Abstract
Interleukin-10 (IL-10) is thought to play an important role in the regulation of microbial immunity. While T-cell-derived IL-10 has been shown to suppress cell-mediated immunity, there has been debate as to whether antigen presenting cell (APC)-derived cytokine can perform the same function in vivo. To assess the influence of APC-produced IL-10 on host resistance to mycobacterial infection, transgenic mice expressing human IL-10 under the control of the major histocompatibility complex class II promoter (hu10Tg) were infected with Mycobacterium avium, and bacterial burdens and immune responses were compared with those observed in wild-type (wt) animals. Hu10Tg mice harbored substantially higher numbers of M. avium and succumbed 16 to 18 weeks postinfection. The granulomas in infected hu10Tg mice showed marked increases in both acid-fast bacilli and host macrophages. In addition, these animals displayed a dramatic increase in hepatic fibrosis. The increased susceptibility of the hu10Tg mice to M. avium infection is independent of T-cell-produced endogenous murine IL-10, since bacterial burdens in mice derived by crossing hu10Tg mice with murine IL-10-deficient mice were not significantly different from those in hu10Tg mice. Importantly, gamma interferon (IFN-γ) responses were not decreased in the infected transgenic animals from those in wt animals, suggesting the normal development of Th1 effector cells. In contrast, mycobacterium-induced macrophage apoptosis as well as production of TNF, nitric oxide, and IL-12p40 were strongly inhibited in hu10Tg mice. Together, these data indicate that APC-derived IL-10 can exert a major inhibitory effect on control of mycobacterial infection by a mechanism involving the suppression of macrophage effector function and apoptosis.
Mycobacteria are intracellular pathogens that primarily invade phagocytes. Activation of infected macrophages and control of mycobacterium replication is essentially dependent on gamma interferon (IFN-γ) produced by T lymphocytes (17). Some bacilli, however, resist killing and survive within macrophages in the face of strong T-cell responses. Mechanisms that alter host immune functions are thought to contribute to the persistence of mycobacterial infection. For example, mycobacteria can inhibit bacterial antigen processing by preventing phagosome maturation (1, 8). In addition, the organisms may evade immune clearance by suppressing macrophage apoptosis (4, 19). Finally, the induction of down-regulatory cytokines, such as interleukin 10 (IL-10) and transforming growth factor β, that can inhibit IFN-γ production by T cells and/or macrophage activation (15, 23, 37, 41), may also contribute to the long-term intracellular survival of the pathogen.
IL-10 is of special interest as a possible evasion strategy because of its strong induction during mycobacterial infection (2, 5, 6, 10, 11). IL-10 profoundly inhibits a broad spectrum of macrophage functions, including monokine synthesis, nitric oxide (NO) production, and expression of costimulatory molecules (31). While IL-10-deficient mice in most studies do not show markedly enhanced resistance to mycobacterial infection, such observations do not rule out a role for IL-10 as one of several redundant mechanisms regulating host resistance to these microorganisms.
Overproduction of IL-10 by T cells has been associated with suppressed immunity and increased susceptibility to mycobacterial infection in both humans and mice (6, 29). In addition to T lymphocytes, antigen-presenting cells (APC) (i.e., dendritic cells, macrophages, and B lymphocytes) are a major source of IL-10 in the immune response to mycobacterial infection (10, 16). IL-10 produced by APC could play a major role as an autocrine regulator of macrophage activation controlling clearance of intracellular bacteria and when triggered during the early stages of host-mycobacterium encounter could strongly influence the generation of effector T cells during the subsequent adaptive response.
In this study we have evaluated the possible influence of APC-derived IL-10 on host resistance to mycobacterial infection by studying the course of Mycobacterium avium infection in transgenic mice expressing human IL-10 (hu10Tg) under the control of the major histocompatibility complex class II (Ea) promoter. These animals constitutively express the cytokine at low levels in macrophages, dendritic cells, and B cells but not in the T-lymphocyte compartment (22). Since human IL-10 is fully functional in mice yet is immunochemically distinct from murine IL-10, one can study its effects in vivo and distinguish them from those of the endogenous cytokine. In addition, because the cytokine is constitutively expressed, these animals offer the opportunity to elucidate the effects of IL-10 when it is present at the onset of mycobacterial infection, a situation that may occur in humans coinfected with other pathogens known to stimulate IL-10 production by macrophages (38).
We found that hu10Tg mice show enhanced susceptibility to M. avium infection as well as augmented immunopathology, effects that appear to result primarily from the inhibition of macrophage apoptosis and activation. These findings indicate that APC-derived IL-10 can be an important factor influencing mycobacterial growth as well as the persistence of the pathogen in latently infected hosts.
MATERIALS AND METHODS
Mice.
BALB/c, IL-10−/− (on BALB/c background), hu10Tg (22), and hu10Tg/mu IL-10−/− mice (generated by crossing hu10Tg and IL-10−/− mice) were bred at Taconic Farms (Germantown, N.Y.). Human IL-10-expressing, heterozygous hu10Tg and hu10Tg/mu IL-10−/− mice were identified by PCR screening with human IL-10-specific primers (22). Female mice, 8 to 12 weeks old, were used in all experiments.
Bacteria and soluble mycobacterial antigens.
M. avium (strain 2-151-SmT) (12) bacilli harvested from infected wild-type (wt) mice were expanded once in Middlebrook 7H9 liquid medium supplemented with albumin-dextrose-catalase (Difco, Detroit, Mich.) for 7 days at 37°C. Aliquots of the bacteria were stored at −70°C. To prepare M. avium antigens (MAVAg) for in vitro assays, the mycobacteria were lysed by repeated sonication and the soluble fraction was collected following centrifugation (2,000 × g, 15 min) of the bacterial lysates. The resulting preparation was sterile filtered, and the protein concentration was determined by a bicinchoninic acid protein assay (Pierce, Rockford, Ill.) according to the manufacturer's instructions.
