Abstract
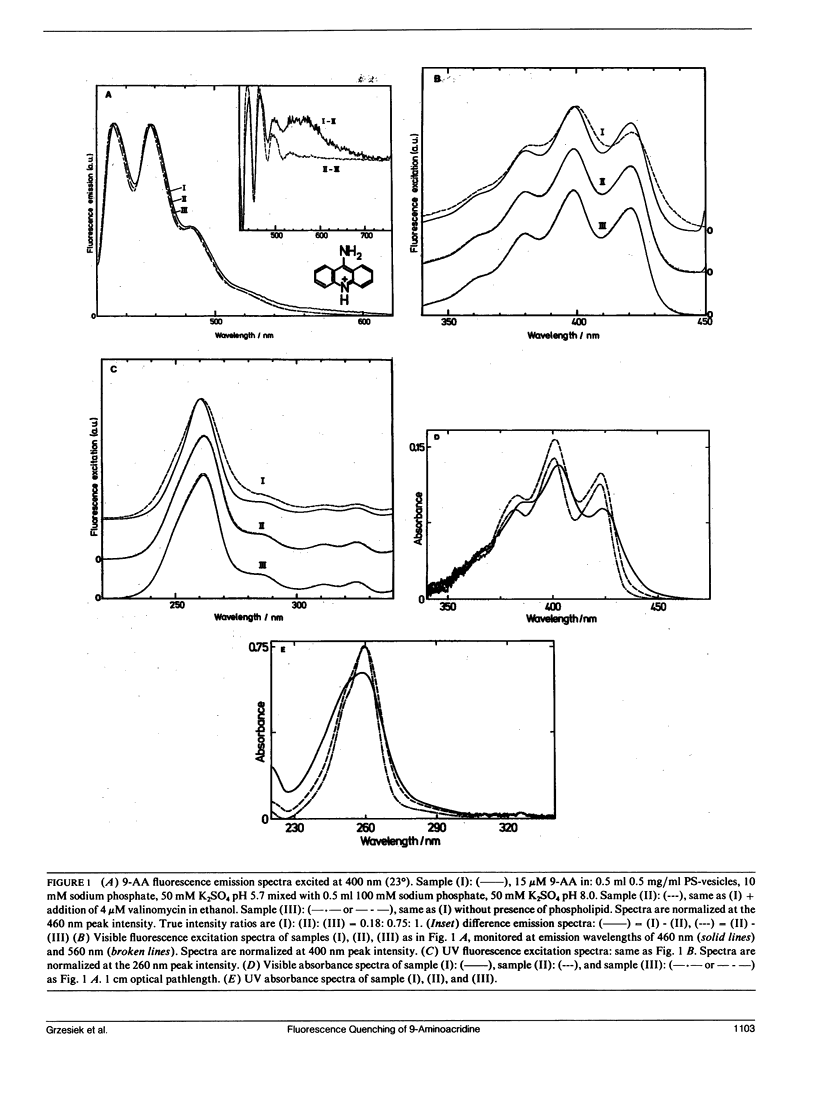
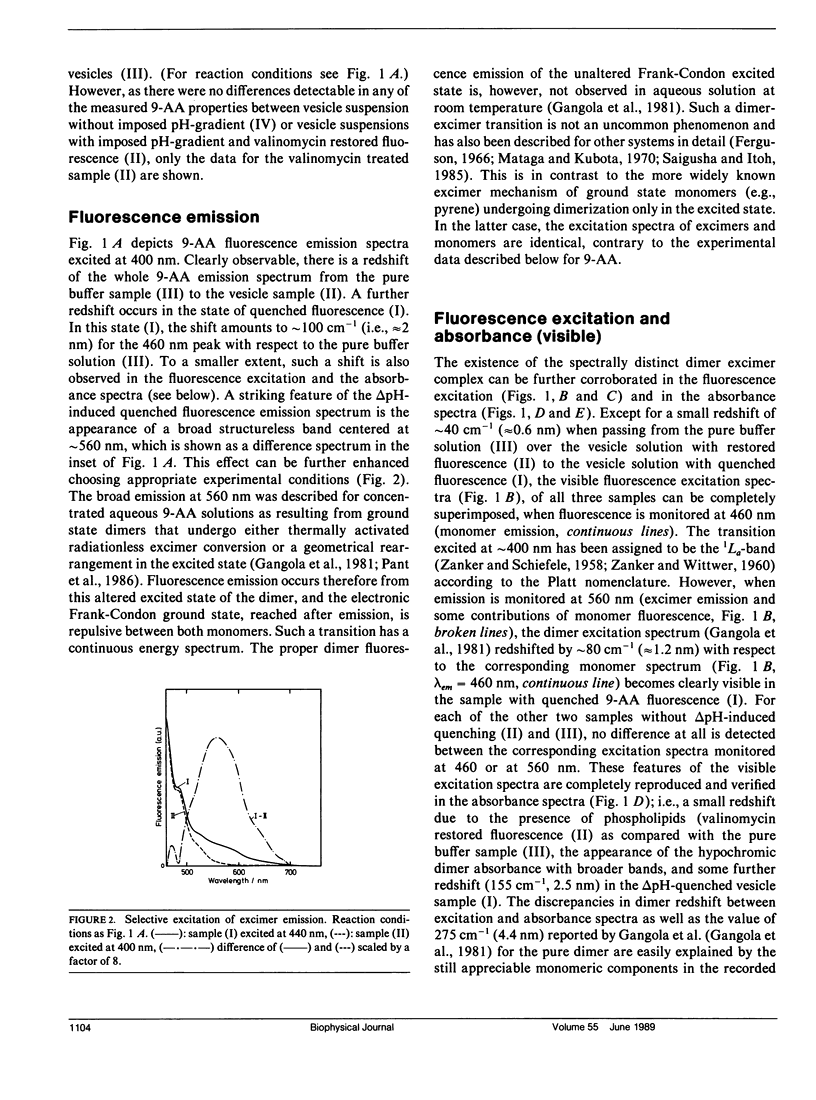
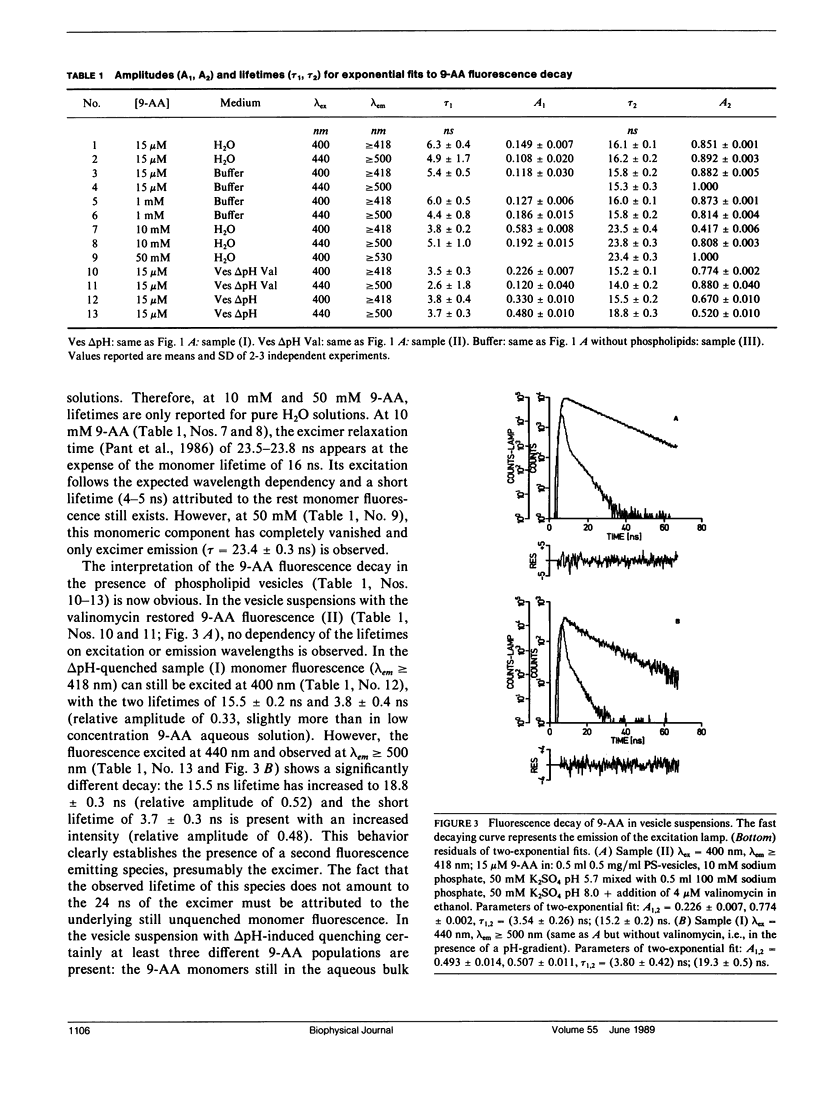
The fluorescence of 9-aminoacridine (9-AA) is quenched in vesicular suspensions containing negatively charged lipid headgroups (e.g., phosphatidylserine) upon imposition of a transmembrane (inside acidic) pH-gradient. It is shown that this fluorescence loss is accompanied by the formation of 9-AA dimers that undergo a transition in the dimer excited state to a dimer-excimer state. This result has been obtained on the basis of the specific dimer fluorescence excitation and hypochromic absorbance spectra that are redshifted by maximally 275 cm-1 (4.4 nm) with respect to the corresponding monomer spectra, as well as by the detection of the characteristic broad excimer emission band, centered at 560 nm. The existence of the spectrally distinct dimer-excimer is further corroborated by fluorescence life-time measurements that indicate an increased lifetime of up to 24 ns for this complex as compared with the normal monomer fluorescence lifetime of 16 ns. The formation of this dimer-excimer complex from the monomers can be reversed completely and the original monomeric spectral properties restored after the abolishment of the electrochemical proton gradient. In addition to the delta pH-induced dimer redshift in absorbance and fluorescence excitation, a further small redshift in monomer absorbance, fluorescence excitation, and emission spectra is observed due solely to the presence of the negatively charged phospholipid headgroups.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bramhall J. Conductance routes for protons across membrane barriers. Biochemistry. 1987 May 19;26(10):2848–2855. doi: 10.1021/bi00384a028. [DOI] [PubMed] [Google Scholar]
