Abstract
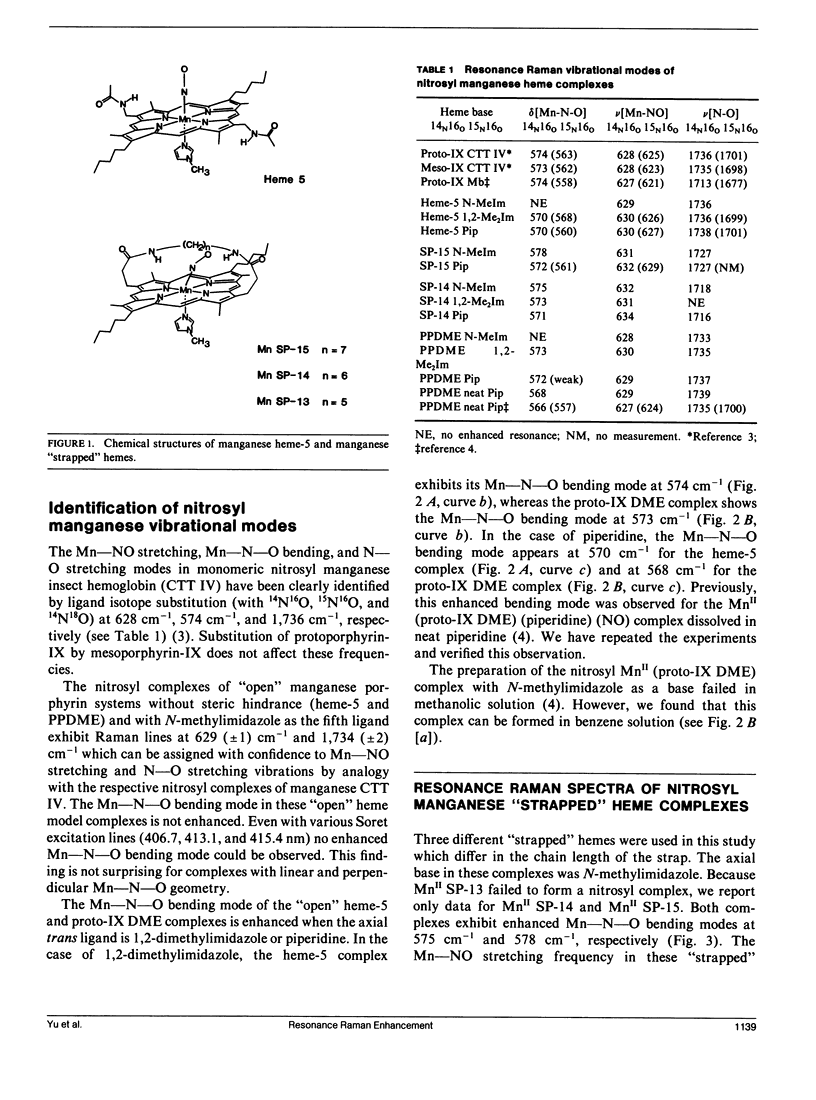
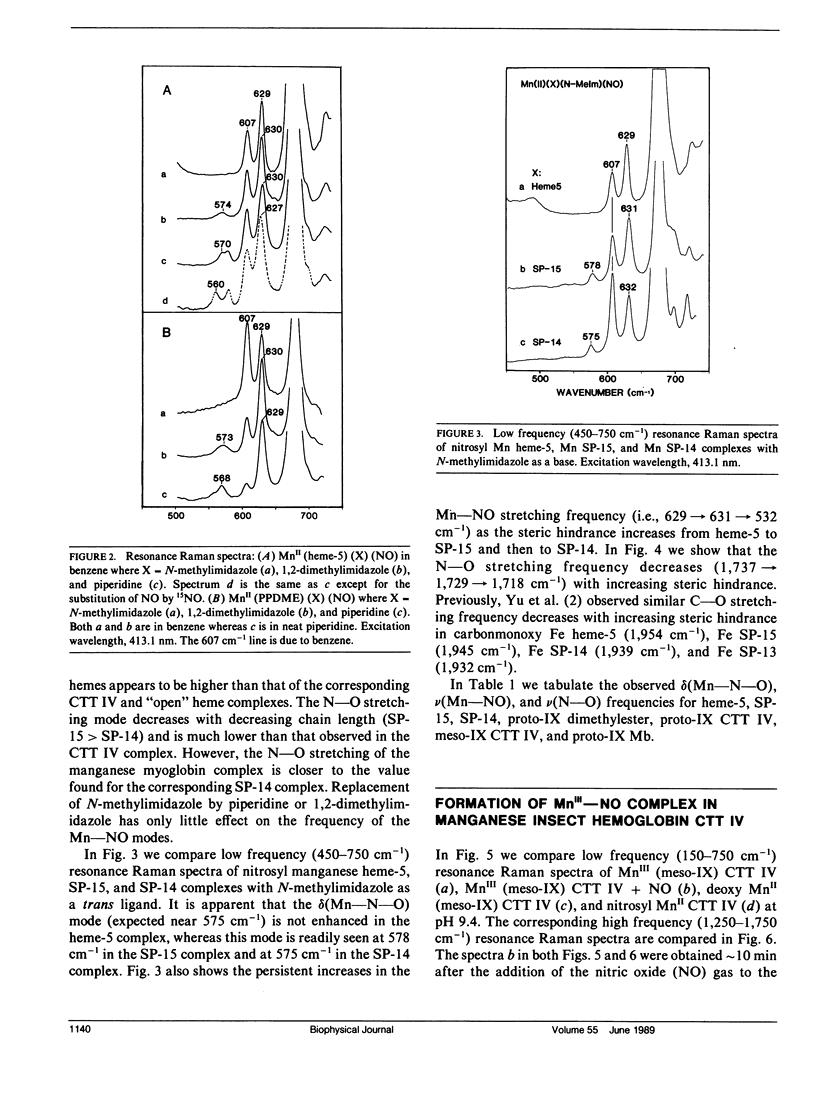
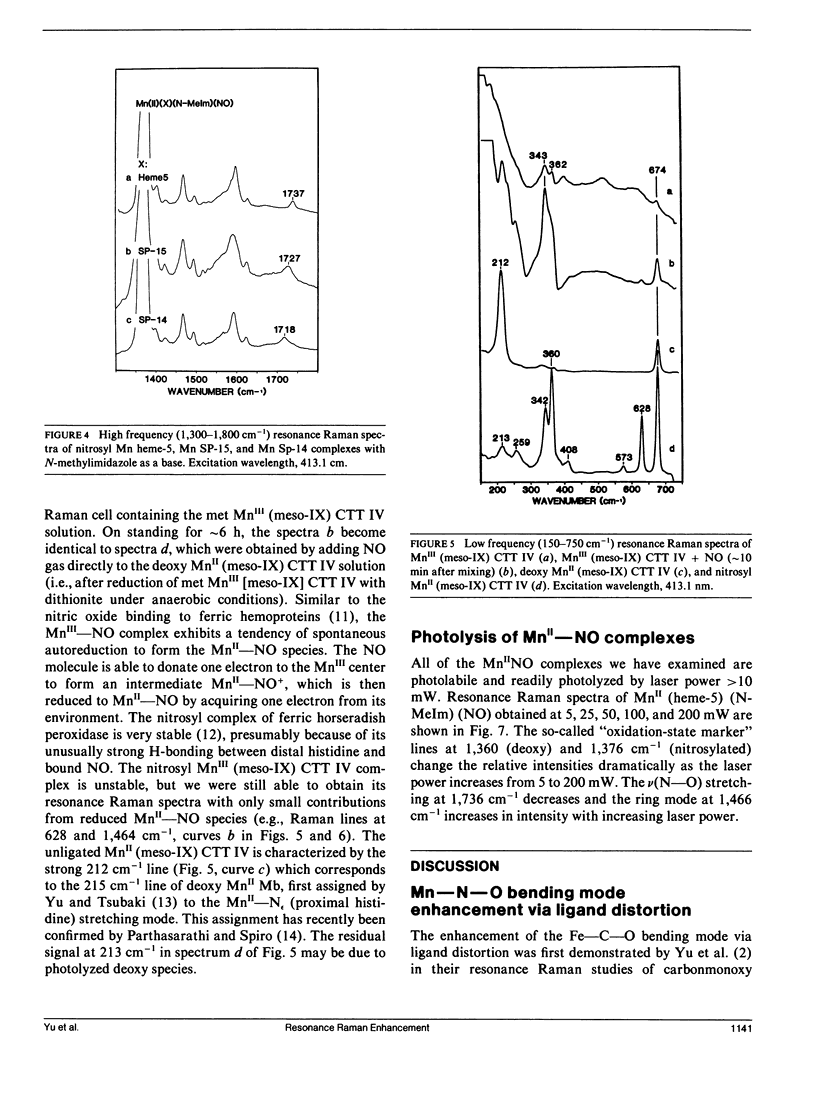
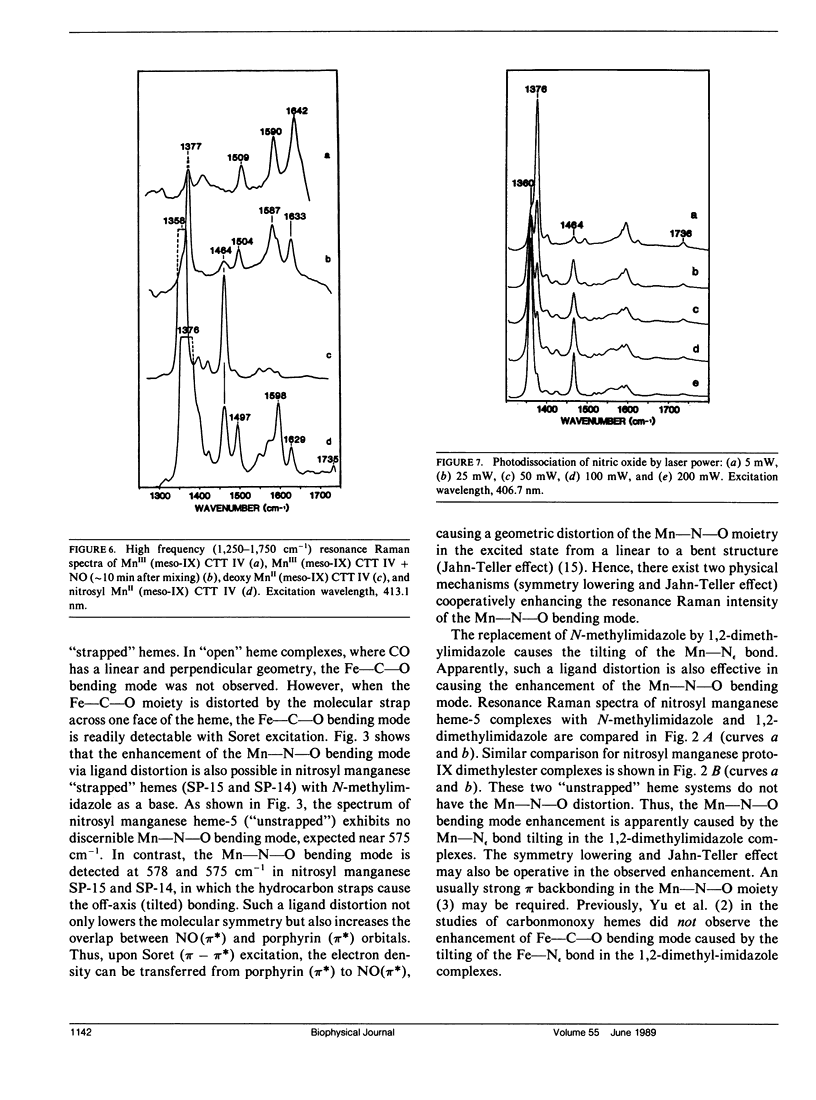
Resonance Raman spectra of the MnII-NO moiety in synthetic nitrosyl manganese heme complexes with and without steric hindrance are reported. The "strapped" hemes having a hydrocarbon strap (variable length) across one face of the heme hinder the perpendicular bonding of a linear ligand. These complexes were employed to investigate the effects of ligand distortion (primarily tilting) on Mn-NO stretching, Mn-N-O bending, and N-O stretching modes. It is demonstrated that ligand distortion in the MnII-NO system is a valid mechanism for causing the resonance enhancement of the Mn-N-O bending mode, similar to that observed in the FeII-CO system (Yu, N.-T., E. A. Kerr, B. Ward, and C. K. Chang. 1983. Biochemistry. 22:4534-4540). More interesting is the observation of the delta(Mn-N-O) enhancement caused by the tilting of the trans Mn-N epsilon bond in the "open" heme complexes (e.g., heme-5 and proto-1X dimethylester) with 1,2-dimethylimidazole or piperidine as a base. The nu(Mn-NO) and nu(N-O) modes exhibit an increase and a decrease, respectively, as the strap length decreases (hence the steric hindrance increases). Both nu(Mn-NO) and