M. avium infection and quantification of bacterial load.
Mice were infected intravenously (i.v.) with 5 × 106 CFU of M. avium. Bacterial loads in infected mice were determined at various time points following infection. Spleens and lungs were homogenized in 2 ml of sterile water, and 100-μl aliquots were used for preparing serial dilution of the bacteria. The diluted homogenates were placed on oleic acid-albumin-dextrose-catalase-supplemented Middlebrook 7H11 Bacto agar (Difco), and colonies were counted visually after 14 days. Bacterial load was expressed as log10 CFU.
Histopathology and quantitation of fibrosis and apoptosis in tissues.
Tissue sections from livers, spleens, and lungs were fixed with formalin, sectioned, and stained with hematoxylin and eosin. The Ziehl-Neelsen method was used to stain acid-fast mycobacteria in tissue sections. Collagen deposition in livers, determined as hydroxyproline, was measured as previously described (7). Apoptosis was measured in formalin-fixed, paraffin-embedded tissues by the terminal deoxynucleotidyl transferase-UTP-nick-end-labeling (TUNEL) assay using a commercial kit (Intergen, New York, N.Y.) in the Pathology/Histotechnology Laboratory (National Cancer Institute, Frederick, Md.).
Cell isolations, tissue cultures, and cytokine assays.
Single-cell suspensions were prepared from spleens of naïve and infected mice. T-cell populations, containing approximately 80% CD3+ cells (data not shown), were purified using T-cell enrichment columns (R & D, Minneapolis, Minn.). To prepare splenic T-cell-depleted adherent cells, Thy1.2+ cells were first removed from total spleen cells by magnetic cell sorting (Miltenyi Biotec GmbH, Auburn, Calif.). The resulting preparation, containing less than 5% CD3+ cells as determined by flow cytometry (data not shown), was incubated in a 24-well plate (5 × 106 cells/ml) for 2 h at 37°C. After removal of nonadherent cells, macrophage-enriched adherent cells were used for further assays. Cells were cultured in RPMI 1640 medium containing 10% heat-inactivated fetal calf serum, 100 U of penicillin/ml, 100 μg of streptomycin/ml, 2 μM glutamine, 10 mM HEPES, and 50 μM 2-mercaptoethanol.
Cytokine secretion profiles were determined in culture supernatants of splenocytes (4 × 106/ml) stimulated with medium, plate-bound anti-CD3 (10 μg/ml), or MAVAg (20 μg/ml) for 72 h. When isolated T cells (106/ml) were used, irradiated splenocytes (4 × 106/ml) from naïve animals were added as a source of APC. IFN-γ, IL-12p40, and murine and human IL-10 (muIL-10 and huIL-10) were measured by enzyme-linked immunosorbent assay (ELISA) with monoclonal antibody (MAb) from BD PharMingen (San Diego, Calif.). TNF was detected using a commercial ELISA kit from Endogen (Woburn, Mass.), and NO was measured by Greiss assays. The limits of detection were 12.5 pg/ml, 6 U/ml, 20 pg/ml, 100 pg/ml, 20 pg/ml and 2 μM for the IFN-γ, muIL-10, huIL-10, IL-12p40, TNF, and NO assays, respectively.
Antibodies, cell surface staining, and flow cytometry.
The following monoclonal antibodies (PharMingen) were used for flow cytometry: Anti-CD44-fluorescein isothiocyanate (FITC) (clone IM7), anti-Gr-1 (Ly-6G)-phycoerythrin (clone RB6-8C5), anti-CD11b-allophycocyanin (clone M1/70), anti-CD4-phycoerythrin or PerCP (clone RM4-5), anti-CD8α-allophycocyanin (clone 53-6.7). Cells were stained for 20 min at 4°C and then washed with fluorescence-activated cell sorter buffer (2% bovine serum albumin and 0.1% NaN3 in phosphate-buffered saline). For apoptosis analysis, cells were surface stained with anti-Gr-1 and anti-CD11b MAb, washed, and then stained with FITC-labeled Annexin V and propidium iodide (PI) (Pharmingen). Cells in early apoptosis were defined as Annexin+ PI−. Data were collected using a FACSCalibur (Becton Dickinson Immunocytometry System, San Jose, Calif.) and analyzed with the CELL-Quest program.
In vivo MAb treatment.
Human IL-10-specific (clone JES3-19F1.1.1., American Type Culture Collection, Manassas, Va.) and control (clone GL113) rat MAb were purified from ascites by two sequential ammonium sulfate precipitations. For in vivo treatment, mice were injected intraperitoneally with 1 mg of MAb on days −1 and +1 of M. avium infection. The treatment was continued for 7 weeks by weekly injection of mice with the same dose of the MAb.
Statistics.
The significance of differences in mycobacterial burdens, tissue collagen, and cytokine levels between wt and hu10Tg mice was determined by means of Student's t test. P values of less than 0.05 were considered significant.
RESULTS
M. avium-infected human IL-10 transgenic mice develop higher bacterial burdens and display accelerated mortality relative to wt animals.