- Capomacchia A. C., Casper J., Schulman S. G. Valence tautomerism of singly protonated 9-aminoacridine and its implications for intercalative interactions with nucleic acids. J Pharm Sci. 1974 Aug;63(8):1272–1276. doi: 10.1002/jps.2600630823. [DOI] [PubMed] [Google Scholar]
- Casadio R., Baccarini-Melandri A., Melandri B. A. On the determination of the transmembrane pH difference in bacterial chromatophores using 9-aminoacridine. Eur J Biochem. 1974 Aug 15;47(1):121–128. doi: 10.1111/j.1432-1033.1974.tb03675.x. [DOI] [PubMed] [Google Scholar]
- Casadio R., Melandri B. A. Calibration of the response of 9-amino acridine fluorescence to transmembrane pH differences in bacterial chromatophores. Arch Biochem Biophys. 1985 Apr;238(1):219–228. doi: 10.1016/0003-9861(85)90159-6. [DOI] [PubMed] [Google Scholar]
- Casadio R., Melandri B. A. The behavior of 9-aminoacridine as an indicator of transmembrane pH difference in liposomes of natural bacterial phospholipids. J Bioenerg Biomembr. 1977 Feb;9(1):17–29. doi: 10.1007/BF00745040. [DOI] [PubMed] [Google Scholar]
- De Young M. B., Nemeth E. F., Scarpa A. Measurement of the internal pH of mast cell granules using microvolumetric fluorescence and isotopic techniques. Arch Biochem Biophys. 1987 Apr;254(1):222–233. doi: 10.1016/0003-9861(87)90098-1. [DOI] [PubMed] [Google Scholar]