nu(N-O) frequencies are insensitive to the strength of the trans base. The results from "strapped" and "open" model heme systems imply that the Mn-N-O geometry is essentially linear and perpendicular in the nitrosyl complexes of monomeric manganese insect hemoglobin CTT IV and sperm whale myoglobin. The unusually low nu(N-O) frequency in the manganese myoglobin complex may be caused by the distal histidine-NO interaction. The delta(Mn-N-O) enhancement in both nitrosyl manganese CTT IV and nitrosyl manganese myoglobin may be caused by a tilting of the Mn"-Nf (proximal histidine) bond.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benko B., Yu N. T. Resonance Raman studies of nitric oxide binding to ferric and ferrous hemoproteins: detection of Fe(III)--NO stretching, Fe(III)--N--O bending, and Fe(II)--N--O bending vibrations. Proc Natl Acad Sci U S A. 1983 Nov;80(22):7042–7046. doi: 10.1073/pnas.80.22.7042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gersonde K., Sick H., Wollmer A., Buse G. Heterotropic allosterism of monomeric haemoglobins of Chironomus thummi thummi. Eur J Biochem. 1972 Jan 31;25(1):181–189. doi: 10.1111/j.1432-1033.1972.tb01683.x. [DOI] [PubMed] [Google Scholar]