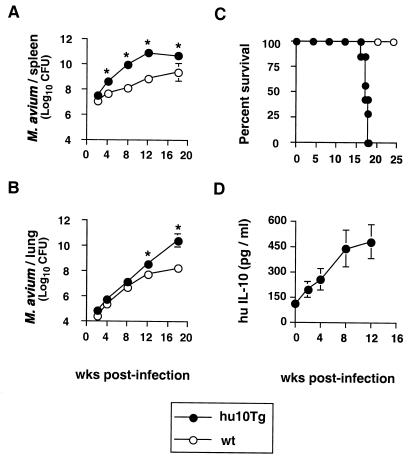
To determine if overexpression of IL-10 in APC can influence control of mycobacterial infection in vivo, hu10Tg mice and control wt BALB/c mice were infected i.v. with M. avium (5 × 106 CFU), and at various time points thereafter, spleens and lungs were collected and bacterial loads were compared. Although results were initially similar, by 4 weeks postinfection (p.i.) transgenic mice displayed significantly higher splenic bacterial burdens than wt animals (Fig. 1A). This difference had increased to approximately 2 logs by weeks 8 and 12. Elevated bacterial loads were also observed in the lungs of hu10Tg mice, but only at later time points (Fig. 1B). The infected transgenic mice showed obvious weight loss at approximately 12 weeks, and all succumbed between 16 to 18 weeks p.i., in contrast to the infected wt animals, which showed 100% survival during the same period (Fig. 1C).
FIG. 1.
Hu10Tg mice display increased bacterial burdens and mortality compared to wt animals. Following i.v. infection with M. avium, bacterial burdens (CFU) in the spleens (A) and lungs (B) of wt and hu10Tg mice were examined at various time points. The means (± SD) for four mice per time point are shown. The significance of the differences in numbers of CFU between hu10Tg and wt mice was determined using the Student t test (∗, P < 0.05). Data are representative results from one of two similar experiments. Survival of wt (n = 10) and hu10Tg (n = 8) mice was monitored following i.v. infection with M. avium (C). Serum human IL-10 of the same hu10Tg mice analyzed in panels A and B was measured by ELISA. The mean (± SD) concentrations of human IL-10 (n = 4) are shown (D).
The late-occurring increase in bacterial burden and abrupt mortality seen in transgenic mice versus wt mice suggested that huIL-10 production might itself be up-regulated during M. avium infection. Indeed, serum levels of the huIL-10 transgene product were found to increase almost fourfold over the first 12 weeks of infection (Fig. 1D), in parallel with the marked elevation in bacterial counts observed in the tissues of the same animals (Fig. 1A and B).
Infected hu10Tg mice develop abnormal granulomas and increased tissue fibrosis.
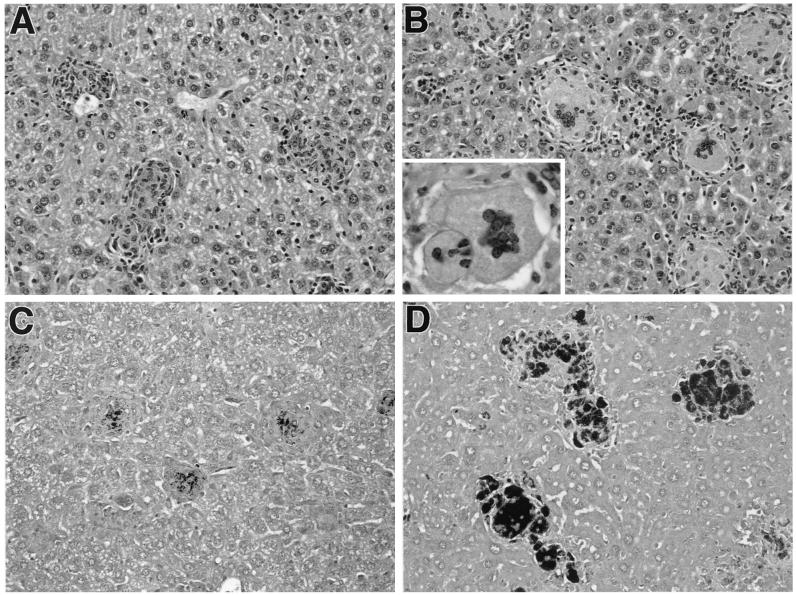
Since the granulomatous response is thought to play a critical role in the control of mycobacterial dissemination, we next examined if granuloma formation and the extent of bacterial replication within these tissue lesions differ between infected wt and transgenic mice. While changes were evident in both pulmonary and splenic granulomas, the lesions in liver presented the most obvious differences because of their compact morphology. In that tissue at 8 weeks of infection, granulomas were significantly (P = 0.04) larger in hu10Tg mice (mean diameter ± standard deviation [SD] = 102.4 ± 19.3 μm) than those in wt animals (71.2 ± 15.6 μm). Moreover, the granulomas in hu10Tg mice typically contained more macrophages (65.8% ± 4.4%) than the wt animals (55.5% ± 6.7%; P = 0.04), with many of those in the transgenic mice displaying a foamy cytoplasm (Fig. 2A and B). A corresponding difference in the level of lymphocytes in the granulomas of the hu10Tg and wt mice was also observed (32.5% ± 2.9% versus 42.0% ± 5.7%; P = 0.03). In addition, multinucleated giant cells were frequently observed in the granulomas of transgenic but not wt animals. Strikingly, the granulomas in hu10Tg mice contained large numbers of bacilli compared to those in wt animals, in which only a few bacilli were detected by acid-fast staining (Fig. 2C and D). The number of mycobacteria per cell also increased in the granulomas of hu10Tg mice, in keeping with the elevated numbers of CFU observed in these animals, suggesting a loss of control of mycobacterial replication within infected macrophages.
FIG. 2.
Hepatic granulomas in M. avium-infected hu10Tg mice contain increased numbers of multinucleated giant cells and abundant mycobacteria. Eight weeks after infection with M. avium, liver tissues from wt (A and C) or hu10Tg (B and D) mice were collected and fixed with formalin. Paraffin-embedded sections were stained with hematoxylin and eosin (A and B; magnification, ×200). The Ziehl-Neelsen method was used to stain acid-fast bacilli (C and D; magnification, ×200). The insert in panel B shows two multinucleated giant cells in hepatic granulomas of hu10Tg mice (magnification, ×630).