- Deamer D. W., Nichols J. W. Proton-hydroxide permeability of liposomes. Proc Natl Acad Sci U S A. 1983 Jan;80(1):165–168. doi: 10.1073/pnas.80.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deamer D. W., Prince R. C., Crofts A. R. The response of fluorescent amines to pH gradients across liposome membranes. Biochim Biophys Acta. 1972 Aug 9;274(2):323–335. doi: 10.1016/0005-2736(72)90180-0. [DOI] [PubMed] [Google Scholar]
- Dell'Antone P., Colonna R., Azzone G. F. The membrane structure studied with cationic dyes. 2. Aggregation, metachromatic effects and pK a shifts. Eur J Biochem. 1972 Jan 21;24(3):566–576. doi: 10.1111/j.1432-1033.1972.tb19719.x. [DOI] [PubMed] [Google Scholar]
- Dell'Antone P., Cusinato O., Volpato O. Mechanism of acridine uptake by submitochondrial particles. Arch Biochem Biophys. 1978 Dec;191(2):413–425. doi: 10.1016/0003-9861(78)90379-x. [DOI] [PubMed] [Google Scholar]
- Fiolet J. W., Bakker E. P., van Dam K. The fluorescent properties of acridines in the presence of chloroplasts or liposomes. On the quantitative relationship between the fluorescence quenching and the transmembrane proton gradient. Biochim Biophys Acta. 1974 Dec 19;368(3):432–445. doi: 10.1016/0005-2728(74)90188-1. [DOI] [PubMed] [Google Scholar]
- Fornasiero D., Kurucsev T. The binding of 9-aminoacridine to calf thymus DNA in aqueous solution. Electronic spectral studies. Biophys Chem. 1985 Nov;23(1-2):31–37. doi: 10.1016/0301-4622(85)80061-2. [DOI] [PubMed] [Google Scholar]
- Grzesiek S., Dencher N. A. The 'delta pH'-probe 9-aminoacridine: response time, binding behaviour and dimerization at the membrane. Biochim Biophys Acta. 1988 Mar 3;938(3):411–424. doi: 10.1016/0005-2736(88)90139-3. [DOI] [PubMed] [Google Scholar]
