Abstract
This work evaluated the kinetics of events that occur in the placenta of Calomys callosus after Toxoplasma gondii infection. Animals on the first day of pregnancy (dop) and virgin nonpregnant females were perorally infected with 20 cysts of T. gondii strain ME49. After 100 days of infection, the virgin animals were mated and received an additional 20 cysts on the first dop. The placentas and the embryos from both acutely and chronically infected animals were analyzed up to day 20 of pregnancy by morphological and immunocytochemical assays. Noninfected and infected animals exhibited placenta with normal morphology. From the seventh dop and infection onwards, liver and spleen cells of the infected animals contained several parasitophorous vacuoles. On the 13th day, the maternal blood present at the placental blood spaces contained T. gondii-infected leukocytes. Infected placental cells were only seen on the 15th dop, being the trophoblast giant cells, the first cell type to contain signs of the parasite internalization, followed by labyrinth zone cells 24 h later and spongiotrophoblast cells only after the 19th dop. Fetal liver and brain were infected by T. gondii concomitantly to the labyrinth cell infection. No signals of infection were observed on placentas and embryos from chronically infected animals. Therefore, considering the sequence of events leading to the infection of the various organs, it could be hypothesized that the placenta is infected later on during pregnancy, which may be related to the defense roles played by this structure. However, trophoblast giant cells are unable to completely stop the progression of T. gondii infection towards the fetal tissues. C. callosus was demonstrated to be a suitable experimental model to study the dynamics of congenital toxoplasmosis.
Trophoblast cells belong to a subset of the embryonic cell population and are directly involved in the process of embryo implantation in the endometrium (13). After crossing the uterine epithelial layer, the trophoblast rapidly spreads out both mesometrially and antimesometrially at the endometrial stroma, phagocytosing the remaining maternal cells to contact and breach the maternal vasculature (3, 4, 13, 31). Particularly in Calomys callosus, this phase is a very early and rapid event, lasting approximately 12 h after the onset of implantation (15). Always in direct contact with maternal blood, after day 9 of pregnancy the polar trophoblast differentiates and together with the mesometrial decidua initiates the placentation process. The mature placenta completely isolates the maternal from the fetal organism, plays different and pivotal roles for embryo development such as nutrition and gas exchange, and participates in the immunoregulation of the pregnancy (22). In addition to being the natural barrier through which infection has to cross to reach the fetal organism, the mature placenta can exhibit mechanisms of defense which are partially or completely effective, depending on the pathogen characteristics and immunological status of the maternal organism (10).
In the present study we explored the possibility of establishing a model for congenital toxoplasmosis, in order to allow a more comprehensive study of the invasion process of a parasite through the placental trophoblast layers and of the role of the trophoblast as an embryo defense barrier.
Congenital toxoplasmosis has been described in the literature in a variety of experimental models (8, 17, 19, 27, 29). However, it is not yet well known what the role of the implanting trophoblast is in relation to the initial events leading to the passage of Toxoplasma gondii from the maternal to embryonic cell population.
In a previous study it was demonstrated that a highly virulent strain of T. gondii (RH) is able to infect trophoblast cells during the early blastocyst-endometrial relationship (16). However, the high virulence of this Toxoplasma strain killed the animals before the embryo had been contaminated, thus making it impossible to recognize the congenital pathway of the infection.
In the present investigation we use a cystogenic strain of T. gondii (ME49) to study the kinetics of events that occur in the placenta of C. callosus animals acutely or chronically infected.
MATERIALS AND METHODS
Animals.
C. callosus of the Canabrava strain came from a resident colony housed at the Institute of Tropical Medicine of São Paulo. This colony was kindly provided by Judith Kloetzel. The animals were kept on a 12-h light, 12-h dark cycle in a temperature-controlled room (25 ± 2°C) with food and water ad libitum. Virgin females aged between 3 and 4 months were caged with males overnight and checked daily as to the presence of a vaginal plug. The day on which this sign was found was designated as the first day of pregnancy (dop).
Parasites.
Cysts of T. gondii strain ME49 were obtained from brains of C. callosus animals. Various groups of animals were perorally infected 45 days earlier. The brains were removed, washed in sterile phosphate-buffered saline (PBS; 0.01 M, pH 7.2), and homogenized with a syringe and a 25- by-8-gauge needle. The brain preparations were further washed by centrifugation at 1,000 × g for 10 min in PBS.
