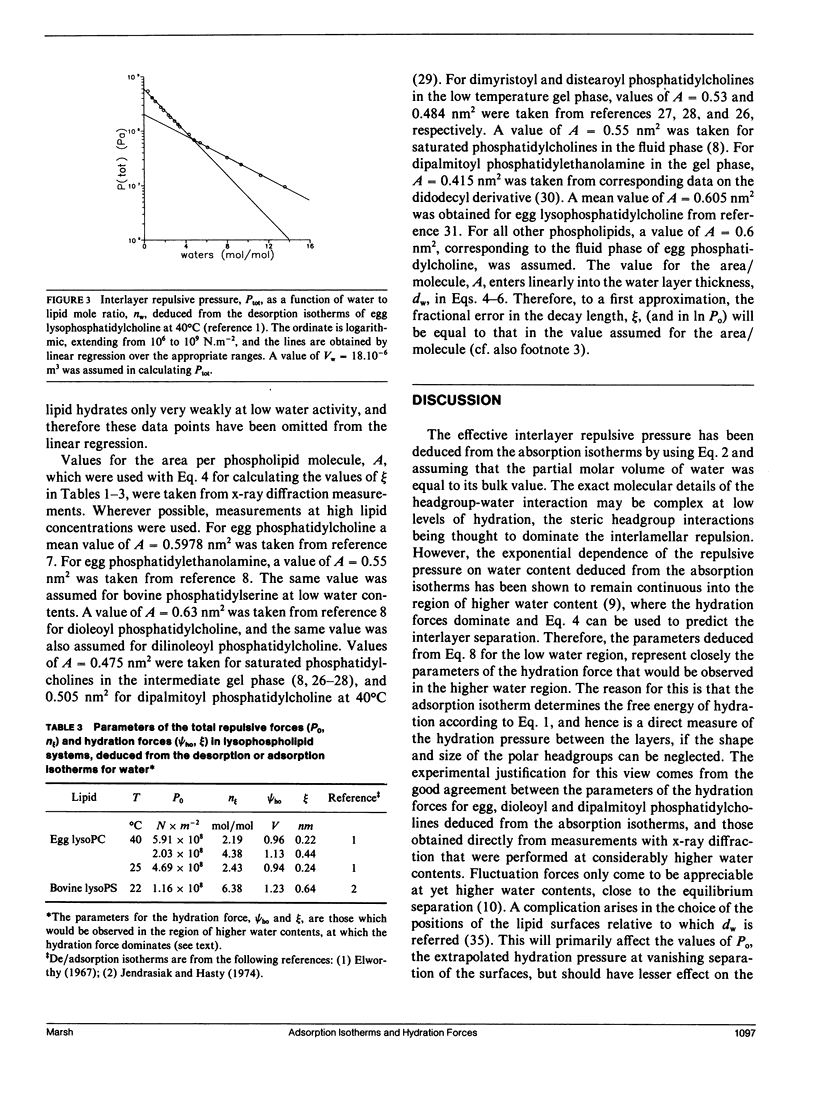
The repulsive forces in a wide range of diacyl and monoacyl phospholipid systems have been obtained from the adsorption isotherms for water. From the exponential dependence of the repulsive pressure on the water content, information has been deduced regarding the hydration force. For diacyl phosphatidylcholines the strength of the hydration force and its characteristic decay length are in good agreement with values previously obtained by x-ray diffraction methods. For natural and synthetic diacyl phosphatidylcholines in the fluid lamellar phase, the hydration force extrapolated to zero layer separation (Po) is in the range 4-5.10(8) N.m-2 and the decay length is approximately 0.3 nm. The results for dimyristoyl, dipalmitoyl, and distearoyl phosphatidylcholines in the gel phase are very similar with Po approximately 2.5.10(8) N.m-2 and decay length of approximately 0.2 nm. Egg monomethyl phosphatidylethanolamine is less strongly hydrated: Po = 2.3.10(9) N.m-2, with a decay length of 0.3 nm. Egg phosphatidylethanolamine and bovine phosphatidylserine hydrate even more weakly with Po approximately 1.3.10(8) N.m-2 and decay length of approximately 0.15 nm. Mixtures with cholesterol or phosphatidylcholine increase both Po and the decay length for phosphatidylethanolamine to values closer to those for phosphatidylcholine. The repulsive forces deduced for egg lysophosphatidylcholine at 40 degrees C display a biphasic water dependence, with the low water phase being similar to lamellar egg phosphatidylcholine, and the phase at higher water content having a smaller value of Po = 2.10(8) N.m-2 but a longer decay length of approximately 0.45 nm, corresponding to a nonlamellar configuration. Bovine lysophosphatidylserine similarly yields values of PO = 1.2.108 N.m-2 and an effective decay length of 0.64 nm. The hydration behavior of the various diacyl phospholipids has been interpreted in terms of the mean-field molecular force theory of lipid hydration, and values deduced for the surface hydration potential of the various lipids. This analysis extends previous results on hydration forces, particularly to lysolipids and nonlamellar phases.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arvidson G., Brentel I., Khan A., Lindblom G., Fontell K. Phase equilibria in four lysophosphatidylcholine/water systems. Exceptional behaviour of 1-palmitoyl-glycerophosphocholine. Eur J Biochem. 1985 Nov 4;152(3):753–759. doi: 10.1111/j.1432-1033.1985.tb09257.x. [DOI] [PubMed] [Google Scholar]
- Cevc G., Marsh D. Hydration of noncharged lipid bilayer membranes. Theory and experiments with phosphatidylethanolamines. Biophys J. 1985 Jan;47(1):21–31. doi: 10.1016/S0006-3495(85)83872-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley A. C., Fuller N. L., Rand R. P., Parsegian V. A. Measurement of repulsive forces between charged phospholipid bilayers. Biochemistry. 1978 Jul 25;17(15):3163–3168. doi: 10.1021/bi00608a034. [DOI] [PubMed] [Google Scholar]