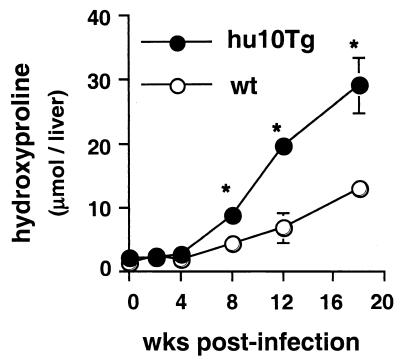
As an additional parameter of the tissue response, we quantified fibrosis (as measured by hydroxyproline levels) in the livers of the two groups of animals. Hepatic fibrosis was found to be dramatically increased in the livers of the infected hu10Tg mice (Fig. 3).
FIG. 3.
Hu10Tg mice develop severe hepatic fibrosis following M. avium infection. Hepatic fibrosis, measured as hydroxyproline, was compared for the wt and hu10Tg mice. The means (± SD) for four animals are shown. The significance of differences between wt and hu10Tg mice was determined using the Student t test (∗, P < 0.05). Data are representative results from one of two similar experiments.
Increased susceptibility of hu10Tg mice to M. avium infection is not the result of dysregulated endogenous murine IL-10 expression.
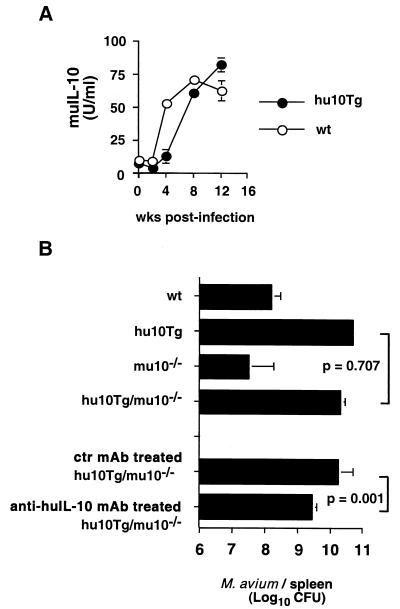
Since hu10Tg mice also express muIL-10, it was possible that the enhanced susceptibility of these animals to M. avium infection was due to abnormal expression of the endogenous murine cytokine. For this reason we compared M. avium-induced muIL-10 production in wt and hu10Tg mice by restimulating splenocytes with MAVAg in vitro and measuring cytokine levels in culture supernatants. As previously reported (2, 11), muIL-10 production by spleen cells steadily increased during the first 3 months of M. avium infection, reaching similar levels in the hu10Tg and wt animals (Fig. 4A). Indeed, if anything, production of muIL-10 was delayed in the hu10Tg mice. Results of T-cell enrichment experiments argued that T lymphocytes are a major cellular source of muIL-10 in the splenic cultures of infected animals, and T-cell depletion using anti-Thy1.2 magnetic-activated cell separation beads resulted in a greater than 95% reduction in the level of murine IL-10 produced by MAVAg-stimulated splenocytes (data not shown).
FIG. 4.
Increased susceptibility of hu10Tg mice to M. avium infection is independent of endogenous production of murine IL-10. Splenocytes of wt and hu10Tg mice isolated at weeks 0, 2, 4, 8, and 12 p.i. were restimulated in vitro with MAVAg. The mean (± SD) muIL-10 secreted in duplicate cultures is shown (A). Eight weeks after i.v. infection with 5 × 106 CFU of M. avium, bacterial loads in the spleens of wt, hu10Tg, muIL-10−/−, and hu10Tg/muIL-10−/− mice were determined (B). Bacterial burdens in hu10Tg/muIL-10−/− mice treated with control (GL113) or anti-huIL-10 (JES3-19F) MAb were also compared. The mean (± SD) numbers of CFU in spleens of 4 to 5 individual mice are shown. The Student t test was used to analyze the statistical difference between group means.
To formally rule out the contribution of muIL-10 to the increased susceptibility of hu10Tg mice, we compared bacterial burdens in these animals with those in hu10Tg/muIL-10−/− mice derived by crossing hu10Tg with IL-10 knockout mice. As shown in Fig. 4B, infected hu10Tg/muIL-10−/− mice developed splenic bacterial burdens indistinguishable from those found in hu10Tg animals in which endogenous muIL-10 expression is intact. Moreover, in vivo treatment of infected hu10Tg/muIL-10−/− mice with neutralizing MAb specific for human IL-10 MAb the day before infection and on a weekly basis thereafter resulted in a reduction in bacterial loads (Fig. 4B).
Infected hu10Tg mice generate normal mycobacterium-specific Th1 responses.