Experimental infections.
A total of 40 C. callosus animals were studied in the present investigation. These animals were divided into six groups of five animals for acute-phase infection and two groups of five animals for chronic-phase infection.
Acute-phase studies.
Seronegative virgin females of C. callosus for T. gondii at the first dop were perorally infected with 20 cysts. These animals were sacrificed from the 12th to 20th dop. Placentas and embryos, as well as blood samples, were collected for morphological and serological analyses.
Chronic-phase studies.
Virgin females were perorally infected with 20 cysts. After 100 days of infection, the group of seropositive animals was mated and divided into two groups. The females of the first group were killed on the 20th dop. The females of the second group were reinfected at the first dop, with the same procedure as carried out for the primary infection. These animals were killed on the 20th dop and their placentas, embryos, and blood samples were collected for morphological and serological analyses. Brains of some embryos from both groups of animals were removed and homogenized. The cell suspensions were obtained and inoculated by the intraperitoneal route into C. callosus animals not yet infected with T. gondii. These animals were killed 30 days after inoculation, and their livers and brains, as well as blood samples, were collected for morphological and serological analyses.
Morphological and immunocytochemical studies.
For the purpose of conventional light microscopy, specimens were fixed by immersion with 10% paraformaldehyde in 0.1 M phosphate buffer (pH 7.0), dehydrated, and embedded in paraffin or methacrylate resin. Sections were stained with toluidine blue or submitted to cytochemical reactions for carbohydrates (periodic acid-Schiff [PAS] stain) (20).
For ultrastructural studies, the material was fixed by immersion in 2.5% glutaraldehyde plus 0.5 M sucrose in 0.1 M phosphate buffer (pH 7.0), dehydrated in ethanol, and embedded in Spurr resin. Ultrathin sections were stained with lead citrate and uranyl acetate, and they were then observed in a JEOL CX II electron microscope.
Immunolocalization of the parasites in paraffin sections placed on glass slides was carried out in accordance with the following protocol: (i) the samples were incubated for 10 min at room temperature with 5% acetic acid to block endogenous alkaline phosphatase; (ii) in order to block nonspecific binding sites, the samples were also treated with 2% normal goat serum diluted in PBS with 0.05 M Tris, pH 7.4 (TBS) for 30 min at 37°C; (iii) the preparations were incubated for 12 h at 4°C with rabbit anti-T. gondii serum. Negative controls were carried out by replacement of the primary antibodies with normal rabbit serum. (iv) The preparations were then rinsed in TBS and incubated with biotinylated goat anti-rabbit immunoglobulin G (Sigma Chemical Co., St. Louis, Mo.) for 30 min at 37°C. (v) The reaction signal was amplified by using the ABC system (Biomeda, Foster City, Calif.), developed with fast red-naphthol (Sigma Chemical Co.), and counterstained with Mayer's hematoxylin.
ELISA and ELISA avidity.
An enzyme-linked immunosorbent assay (ELISA) was carried out with some modifications (21). Briefly, polystyrene microtiter plates (Interlab, São Paulo, Brazil) were coated overnight at 4°C with soluble antigen from the RH strain of T. gondii at a protein concentration of 10 μg/ml in sodium carbonate buffer (0.06 M, pH 9.6). The plates were washed three times with PBS plus 0.1% Tween 20 (PBST) and incubated with serum samples of C. callosus, in duplicate, diluted at 1:32 in PBST. After incubation for 45 min at 37°C, the plates were incubated with an immunoenzymatic conjugate consisting of antibodies to gamma globulin from C. callosus linked to horseradish peroxidase type VI (Sigma Chemical Co.). After new washes, the reaction was developed with a substrate solution consisting of hydrogen peroxide (0.04%) and o-phenylenediamine (0.5 mg/ml) diluted in citrate-phosphate buffer (0.1 M, pH 5.0). After incubation for 15 min at room temperature, the reaction was stopped with 2 N H2SO4. The absorbances were measured at 492 nm by using a plate reader system (Titertek Multiskan Plus; Flow Laboratories, Geneva, Switzerland). The results were expressed by using the following ELISA index (EI) formula: EI = [(ABSx − ABSn)/(ABSp − ABSn)] × 100, where EI is the ELISA index, ABSx is the sample mean absorbance, ABSn is the negative control mean absorbance, and ABSp is the positive control mean absorbance (35).