- Evans E. A., Parsegian V. A. Thermal-mechanical fluctuations enhance repulsion between bimolecular layers. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7132–7136. doi: 10.1073/pnas.83.19.7132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruner S. M., Parsegian V. A., Rand R. P. Directly measured deformation energy of phospholipid HII hexagonal phases. Faraday Discuss Chem Soc. 1986;(81):29–37. doi: 10.1039/dc9868100029. [DOI] [PubMed] [Google Scholar]
- Janiak M. J., Small D. M., Shipley G. G. Nature of the Thermal pretransition of synthetic phospholipids: dimyristolyl- and dipalmitoyllecithin. Biochemistry. 1976 Oct 19;15(21):4575–4580. doi: 10.1021/bi00666a005. [DOI] [PubMed] [Google Scholar]
- Janiak M. J., Small D. M., Shipley G. G. Temperature and compositional dependence of the structure of hydrated dimyristoyl lecithin. J Biol Chem. 1979 Jul 10;254(13):6068–6078. [PubMed] [Google Scholar]
- Jendrasiak G. L., Hasty J. H. The hydration of phospholipids. Biochim Biophys Acta. 1974 Jan 23;337(1):79–91. doi: 10.1016/0005-2760(74)90042-3. [DOI] [PubMed] [Google Scholar]
- Jendrasiak G. L., Mendible J. C. The effect of the phase transition on the hydration and electrical conductivity of phospholipids. Biochim Biophys Acta. 1976 Feb 23;424(2):133–148. doi: 10.1016/0005-2760(76)90183-1. [DOI] [PubMed] [Google Scholar]
- Jendrasiak G. L., Mendible J. C. The phospholipid head-group orientation: effect on hydration and electrical conductivity. Biochim Biophys Acta. 1976 Feb 23;424(2):149–158. doi: 10.1016/0005-2760(76)90184-3. [DOI] [PubMed] [Google Scholar]
- LeNeveu D. M., Rand R. P. Measurement and modification of forces between lecithin bilayers. Biophys J. 1977 May;18(2):209–230. doi: 10.1016/S0006-3495(77)85608-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lis L. J., McAlister M., Fuller N., Rand R. P., Parsegian V. A. Interactions between neutral phospholipid bilayer membranes. Biophys J. 1982 Mar;37(3):657–665. [PMC free article] [PubMed] [Google Scholar]
- McIntosh T. J., Magid A. D., Simon S. A. Steric repulsion between phosphatidylcholine bilayers. Biochemistry. 1987 Nov 17;26(23):7325–7332. doi: 10.1021/bi00397a020. [DOI] [PubMed] [Google Scholar]
- McIntosh T. J., Simon S. A. Area per molecule and distribution of water in fully hydrated dilauroylphosphatidylethanolamine bilayers. Biochemistry. 1986 Aug 26;25(17):4948–4952. doi: 10.1021/bi00365a034. [DOI] [PubMed] [Google Scholar]
- McIntosh T. J., Simon S. A. Hydration force and bilayer deformation: a reevaluation. Biochemistry. 1986 Jul 15;25(14):4058–4066. doi: 10.1021/bi00362a011. [DOI] [PubMed] [Google Scholar]