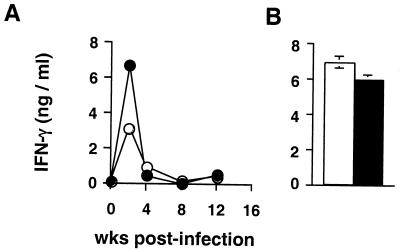
Since IFN-γ produced by Th1 cells is essential for the activation of macrophages to control mycobacterial replication, we examined if the increased susceptibility of hu10Tg mice to M. avium infection is the result of effects of huIL-10 on the generation of Th1 responses. We first examined IFN-γ production by unfractionated splenocytes restimulated in vitro with MAVAg. As shown by previous studies (2, 11), IFN-γ levels rapidly increased, reaching peak levels at 2 weeks p.i., and then returned to near baseline levels. The same kinetics of IFN-γ production was observed in wt and hu10Tg mice, the latter animals producing more IFN-γ at 2 weeks than the former. This suggests that suppression of IFN-γ production is not the primary cause of the reduced control of M. avium infection observed (Fig. 5A). To directly compare intrinsic T-lymphocyte responses in the two groups of animals, enriched splenic T cells were restimulated with MAVAg in the presence of APC from uninfected mice. Interestingly, while production of the cytokine was suppressed in cultures of unfractionated spleen cells at 8 weeks p.i., enriched T cells from the same spleens produced high levels of IFN-γ when provided with APC from naïve animals, and this response was comparable in the wt and transgenic animals (Fig. 5B). The latter observations suggest that the suppressed IFN-γ production observed during late infection is the result of altered APC function. Attempts to reverse this suppression by addition of anti-IL-10R MAb were unsuccessful (data not shown), indicating that IL-10 production by APC is not in itself the explanation for the observed down-regulation of IFN-γ production. To further confirm the normal activation of effector CD4+ T lymphocytes in the infected hu10Tg mice, we quantitated the numbers of activated (CD44hi) CD4+ T cells in spleens. No significant differences were observed between the wt and transgenic animals at either week 2 (6.2 × 106 ± 0.2 × 106 versus 5.9 × 106 ± 0.3 × 106; n = 4) or week 8 (6.8 × 106 ± 0.4 × 106 versus 7.1 × 106 ± 0.5 × 106; n = 4) p.i.
FIG. 5.
M. avium-induced IFN-γ production is not impaired in hu10Tg mice. Total splenocytes (A) or purified T cells (B) from wt (open) and hu10Tg (closed) mice were restimulated with MAVAg in vitro for 72 h. IFN-γ in the culture supernatants was then measured by ELISA. The cells stimulated in panel B were T lymphocytes isolated from spleens of wt and hu10Tg animals by T-cell enrichment column at 8 weeks p.i. IFN-γ levels were below the limit of detection in cultures of MAVAg-stimulated T cells purified from naïve animals (data not shown). The mean (± SD) concentration of IFN-γ produced in duplicate cultures is shown. The experiment shown is representative of two performed.
Splenic macrophages from infected hu10Tg mice fail to undergo apoptosis and produce lower levels of proinflammatory mediators.
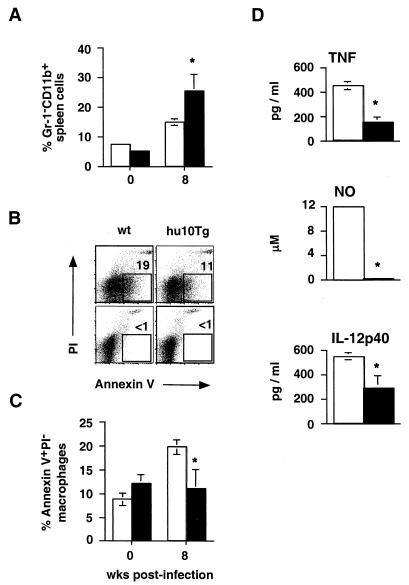
Since Th1 development and lymphokine production appear to be normal in hu10Tg mice, we next asked whether defects in macrophage survival and/or function could explain the increased susceptibility to M. avium infection. To compare macrophage numbers, splenocytes from wt and transgenic animals were stained with anti-Gr-1 and CD11b MAb ex vivo and analyzed by flow cytometry. Although initially similar, by 8 weeks p.i., spleens of transgenic mice contained approximately twice the percentage of Gr-1− CD11b+ cells as their wt counterparts (Fig. 6A). To investigate whether this increase is due to a difference in macrophage survival in M. avium-infected hu10Tg mice, we measured apoptosis of splenic Gr-1− CD11b+ cells in infected wt and transgenic animals by flow-cytometric staining utilizing Annexin V-FITC. The percentage of apoptotic splenic macrophages in infected wt mice had increased more than twofold by 8 weeks p.i., while no increase was observed in the same populations from infected hu10Tg animals (Fig. 6B and C). Examination of tissue sections using TUNEL staining confirmed that, in contrast to the situation in wt mice, there was no significant difference in the number of TUNEL-positive cells in the spleens of transgenic mice before and 8 weeks after M. avium infection (data not shown).
FIG. 6.
Splenic macrophages from M. avium-infected hu10Tg mice fail to undergo apoptosis and produce less TNF, NO, and IL-12p40 than their wt counterparts. Percentages of Gr-1− CD11b+ macrophages in spleens (n = 4) of wt (open bars) and hu10Tg (closed bars) mice were compared ex vivo by flow cytometry before infection and 8 weeks p.i. (A). Splenocytes from the same animals were also costained with Annexin V FITC and PI, as well as MAb to Gr-1 and CD11b to determine the percentage of apoptotic macrophages (B and C). Representative FACS profiles showing costaining of Annexin V-FITC and PI on splenic Gr-1− CD11b+ macrophages from mice at 8 weeks p.i. (B, top panels) are presented. The numbers indicate the percentages of Annexin V-FITC+ PI− Gr-1+ CD11+ cells. Preincubation of the same cells with purified recombinant Annexin V blocked Annexin V-FITC binding, confirming the specificity of the staining (B, lower panels). The mean (± SD) percentages of apoptotic macrophages (n = 4) in spleens of wt and hu10Tg mice before infection and 8 wk p.i. are shown in Fig. 6C. To measure macrophage production of proinflammatory mediators (D), T-cell-depleted, splenic adherent cells isolated from wt (open) or hu10Tg (closed) mice 2 wk after infection with M. avium were restimulated with MAVAg in vitro for 72 h. Secreted TNF and IL-12 was measured by ELISA, and NO was measured by Greiss reaction. The data shown are the means (± SD) for triplicate cultures performed on pooled cells from three animals. The significance of differences between wt and hu10Tg mice was determined using the Student t test (∗, P < 0.05).