Based on screening tests performed with negative and positive controls, the value of 15 was established as the cutoff for the ELISA index. Thus, samples that presented an EI above or equal to 15 were considered reactive samples.
The ELISA avidity assay was carried out as described above for the conventional ELISA, except for a modification in the serial washing step after incubation with serum samples. This modification consisted of the addition of 6 M urea to the diluent (PBST) for 10 min at room temperature, in parallel with microplates submitted to the conventional ELISA. The serum samples, as well as positive and negative controls, were tested in quadruplicate at a dilution of 1:32.
The avidity index (AI) was the ratio between the absorbance (ABS) obtained for the microplates washed with urea (U+) and the microplates washed without urea (U−). The AI values were expressed in percentages, as described elsewhere (9, 18), and calculated according to the following formula: AI = [ABS(U+)/ABS(U−)] × 100.
RESULTS
Morphological and immunocytochemical studies of the acute phase of T. gondii infection.
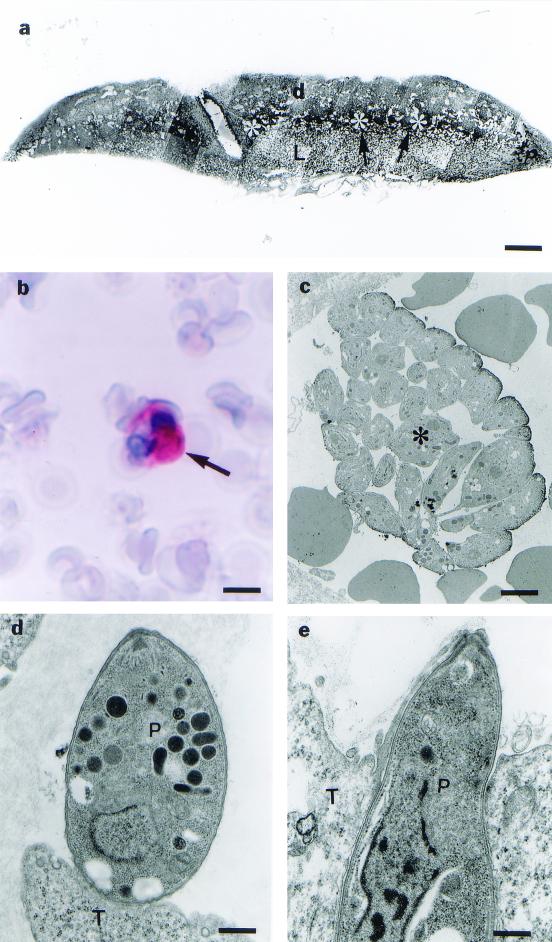
Placentas of infected C. callosus animals showed a normal appearance, containing basal decidua, spongiotrophoblast cells, giant trophoblastic cells, and a labyrinth zone (Fig. 1a).
FIG. 1.
(a) Photomicrograph of a 21-day placenta from C. callosus, showing the labyrinth area (L), the region of giant trophoblastic cells (arrowhead), spongiotrophoblasts (asterisk) and decidua (d). Bar, 1.57 μm. (b) Photomicrograph of the neutrophil containing T. gondii after 13 days of infection, assayed by immunohistochemistry using alkaline phosphatase (arrow). Bar, 4.61 μm. (c) Electromicrograph of T. gondii cells at the materno-placental interface, after 14 days of infection, showing a group of parasites (✻) at the maternal blood vessel. Bar, 1.85 μm. (d) Electromicrograph of T. gondii (P) cells attached at the surface of giant trophoblastic cells (T). Bar, 0.71 μm. (e) Electromicrograph of T. gondii (P) cells partially internalized in a giant trophoblastic cell (T) after the 15th dop and infection. Bar, 0.71 μm.