- Parsegian V. A., Fuller N., Rand R. P. Measured work of deformation and repulsion of lecithin bilayers. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2750–2754. doi: 10.1073/pnas.76.6.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand R. P., Fuller N., Parsegian V. A., Rau D. C. Variation in hydration forces between neutral phospholipid bilayers: evidence for hydration attraction. Biochemistry. 1988 Oct 4;27(20):7711–7722. doi: 10.1021/bi00420a021. [DOI] [PubMed] [Google Scholar]
- Rand R. P. Interacting phospholipid bilayers: measured forces and induced structural changes. Annu Rev Biophys Bioeng. 1981;10:277–314. doi: 10.1146/annurev.bb.10.060181.001425. [DOI] [PubMed] [Google Scholar]
- Reiss-Husson F. Structure des phases liquide-cristallines de différents phospholipides, monoglycérides, sphingolipides, anhydres ou en présence d'eau. J Mol Biol. 1967 May 14;25(3):363–382. doi: 10.1016/0022-2836(67)90192-1. [DOI] [PubMed] [Google Scholar]
- Scherer J. R. The partial molar volume of water in biological membranes. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7938–7942. doi: 10.1073/pnas.84.22.7938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddon J. M., Cevc G., Kaye R. D., Marsh D. X-ray diffraction study of the polymorphism of hydrated diacyl- and dialkylphosphatidylethanolamines. Biochemistry. 1984 Jun 5;23(12):2634–2644. doi: 10.1021/bi00307a015. [DOI] [PubMed] [Google Scholar]
- Seddon J. M., Cevc G., Kaye R. D., Marsh D. X-ray diffraction study of the polymorphism of hydrated diacyl- and dialkylphosphatidylethanolamines. Biochemistry. 1984 Jun 5;23(12):2634–2644. doi: 10.1021/bi00307a015. [DOI] [PubMed] [Google Scholar]
- Seddon J. M., Harlos K., Marsh D. Metastability and polymorphism in the gel and fluid bilayer phases of dilauroylphosphatidylethanolamine. Two crystalline forms in excess water. J Biol Chem. 1983 Mar 25;258(6):3850–3854. [PubMed] [Google Scholar]
- Tardieu A., Luzzati V., Reman F. C. Structure and polymorphism of the hydrocarbon chains of lipids: a study of lecithin-water phases. J Mol Biol. 1973 Apr 25;75(4):711–733. doi: 10.1016/0022-2836(73)90303-3. [DOI] [PubMed] [Google Scholar]
- White S. H., Jacobs R. E., King G. I. Partial specific volumes of lipid and water in mixtures of egg lecithin and water. Biophys J. 1987 Oct;52(4):663–665. doi: 10.1016/S0006-3495(87)83259-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White S. H., King G. I. Molecular packing and area compressibility of lipid bilayers. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6532–6536. doi: 10.1073/pnas.82.19.6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson D. A., Morowitz H. J., Prestegard J. H. Hydration of phosphatidylocholine. Adsorption isotherm and proton nuclear magnetic resonance studies. Biophys J. 1977 Nov;20(2):169–179. doi: 10.1016/S0006-3495(77)85542-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