- Gersonde K., Twilfer H., Overkamp M. Bohr-effect and pH-dependence of electron spin resonance spectra of a cobalt-substituted monomeric insect haemoglobin. Biophys Struct Mech. 1982;8(3):189–211. doi: 10.1007/BF00535459. [DOI] [PubMed] [Google Scholar]
- La Mar G. N., Anderson R. R., Chacko V. P., Gersonde K. High-resolution proton NMR as indicator of a silent mutation in the haem cavity of a monomeric allosteric haemoglobin. Eur J Biochem. 1983 Oct 17;136(1):161–166. doi: 10.1111/j.1432-1033.1983.tb07721.x. [DOI] [PubMed] [Google Scholar]
- Lin S. H., Yu N. T., Gersonde K. Resonance Raman evidence for an unusually strong exogenous ligand-metal bond in a monomeric nitrosyl manganese hemoglobin. FEBS Lett. 1988 Mar 14;229(2):367–371. doi: 10.1016/0014-5793(88)81158-x. [DOI] [PubMed] [Google Scholar]
- Overkamp M., Twilfer H., Gersonde K. Conformation-controlled trans-effect of the proximal histidine in haemoglobins. An electron spin resonance study of monomeric nitrosyl-57Fe-haemoglobins. Z Naturforsch C. 1976 Sep-Oct;31(9-10):524–533. doi: 10.1515/znc-1976-9-1009. [DOI] [PubMed] [Google Scholar]
- Peng S. M., Ibers J. A. Stereochemistry of carbonylmetalloporphyrins. The structure of (pyridine)(carbonyl)(5, 10, 15, 20-tetraphenylprophinato)iron(II). J Am Chem Soc. 1976 Dec 8;98(25):8032–8036. doi: 10.1021/ja00441a025. