Abstract
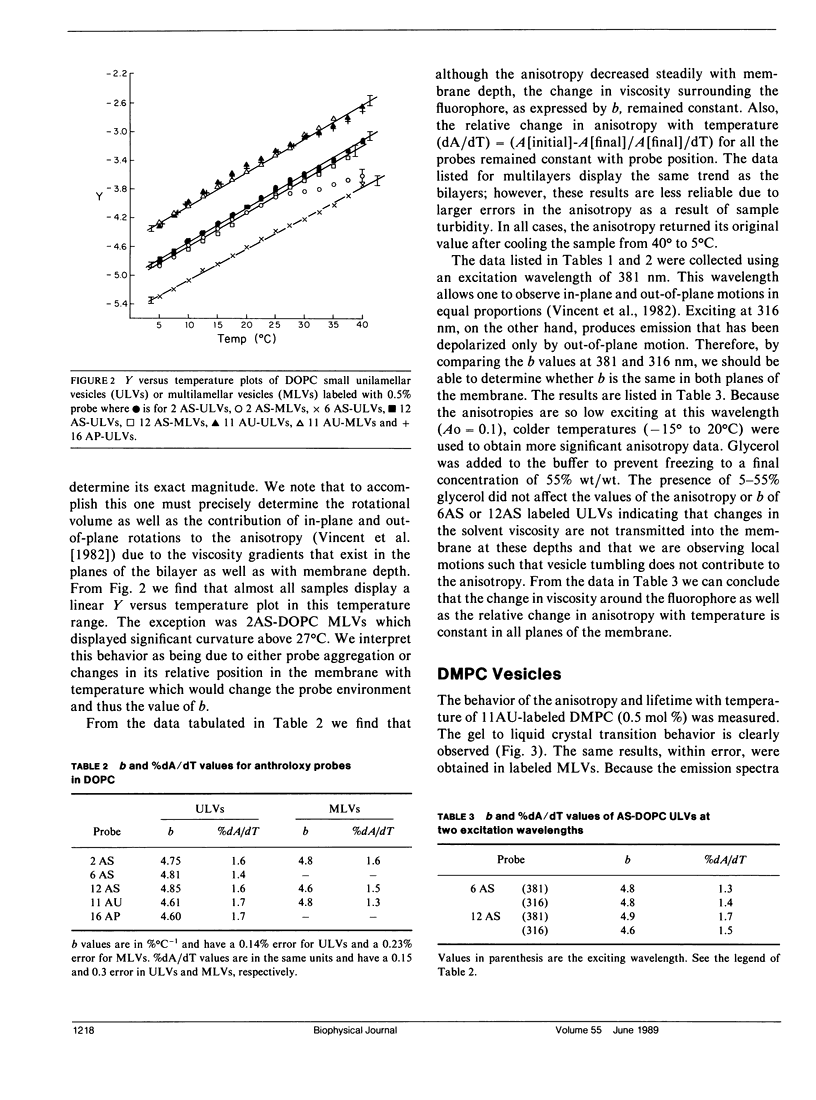
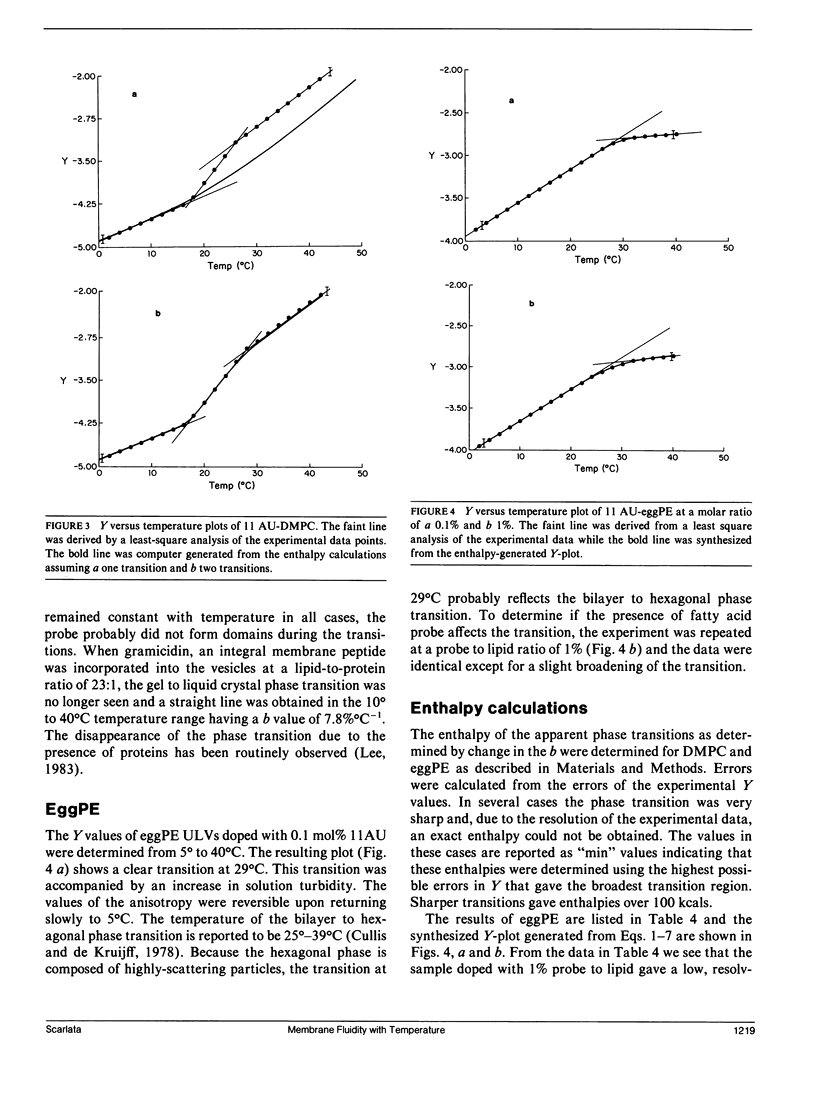
The thermal coefficient of the frictional resistance to fluorophore rotation (b), a parameter related to the change in the local viscosity with temperature, was determined for anthroyloxy-fatty acid probes in micelles and dimyristoyl lecithin (DMPC) and dioleoyl lecithin (DOPC) unilamellar and multilamellar vesicles. The value of b and the percent change in anisotropy with temperature (%dA/dT) remained constant with membrane depth and only depended on composition. These parameters were also the same when either in-plane, or in-plane and out-of-plane fluorophore motions were observed. This result indicates that the membranes expand isotropically. The magnitude of b was found to be primarily dependent on the packing of the hydrocarbon chains with higher b values relating to more closely-packed chains. b was responsive to the gel to liquid crystal phase transition of DMPC and the bilayer to hexagonal phase transition of egg-phosphatidylethanolamine. When the enthalpy values for the fluorophore transfer from one phase to another are calculated, the values are larger than those measured by calorimetry and reflect a discrepancy between the microscopic enthalpy experienced by the fluorophore due to a change in environment versus the macroscopic enthalpy of the system as a whole.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chong P. L., Thompson T. E. Depolarization of dehydroergosterol in phospholipid bilayers. Biochim Biophys Acta. 1986 Dec 1;863(1):53–62. doi: 10.1016/0005-2736(86)90386-x. [DOI] [PubMed] [Google Scholar]
- Cullis P. R., de Kruijff B. The polymorphic phase behaviour of phosphatidylethanolamines of natural and synthetic origin. A 31P NMR study. Biochim Biophys Acta. 1978 Oct 19;513(1):31–42. doi: 10.1016/0005-2736(78)90109-8. [DOI] [PubMed] [Google Scholar]