On the 13th dop, leukocytes located in the maternal vasculature exhibited parasitophorous vacuoles with T. gondii in their cytoplasm (Fig. 1b), although other organs such as the liver and spleen were infected earlier, at the 7th dop. Figures resembling discharge of parasites in the maternal vascular bed could also be observed (Fig. 1c). Exclusively on the 15th dop, steps of the invasion of the trophoblast giant cells by T. gondii could be identified (Fig. 1d and e). No other trophoblast cell population presented signals of infection in this particular period of the pregnancy (Fig. 2a).
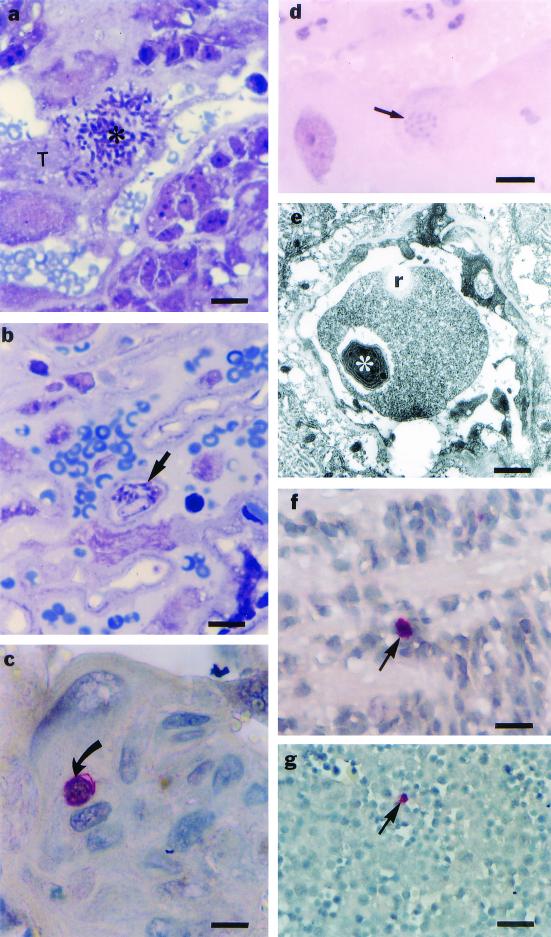
FIG. 2.
Photomicrographs showing the kinetics of infection of the trophoblastic cell populations from placentas of C. callosus and fetal tissues. (a) Rupture of a giant trophoblastic cell (T) by T. gondii (asterisk) after the 15th dop and infection. Bar, 11 μm. (b) Presence of T. gondii (arrow) in the region of labyrinth after the 16th dop. Bar, 11 μm. (c) Presence of T. gondii (arrow) in a spongiotrophoblast at the 19th dop. Bar, 11 μm. (d) T. gondii inside a giant trophoblastic cell at the 20th dop, showing that the parasite was unstained by PAS (arrow). Bar, 11 μm. (e) Electromicrograph from a fetal blood vessel in the labyrinth region at the 20th dop, showing the presence of T. gondii (asterisk) inside a reticulocyte (r). Bar, 1.30 μm. (f) Fetal nervous tissue containing T. gondii stained by alkaline phosphatase substrate in the immunohistochemistry assay (arrow) at the 16th dop. Bar, 11 μm. (g) Fetal liver containing T. gondii inside a hepatocyte stained by alkaline phosphatase substrate in the immunohistochemistry assay (arrow). Bar, 48 μm.
Infection of labyrinth zone cells occurred on the 16th dop (Fig. 2b) concomitantly with infection of the brain and liver, and infected spongiotrophoblast cells were only observed close to the end of pregnancy (19th dop) (Fig. 2c). In any of the trophoblast cell populations, the parasites exhibited low reactivity to a PAS reaction (Fig. 2d).
Signals of infection of fetal tissue by T. gondii occurred concomitantly with the infection of the labyrinth zone on the 16th dop. At this time of the pregnancy, T. gondii infection could be found in fetal reticulocytes (Fig. 2e) and liver and brain (Fig. 2f and g).
The above-mentioned findings were consistently observed in all animals from all groups in the acute-phase study.
Serological analysis of the acute phase of T. gondii infection.