We next assessed the production of the proinflammatory mediators TNF, NO, and IL-12 as markers of macrophage activation. MAVAg stimulated spleen cells from hu10Tg mice produced significantly less TNF, NO, and IL-12p40 than those from wt animals, particularly at the early stages of infection (data not shown). To confirm that these differences are not the result of impaired T-cell function, we analyzed the production of the same mediators by a macrophage-enriched, T-cell-depleted, adherent subpopulation of spleens from mice at 2 weeks p.i. When derived from hu10Tg mice, these cells produced lower levels of TNF, NO, and IL-12p40 in vitro than the equivalent macrophage-enriched fraction from wt animals (Fig. 6D). These observations suggest that the decreased resistance of hu10Tg mice to M. avium infection is the result of IL-10-mediated deactivation of macrophages combined with increased survival of these cells, a conclusion consistent with the histopathologic observation of increased numbers of macrophages as well as bacilli per macrophage within granulomas in these animals (Fig. 2D).
DISCUSSION
IL-10 is a prominent cytokine in the immune responses of both humans and mice to mycobacteria (38). Both T cells and APC produce IL-10 in response to mycobacterial infection (10, 21, 29, 32, 41). Interestingly, although originally described as a Th2 cytokine, IL-10 appears to be produced in large amounts during mycobacterial infection by Th1-type interferon-producing CD4+ lymphocytes (20, 28). That T-cell-derived IL-10 can play a role in regulating host resistance to mycobacterial infection was confirmed in a study demonstrating enhanced susceptibility to Mycobacterium bovis BCG infection of transgenic mice overproducing IL-10 in the T-lymphocyte compartment (34). While the induction of T cells producing IL-10 can be readily measured by in vitro recall with mycobacterial antigen, secretion of the cytokine by pathogen-stimulated APC can be assayed only as constitutive production, which is often difficult to detect in vitro. An important question concerns whether IL-10 produced by APC can also influence the outcome of mycobacterial infection. In the present study, we used transgenic mice expressing huIL-10 under the control of the major histocompatibility complex class II promoter to address this issue. Because human IL-10 is fully functional in mice, the relative contributions of APC and T-cell-derived IL-10 can be distinguished in vivo.
M. avium-infected hu10Tg mice developed higher bacterial burdens than control animals and succumbed precipitously at 16 to 18 weeks of infection (Fig. 1), an outcome unusual for mice exposed to this normally nonlethal bacterial strain. In addition, hu10Tg/muIL-10−/− mice, in which APC provide the only source of IL-10, harbored levels of mycobacterium similar to those for hu10Tg mice (Fig. 4), establishing that APC-derived IL-10 alone is sufficient to down-regulate antimycobacterial immunity. Also striking was the increased tissue fibrosis seen in the infected tissues of transgenic mice (Fig. 3). This observation was surprising, since in other models, such as murine schistosomiasis, IL-10 is typically associated with suppression of granuloma-associated tissue fibrosis (24). The probable explanation of this discrepancy is that the increased fibrosis observed in hu10Tg mice is a direct result of the increased bacterial loads and ensuing tissue damage occurring in these animals.
IL-10 is known to have an indirect suppressive effect on the development of Th1 cells and on their expression of IFN-γ (9, 14). That this mechanism might be operating in mycobacterial infection is suggested by our observations (Fig. 4 and 5) and those of others (2, 11) showing that T-cell-derived IFN-γ production in mice infected with M. avium decreases as IL-10 levels increase. Nevertheless, a number of other findings argue that suppression by IL-10 is not the explanation of this decline in IFN-γ production. For example, IL-10−/− mice infected with either BCG (35) or M. avium (D. Jankovic, unpublished data) do not show markedly increased levels of IFN-γ during chronic infection. The results reported here demonstrate that the increased APC-derived IL-10 in our hu10Tg mice fails to suppress the early T-cell-derived IFN-γ response induced by M. avium infection (Fig. 5). Taken together, these observations indicate that the down-regulation of IFN-γ production seen in M. avium infection must be the result of immunoregulatory mechanisms distinct from IL-10 production but dependent on APC, since replacement of APC from infected mice with those from naïve animals restores in vitro IFN-γ production to high levels (Fig. 5B). These findings also argue that any effects of IL-10 on host resistance must operate at a stage of the antimycobacterial effector mechanism distinct from the T-cell response.
Histological and flow-cytometric examination of infected livers and spleens revealed several important clues concerning the mechanisms underlying the increased susceptibility of hu10Tg mice to M. avium infection. First, macrophage recruitment into granulomas in the transgenic animals is clearly enhanced, with many of these cells displaying extended, foamy cytoplasm. This elevation in macrophage numbers was associated with a block in apoptosis (Fig. 6). Recent findings demonstrating that virulent mycobacterial strains induce significantly less macrophage apoptosis than the attenuated strains suggest that the induction of apoptosis in infected macrophages is an important innate defense mechanism against mycobacterial infections (18, 26, 30). Apoptosis prevents the release of intracellular components and the spread of the infection by sequestering the pathogens within apoptotic bodies. It has been previously established that IL-10 produced by mycobacterium-infected macrophages inhibits apoptosis of these cells by suppressing TNF function (3, 4). Consistent with these in vitro studies, the attenuated macrophage apoptosis in infected hu10Tg mice is associated with overproduction of IL-10 by APC and reduced secretion of TNF by splenic macrophages (Fig. 6). APC-derived IL-10 therefore protects M. avium from immune clearance by providing a supportive environment for its rapid growth and spread.