- Gruner S. M., Cullis P. R., Hope M. J., Tilcock C. P. Lipid polymorphism: the molecular basis of nonbilayer phases. Annu Rev Biophys Biophys Chem. 1985;14:211–238. doi: 10.1146/annurev.bb.14.060185.001235. [DOI] [PubMed] [Google Scholar]
- Kawato S., Kinosita K., Jr, Ikegami A. Dynamic structure of lipid bilayers studied by nanosecond fluorescence techniques. Biochemistry. 1977 May 31;16(11):2319–2324. doi: 10.1021/bi00630a002. [DOI] [PubMed] [Google Scholar]
- Kutchai H., Chandler L. H., Zavoico G. B. Effects of cholesterol on acyl chain dynamics in multilamellar vesicles of various phosphatidylcholines. Biochim Biophys Acta. 1983 Dec 21;736(2):137–149. doi: 10.1016/0005-2736(83)90277-8. [DOI] [PubMed] [Google Scholar]
- Lentz B. R., Barenholz Y., Thompson T. E. Fluorescence depolarization studies of phase transitions and fluidity in phospholipid bilayers. 1. Single component phosphatidylcholine liposomes. Biochemistry. 1976 Oct 5;15(20):4521–4528. doi: 10.1021/bi00665a029. [DOI] [PubMed] [Google Scholar]
- Massey V., Curti B., Ganther H. A temperature-dependent conformational change in D-amino acid oxidase and its effect on catalysis. J Biol Chem. 1966 May 25;241(10):2347–2357. [PubMed] [Google Scholar]
- Scarlata S. F. The effects of viscosity on gramicidin tryptophan rotational motion. Biophys J. 1988 Dec;54(6):1149–1157. doi: 10.1016/S0006-3495(88)83049-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinitzky M., Barenholz Y. Fluidity parameters of lipid regions determined by fluorescence polarization. Biochim Biophys Acta. 1978 Dec 15;515(4):367–394. doi: 10.1016/0304-4157(78)90010-2. [DOI] [PubMed] [Google Scholar]