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Yu N. T., Chang C. K. Resonance Raman studies of sterically hindered cyanomet "strapped" hemes. Effects of ligand distortion and base tension on iron-carbon bond. Biophys J. 1987 Nov;52(5):801–805. doi: 10.1016/S0006-3495(87)83274-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonetani T., Yamamoto H., Erman J. E., Leigh J. S., Jr, Reed G. H. Electromagnetic properties of hemoproteins. V. Optical and electron paramagnetic resonance characteristics of nitric oxide derivatives of metalloporphyrin-apohemoprotein complexes. J Biol Chem. 1972 Apr 25;247(8):2447–2455. [PubMed] [Google Scholar]
- Yu N. T., Kerr E. A., Ward B., Chang C. K. Resonance Raman detection of Fe-CO stretching and Fe-C-O bending vibrations in sterically hindered carbonmonoxy "strapped hemes". A structural probe of Fe-C-O distortion. Biochemistry. 1983 Sep 13;22(19):4534–4540. doi: 10.1021/bi00288a028. [DOI] [PubMed] [Google Scholar]
- Yu N. T. Resonance Raman studies of ligand binding. Methods Enzymol. 1986;130:350–409. doi: 10.1016/0076-6879(86)30018-1. [DOI] [PubMed] [Google Scholar]
- Yu N. T., Tsubaki M. Resonance Raman spectra of manganese myoglobin and its azide complex. Assignment of a new charge-transfer band to azide (pi) to porphyrin (pi) transition. Biochemistry. 1980 Sep 30;19(20):4647–4653. doi: 10.1021/bi00561a017. [DOI] [PubMed] [Google Scholar]