- Haraux F., de Kouchkovsky Y. Measurement of chloroplast internal protons with 9-aminoacridine. Probe binding, dark proton gradient, and salt effects. Biochim Biophys Acta. 1980 Aug 5;592(1):153–168. doi: 10.1016/0005-2728(80)90122-x. [DOI] [PubMed] [Google Scholar]
- Heitzman E. R., Ferguson J. H. Mass in right lung. N Y State J Med. 1966 Oct 15;66(20):2682–2683. [PubMed] [Google Scholar]
- Huang C. S., Kopacz S. J., Lee C. P. Mechanistic differences in the energy-linked fluorescence decreases of 9-aminoacridine dyes associated with bovine heart submitochondrial membranes. Biochim Biophys Acta. 1983 Jan 13;722(1):107–115. doi: 10.1016/0005-2728(83)90163-9. [DOI] [PubMed] [Google Scholar]
- Lee H. C., Forte J. G. A study of H+ transport in gastric microsomal vesicles using fluorescent probes. Biochim Biophys Acta. 1978 Apr 4;508(2):339–356. doi: 10.1016/0005-2736(78)90336-x. [DOI] [PubMed] [Google Scholar]
- Pfister V. R., Homann P. H. Intrinsic and artifactual pH buffering in chloroplast thylakoids. Arch Biochem Biophys. 1986 May 1;246(2):525–530. doi: 10.1016/0003-9861(86)90307-3. [DOI] [PubMed] [Google Scholar]
- Schuldiner S., Rottenberg H., Avron M. Determination of pH in chloroplasts. 2. Fluorescent amines as a probe for the determination of pH in chloroplasts. Eur J Biochem. 1972 Jan 31;25(1):64–70. doi: 10.1111/j.1432-1033.1972.tb01667.x. [DOI] [PubMed] [Google Scholar]
- Searle G. F., Barber J. The involvement of the electrical double layer in the quenching of 9-aminoacridine fluorescence by negatively charged surfaces. Biochim Biophys Acta. 1978 May 10;502(2):309–320. doi: 10.1016/0005-2728(78)90052-x. [DOI] [PubMed] [Google Scholar]