- Straume M., Litman B. J. Equilibrium and dynamic structure of large, unilamellar, unsaturated acyl chain phosphatidylcholine vesicles. Higher order analysis of 1,6-diphenyl-1,3,5-hexatriene and 1-[4-(trimethylammonio)phenyl]- 6-phenyl-1,3,5-hexatriene anisotropy decay. Biochemistry. 1987 Aug 11;26(16):5113–5120. doi: 10.1021/bi00390a033. [DOI] [PubMed] [Google Scholar]
- Sturtevant J. M., Mateo P. L. Proposed temperature-dependent conformational transition in D-amino acid oxidase: a differential scanning microcalorimetric study. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2584–2587. doi: 10.1073/pnas.75.6.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thulborn K. R., Sawyer W. H. Properties and the locations of a set of fluorescent probes sensitive to the fluidity gradient of the lipid bilayer. Biochim Biophys Acta. 1978 Aug 4;511(2):125–140. doi: 10.1016/0005-2736(78)90308-5. [DOI] [PubMed] [Google Scholar]
- Tilley L., Thulborn K. R., Sawyer W. H. An assessment of the fluidity gradient of the lipid bilayer as determined by a set of n-(9-anthroyloxy) fatty acids (n = 2, 6, 9, 12, 16). J Biol Chem. 1979 Apr 25;254(8):2592–2594. [PubMed] [Google Scholar]
- Van Dijck P. W., De Kruijff B., Van Deenen L. L., De Gier J., Demel R. A. The preference of cholesterol for phosphatidylcholine in mixed phosphatidylcholine-phosphatidylethanolamine bilayers. Biochim Biophys Acta. 1976 Dec 2;455(2):576–587. doi: 10.1016/0005-2736(76)90326-6. [DOI] [PubMed] [Google Scholar]
- Vincent M., de Foresta B., Gallay J., Alfsen A. Nanosecond fluorescence anisotropy decays of n-(9-anthroyloxy) fatty acids in dipalmitoylphosphatidylcholine vesicles with regard to isotropic solvents. Biochemistry. 1982 Feb 16;21(4):708–716. doi: 10.1021/bi00533a019. [DOI] [PubMed] [Google Scholar]