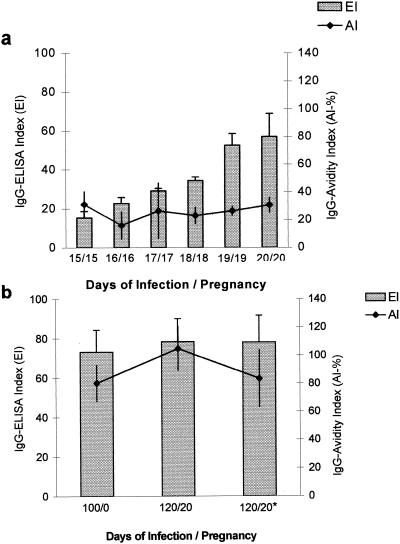
The results concerning the EI of the antibody showed ELISA indices higher than 15. Therefore, the animals were considered positive for T. gondii. Furthermore, the AIs were also very low (24.39%). These results are depicted in Fig. 3a, in which an acute phase of toxoplasmosis is characterized.
FIG. 3.
(a) Acute phase of infection by T. gondii in C. callosus; shown are means of EI and AI values for antibodies in C. callosus from the 15th to 20th dop and inoculation with T. gondii strain ME49. (b) Chronic phase of infection by T. gondii in C. callosus; shown are EIs and AIs for antibodies in C. callosus after 100 days of infection with T. gondii strain ME49 and before pregnancy (100/0); after 120 days of infection and the 20th dop (120/20); and after 120 days of infection and the 20th dop with also reinfection with 20 cysts of T. gondii strain ME49 (120/20∗).
Serological, morphological, and immunocytochemical studies of the chronic phase of T. gondii infection. (i) Serological analysis before pregnancy.
All animals that remained infected with T. gondii for 100 days or more showed EIs higher than 15 and high AIs (80.34%), characterizing a chronic phase of toxoplasmosis (Fig. 3b).
(ii) Immunocytochemical study.
All females after 100 days of infection had cysts in their brains. Placentas and embryos obtained from these females, however, did not show signals of infection upon immunocytochemical assay. In the same manner, placentas and embryos from females of the group that received a second reinfection on day 1 of pregnancy, after 100 days of the primary infection, were not infected. The above-mentioned findings were consistently observed in all animals from both groups in the chronic-phase study.
Animals serologically negative for T. gondii, which received brain homogenates of embryos obtained from females that received a second infection on the first dop also did not show signs of infection in the liver. The serum analysis of these animals revealed EIs lower than 15, indicating that there was not congenital transmission to those embryos.
Serological analysis after pregnancy.
As shown in Fig. 3b, the serum analysis presented EIs higher than 15 and high AIs at 120 days of infection and the 20th dop. Very similar results were also observed in the females reinoculated 100 days after the primary infection with 20 cysts of T. gondii and analyzed at the 20th dop. Serum samples from these animals exhibited EIs higher than 15 and high AIs (Fig. 3b).
DISCUSSION
Experimental models of congenital toxoplasmosis have been described in the literature since the early 1950s (2, 8, 17, 19, 23, 26, 27). However, there is no information concerning the kinetics of events leading to the understanding of how T. gondii tachyzoites reach the fetal tissues. In addition, no data describing the role played by trophoblast cells interacting with the parasites in the placenta from infected mothers have been published.
In this study we present the results from experiments showing the serial events in different compartments of the placentas from C. callosus animals infected with the ME-49 strain of T. gondii. Our data demonstrated that the infection of the placenta starts with giant trophoblastic cells, at the 15th dop, followed by cells from the labyrinth at dop 16 and spongiotrophoblasts at dop 19.
These findings are anatomically comprehensive and have also been found in other congenital infection models (1, 5, 34). The progression of chlamydial infection in the mouse placenta shows that the first cell population to be affected is the decidua and neutrophils located at the limit of the maternal and fetal tissues. On the embryonic side, the first fetal invaded area is the giant trophoblastic cell layer (5). In a similar manner, other intracellular pathogens, such as Coxiella burnetti and Brucella abortus, also preferentially first invade this cell population (1, 34).
In rodents, at least two points seem to explain these findings. First, the boundary region between the fetal and maternal placenta is characterized by the presence of a low number of macrophages and T cells, making this region more susceptible to pathogen infections (25). Furthermore, the giant cells of the junctional zone of the placenta form a mesh which receives the maternal blood directly from the uterine vessels (38). The blood goes through the spaces of this mesh before reaching the labyrinth zone for molecular exchange.