A second important histological finding is the appearance of greatly increased numbers of bacilli at both the single-macrophage and whole-granuloma levels in the tissues of transgenic mice (Fig. 2), suggesting a failure to restrict bacterial growth rather than defective containment of the organism within these lesions (Fig. 2). This finding is consistent with the direct suppression of macrophage control of mycobacterial growth by APC-derived IL-10, a hypothesis supported by our observation of reduced production of TNF, NO, and IL-12p40 by macrophages from infected IL-10-transgenic mice. These inflammatory mediators, while not directly implicated in the mechanism of M. avium (as opposed to BCG and M. tuberculosis) growth restriction, are nevertheless useful markers of macrophage activation.
Since IL-10 has a major down-regulatory effect on cell-mediated immunity, it has been hypothesized that the production of this cytokine helps promote the long-term survival of mycobacteria in chronically infected hosts (33, 38, 40). Studies directed at defining the function of IL-10 in mycobacterial function have in general supported this concept but failed to establish a major function for IL-10 production in the prevention of bacterial clearance. Thus, mice deficient in IL-10 show increased resistance to M. avium (39) and in some (25, 35, 39) but not all (13, 36) studies display enhanced early control of M. tuberculosis and BCG infection. Although IL-10−/− mice have never been shown to exhibit dramatically reduced bacterial burdens, our observations and those of others (27, 34) that excess IL-10 production can promote intracellular pathogen growth in macrophages argue that IL-10-mediated immune down-regulation may contribute to the maintenance of latency in chronic mycobacterial infection, possibly as one of several redundant mechanisms.
Our conclusion that infected hu10Tg mice display impaired macrophage effector function while developing normal Th1 responses is in agreement with results of two recent studies employing transgenic mice in which IL-10 was overexpressed under the control of either the CD2 enhancer (34) or the macrophage cell surface molecule CD68 (27). These animals displayed IL-10-mediated deactivation of macrophage function but unimpaired IFN-γ responses and exhibited decreased resistance to BCG infection. Together, the present and previous studies support the argument that the immunosuppressive effects of IL-10 occur primarily at the level of effector cell activity rather than lymphokine-producing T cells (31). This conclusion has important implications for the measurement of protective antimycobacterial responses. Thus, as clearly demonstrated here and in the previous studies of mycobacterial infection in IL-10 transgenic mice, the assay of T-cell IFN-γ production may not accurately reflect host immune status when macrophage down-regulatory cytokines are simultaneously induced. This concept needs to be considered when using T-cell function as a read-out in vaccination studies.
Acknowledgments
We thank Sara Hieny for purification of the MAb. We are also grateful to Yasmine Belkaid, Matthias Hesse, and Charles Scanga for their helpful comments and suggestions during the course of this project.
Editor: J. M. Mansfield
REFERENCES
- 1.Armstrong, J. A., and P. D. Hart. 1975. Phagosome-lysosome interactions in cultured macrophages infected with virulent tubercle bacilli. Reversal of the usual nonfusion pattern and observations on bacterial survival. J. Exp. Med. 142:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azouaou, N., M. Petrofsky, L. S. Young, and L. E. Bermudez. 1997. Mycobacterium avium infection in mice is associated with time-related expression of Th1 and Th2 CD4+ T-lymphocyte response. Immunology 91:414-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balcewicz-Sablinska, M. K., H. Gan, and H. G. Remold. 1999. Interleukin 10 produced by macrophages inoculated with Mycobacterium avium attenuates mycobacteria-induced apoptosis by reduction of TNF-alpha activity. J. Infect. Dis. 180:1230-1237. [DOI] [PubMed] [Google Scholar]
- 4.Balcewicz-Sablinska, M. K., J. Keane, H. Kornfeld, and H. G. Remold. 1998. Pathogenic Mycobacterium tuberculosis evades apoptosis of host macrophages by release of TNF-R2, resulting in inactivation of TNF-alpha. J. Immunol. 161:2636-2641. [PubMed] [Google Scholar]
- 5.Bermudez, L. E., and J. Champsi. 1993. Infection with Mycobacterium avium induces production of interleukin-10 (IL-10), and administration of anti-IL-10 antibody is associated with enhanced resistance to infection in mice. Infect. Immun. 61:3093-3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boussiotis, V. A., E. Y. Tsai, E. J. Yunis, S. Thim, J. C. Delgado, C. C. Dascher, A. Berezovskaya, D. Rousset, J. M. Reynes, and A. E. Goldfeld. 2000. IL-10-producing T cells suppress immune responses in anergic tuberculosis patients. J. Clin. Investig. 105:1317-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheever, A. W., M. E. Williams, T. A. Wynn, F. D. Finkelman, R. A. Seder, T. M. Cox, S. Hieny, P. Caspar, and A. Sher. 1994. Anti-IL-4 treatment of Schistosoma mansoni-infected mice inhibits development of T cells and non-B, non-T cells expressing Th2 cytokines while decreasing egg-induced hepatic fibrosis. J. Immunol. 153:753-759. [PubMed] [Google Scholar]