As already described by Fadul et al. (14), T. gondii is systemically spread through maternal leukocytes and, when these cells are lysed at the maternal-fetal interface, the parasites may infect the giant trophoblastic cells. The labyrinth area is the main region of exchange of nutrients between maternal and fetal blood and, therefore, once infected by pathogens there is no difficulty for the pathogens to reach the fetal tissues. This hypothesis is compatible with our experimental model, since a concurrence between the infection of the labyrinth area and the fetal tissues was observed. However, the infection of the labyrinth area started only after the infection of the giant trophoblastic cells. The spreading of the parasites toward fetal tissues occurred very quickly right after the infection of the labyrinth zone. Probably cells from an erythrocytic lineage also contribute to carry the parasites to fetal tissues, as described by Tanabe et al. (32).
Concerning the late infection of the spongiotrophoblast, one could hypothesize that this phenomenon was due to the presence of a wide layer of giant trophoblast cells protecting those maternal blood cells. However, the rationale for a late infection of the spongiotrophoblast remains unclear and deserves more investigation, considering that these cells present endocrine and stock functions (7, 24, 30). Therefore, considering the sequence of events leading to the infection of the various organs, it could be hypothesized that the placenta is infected later on during pregnancy, which may be related to the defense roles played by this structure.
The serological evaluation of T. gondii infection in relation to its time of occurrence is very important to define the acute phase, considering that it is in this phase that the major problems of congenital infection in humans and experimental models occur.
Determination of the low avidity of immunoglobulin G antibodies to T. gondii is a very useful tool for evaluating acute infection, reflecting the degree of maturation of these molecules during the immune response (6). Our results clearly demonstrate that congenital transmission only occurs when the antibodies present have low avidity, and these findings were correlated with the acute phase of infection, when the parasites inside the giant trophoblasts were unstained by PAS, characterizing the presence of tachyzoites (12).
Among the experimental models, rats, unlike mice, are good animals for the study of congenital toxoplasmosis. Similarly as occurs in humans, chronically infected pregnant rats protect their fetuses from congenital infection (23, 26, 33, 37), even though it was found that the occurrence of a low percentage of congenital transmission of T. gondii occurs during the chronic phase of maternal infection (11). Our results showed that all females in the chronic phase of infection, which were monitored by the presence of PAS-positive cerebral cysts and high-avidity antibodies to T. gondii, did not transmit the infection to their fetuses.
Although the macaque model is considered the closest model to human congenital toxoplasmosis (28, 36), there are many restrictions to working with primates and, therefore, the search for alternative experimental models to study congenital toxoplasmosis is very useful. Taken together, the results presented herein demonstrate that C. callosus is an appropriate experimental model to study the dynamics of congenital toxoplasmosis that occurs in humans and in a wide range of mammals.
Acknowledgments
This work was financially supported by Brazilian Research Agencies (FAPEMIG, FAPESP, CNPq, and CAPES).
We thank Nelly Patriarcha for revising the manuscript.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Baumgärtner, W., and S. Bachmann. 1992. Histological and immunocytochemical characterization of Coxiella burnetti-associated lesions in the murine uterus and placenta. Infect. Immun. 60:5232-5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bervely, J. K. A. 1959. Congenital transmission of toxoplasmosis through successive generations of mice. Nature 183:1348-1349. [DOI] [PubMed] [Google Scholar]
- 3.Bevilacqua, E., and P. A. Abrahamsohn. 1989. Trophoblast invasion during implantation of the mouse embryo. Arch. Biol. Med. Exp. 22:107-118. [PubMed] [Google Scholar]
- 4.Billington, W. D. 1971. Biology of trophoblast. Adv. Reprod. Physiol. 5:27-66. [PubMed] [Google Scholar]
- 5.Buendía, A. J., J. Sánchez, M. C. Martínez, P. Cámara, J. A. Navarro, A. Rodolakis, and J. Salinas. 1988. Kinetics of infection and effects on placental cell population in murine model of Chlamydia psittaci-induced abortion. Infect. Immun. 66:2128-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Camargo, M. E., S. M. Silva, P. G. Leser, and C. H. Granato. 1991. Avidez de anticorpos IgG específicos como marcadores de infecção primária recente pelo Toxoplasma gondii. Rev. Inst. Med. Trop. S. Paulo 33:213-218. [PubMed] [Google Scholar]
- 7.Campbell, W. J., S. Deb, S. C. Kwok, J. A. Joslin, and M. J. Soares. 1989. Differential expression of placental lactogen II and prolactin-like protein-A in the rat chorioallantoic placenta. Endocrinology 125:1565-1574. [DOI] [PubMed] [Google Scholar]
- 8.Cowen, D., and A. Wolf. 1950. Experimental congenital toxoplasmosis I. The vagina as a portal of entry of Toxoplasma in the mouse. J. Exp. Med. 92:393-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cozon, G. J. N., J. Ferrandiz, H. Nebhi, M. Wallon, and F. Peyron. 1998. Estimation of the avidity of immunoglobulin G for routine diagnosis of chronic Toxoplasma gondii infection in pregnant women. Eur. J. Clin. Microbiol. Infect. Dis. 17:32-36. [DOI] [PubMed] [Google Scholar]
- 10.Djurkovic-Djakovic, O. 1995. Toxoplasma infection and pathological outcome of pregnancy. Gynecol. Obstet. Investig. 40:36-41. [DOI] [PubMed] [Google Scholar]
- 11.Dubey, J. P., S. K. Shen, O. C. H. Knok, and P. Thulliez. 1997. Toxoplasmosis in rats (Ratus norvegicus): congenital transmission to first and second generation offspring and isolation of Toxoplasma gondii from seronegative rats. Parasitology 115:9-14. [DOI] [PubMed] [Google Scholar]
- 12.Dubey, J. P., D. S. Lindsay, and C. A. Speer. 1998. Structures of Toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology and development of tissue cyst. Clin. Microbiol. Rev. 11:267-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Enders, A. C., and S. Schlafke. 1969. Cytological aspects of trophoblast-uterine interaction in early implantation. Am. J. Anat. 125:1-30. [DOI] [PubMed] [Google Scholar]
- 14.Fadul, C. E., J. Y. Channon, and L. H. Kasper. 1995. Survival of immunoglobulin G-opsonized Toxoplasma gondii in nonadherent human monocytes. Infect. Immun. 63:4290.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferro, E. A. V., and E. Bevilacqua. 1994. Trophoblastic invasion of the uterine epithelium in Calomys callosus (Rodentia, Cricetidae). J. Morphol. 221:139-152. [DOI] [PubMed] [Google Scholar]
- 16.Ferro, E. A. V., E. Bevilacqua, S. Favoreto, Jr., D. A. O. Silva, R. A. Mortara, and J. R. Mineo. 1999. Calomys callosus (Rodentia: Cricetidae) trophoblast cells as host cells to Toxoplasma gondii in early pregnancy. Parasitol. Res. 85:647-654. [DOI] [PubMed] [Google Scholar]
- 17.Graham, D. I., J. Hay, W. M. Hutchison, and J. C. Siim. 1984. Encephalitis in mice with congenital ocular toxoplasmosis. J. Pathol. 142:265-277. [DOI] [PubMed] [Google Scholar]
- 18.Holliman, R. E., R. Raymond, N. Renton, and J. D. Johnson. 1994. The diagnosis of toxoplasmosis using IgG avidity. Epidemiol. Infect. 112:399-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hutchison, W. M., J. Hay, W. R. Lee, and J. C. Siim. 1982. A study of cataract in murine congenital toxoplasmosis. Ann. Trop. Med. Parasitol. 76:53-70. [DOI] [PubMed] [Google Scholar]
- 20.McMannus, J. F. A. 1948. Histological and histochemical use of periodic acid. Stain Technol. 23:99-108. [DOI] [PubMed] [Google Scholar]
- 21.Mineo, J. R., M. E. Camargo, and A. W. Ferreira. 1980. Enzyme-linked immunosorbent assay for antibodies to Toxoplasma gondii polysaccharides in human toxoplasmosis. Infect. Immun. 27:283-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mossman, H. W. 1987. Vertebrate fetal membranes. Rutgers University Press, New Brunswick, N.J.
- 23.Paulino, J. P., and R. W. A. Vitor. 1999. Experimental congenital toxosplasmosis in Wistar and Holtzmann rats. Parasite 6:63-66. [DOI] [PubMed] [Google Scholar]
- 24.Rasmusssen, C. A., K. E. Orwig, S. Vellucci, and M. J. Soares. 1997. Dual expression of prolactin related protein in decidua and trophoblast tissue pregnancy in rats. Biol. Reprod. 56:647-654. [DOI] [PubMed] [Google Scholar]
- 25.Redline, R. W., and C. Y. Lu. 1988. Specific defects in the anti-listerial immune response in discrete regions of the murine uterus and placenta account for susceptibility to infection. J. Immunol. 140:3947-3955. [PubMed] [Google Scholar]
- 26.Remington, J. S., L. Jabobs, and L. Melton. 1961. Congenital transmission of toxoplasmosis from mother animals with acute and chronic infection. J. Infect. Dis. 108:163-173. [DOI] [PubMed] [Google Scholar]
- 27.Roberts, C. W., and J. Alexander. 1992. Studies on a murine model of congenital toxoplasmosis: vertical disease transmission only occurs in BALB/c mice infected for the first time during pregnancy. Parasitology 104:19-23. [DOI] [PubMed] [Google Scholar]
- 28.Schoondermarkvandeven, E., W. Melchers, J. Galama, W. Camps, T. Eskes, and J. Meuwissen. 1993. Congenital toxoplasmosis: an experimental study in rhesus monkeys for transmission and prenatal diagnosis. Exp. Parasitol. 77:200-211. [DOI] [PubMed] [Google Scholar]
- 29.Sims, T. A., J. Hay, and I. C. Talbot. 1988. Host-parasite relationship in the brains of mice with congenital toxoplasmosis. J. Pathol. 156:255-261. [DOI] [PubMed] [Google Scholar]
- 30.Soares, M. J., B. M. Chapman, C. A. Rasmussen, G. Daí, T. Kamei, and K. E. Orwing. 1996. Differentiation of trophoblast endocrine cells. Placenta 17:277-289. [DOI] [PubMed] [Google Scholar]
- 31.Tachi, S., C. Tachi, and H. R. Lindner. 1970. Ultrastructural features of blastocyst attachment and trophoblastic invasion in the rat. J. Reprod. Fert. 21:37-56. [DOI] [PubMed] [Google Scholar]
- 32.Tanabe, K., T. Asi, I. Kimata, and S. Takada. 1979. Penetration of maturating red blood cells by Toxoplasma gondii. J. Gen. Microbiol. 113:433-437. [DOI] [PubMed] [Google Scholar]
- 33.Thiermann, I. E. 1957. Transmission congenita del Toxoplasma gondii en ratas com infection leve. Biologica (Santiago) 23:59-67. [Google Scholar]
- 34.Tobias, L., D. O. Cordes, and G. G. Schurig. 1993. Placental pathology of the pregnant mouse inoculated with Brucella abortus strain 2308. Vet. Pathol. 30:119-129. [DOI] [PubMed] [Google Scholar]
- 35.Turunen, H., K. A. Vuorio, and P. O. Leiniki. 1983. Determination of IgG, IgM and IgA antibody responses in human toxoplasmosis by enzyme-linked immunosorbent assay (ELISA). Scand. J. Dis. 15:307-311. [DOI] [PubMed] [Google Scholar]
- 36.Wong, M. M., W. J. Kozek, S. L. Karr, Jr., M. A. Brayton, J. H. Theis, and A. G. Hendrickx. 1979. Experimental congenital infection of Toxoplasma gondii in Macaca arctoides. Asian J. Infect. Dis. 3:61-67. [PubMed] [Google Scholar]
- 37.Zenner, L., J. Estaquier, F. Darcy, P. Maes, A. Capron, and M. F. Cesbron-Delaw. 1999. Protective immunity in the rat model of congenital toxoplasmosis and the potential of excreted-secreted antigens as vaccine components. Parasite Immunol. 21:261-272. [DOI] [PubMed] [Google Scholar]
- 38.Zuckermann, F., and J. R. Head. 1986. Isolation and characterization of trophoblast from murine placenta. Placenta 7:349-364. [DOI] [PubMed] [Google Scholar]