- 8.Clemens, D. L., and M. A. Horwitz. 1995. Characterization of the Mycobacterium tuberculosis phagosome and evidence that phagosomal maturation is inhibited. J. Exp. Med. 181:257-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Andrea, A., M. Aste-Amezaga, N. M. Valiante, X. Ma, M. Kubin, and G. Trinchieri. 1993. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J. Exp. Med. 178:1041-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demangel, C., P. Bertolino, and W. J. Britton. 2002. Autocrine IL-10 impairs dendritic cell (DC)-derived immune responses to mycobacterial infection by suppressing DC trafficking to draining lymph nodes and local IL-12 production. Eur. J. Immunol. 32:994-1002. [DOI] [PubMed] [Google Scholar]
- 11.Denis, M., and E. Ghadirian. 1993. IL-10 neutralization augments mouse resistance to systemic Mycobacterium avium infections. J. Immunol. 151:5425-5430. [PubMed] [Google Scholar]
- 12.Doherty, T. M., and A. Sher. 1997. Defects in cell-mediated immunity affect chronic, but not innate, resistance of mice to Mycobacterium avium infection. J. Immunol. 158:4822-4831. [PubMed] [Google Scholar]
- 13.Erb, K. J., J. Kirman, B. Delahunt, W. Chen, and G. Le Gros. 1998. IL-4, IL-5 and IL-10 are not required for the control of M. bovis-BCG infection in mice. Immunol. Cell Biol. 76:41-46. [DOI] [PubMed] [Google Scholar]
- 14.Fiorentino, D. F., A. Zlotnik, P. Vieira, T. R. Mosmann, M. Howard, K. W. Moore, and A. O'Garra. 1991. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J. Immunol. 146:3444-3451. [PubMed] [Google Scholar]
- 15.Flesch, I. E., J. H. Hess, I. P. Oswald, and S. H. Kaufmann. 1994. Growth inhibition of Mycobacterium bovis by IFN-gamma stimulated macrophages: regulation by endogenous tumor necrosis factor-alpha and by IL-10. Int. Immunol. 6:693-700. [DOI] [PubMed] [Google Scholar]
- 16.Flesch, I. E., and S. H. Kaufmann. 1994. Role of macrophages and alpha beta T lymphocytes in early interleukin 10 production during Listeria monocytogenes infection. Int. Immunol. 6:463-468. [DOI] [PubMed] [Google Scholar]
- 17.Flynn, J. L., and J. Chan. 2001. Immunology of tuberculosis. Annu. Rev. Immunol. 19:93-129. [DOI] [PubMed] [Google Scholar]
- 18.Fratazzi, C., R. D. Arbeit, C. Carini, M. K. Balcewicz-Sablinska, J. Keane, H. Kornfeld, and H. G. Remold. 1999. Macrophage apoptosis in mycobacterial infections. J. Leukoc. Biol. 66:763-764. [DOI] [PubMed] [Google Scholar]
- 19.Fratazzi, C., R. D. Arbeit, C. Carini, and H. G. Remold. 1997. Programmed cell death of Mycobacterium avium serovar 4-infected human macrophages prevents the mycobacteria from spreading and induces mycobacterial growth inhibition by freshly added, uninfected macrophages. J. Immunol. 158:4320-4327. [PubMed] [Google Scholar]
- 20.Gerosa, F., C. Nisii, S. Righetti, R. Micciolo, M. Marchesini, A. Cazzadori, and G. Trinchieri. 1999. CD4+ T cell clones producing both interferon-gamma and interleukin-10 predominate in bronchoalveolar lavages of active pulmonary tuberculosis patients. Clin. Immunol. 92:224-234. [DOI] [PubMed] [Google Scholar]
- 21.Giacomini, E., E. Iona, L. Ferroni, M. Miettinen, L. Fattorini, G. Orefici, I. Julkunen, and E. M. Coccia. 2001. Infection of human macrophages and dendritic cells with Mycobacterium tuberculosis induces a differential cytokine gene expression that modulates T cell response. J. Immunol. 166:7033-7041. [DOI] [PubMed] [Google Scholar]
- 22.Groux, H., F. Cottrez, M. Rouleau, S. Mauze, S. Antonenko, S. Hurst, T. McNeil, M. Bigler, M. G. Roncarolo, and R. L. Coffman. 1999. A transgenic model to analyze the immunoregulatory role of IL-10 secreted by antigen-presenting cells. J. Immunol. 162:1723-1729. [PubMed] [Google Scholar]
- 23.Hirsch, C. S., R. Hussain, Z. Toossi, G. Dawood, F. Shahid, and J. J. Ellner. 1996. Cross-modulation by transforming growth factor beta in human tuberculosis: suppression of antigen-driven blastogenesis and interferon gamma production. Proc. Natl. Acad. Sci. USA 93:3193-3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffmann, K. F., A. W. Cheever, and T. A. Wynn. 2000. IL-10 and the dangers of immune polarization: excessive type 1 and type 2 cytokine responses induce distinct forms of lethal immunopathology in murine schistosomiasis. J. Immunol. 164:6406-6416. [DOI] [PubMed] [Google Scholar]
- 25.Jacobs, M., N. Brown, N. Allie, R. Gulert, and B. Ryffel. 2000. Increased resistance to mycobacterial infection in the absence of interleukin-10. Immunology 100:494-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keane, J., H. G. Remold, and H. Kornfeld. 2000. Virulent Mycobacterium tuberculosis strains evade apoptosis of infected alveolar macrophages. J. Immunol. 164:2016-2020. [DOI] [PubMed] [Google Scholar]
- 27.Lang, R., R. L. Rutschman, D. R. Greaves, and P. J. Murray. 2002. Autocrine deactivation of macrophages in transgenic mice constitutively overexpressing IL-10 under control of the human CD68 promoter. J. Immunol. 168:3402-3411. [DOI] [PubMed] [Google Scholar]
- 28.Lyadova, I., V. Yeremeev, K. Majorov, B. Nikonenko, S. Khaidukov, T. Kondratieva, N. Kobets, and A. Apt. 1998. An ex vivo study of T lymphocytes recovered from the lungs of I/St mice infected with and susceptible to Mycobacterium tuberculosis. Infect. Immun. 66:4981-4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lyadova, I. V., E. B. Eruslanov, S. V. Khaidukov, V. V. Yeremeev, K. B. Majorov, A. V. Pichugin, B. V. Nikonenko, T. K. Kondratieva, and A. S. Apt. 2000. Comparative analysis of T lymphocytes recovered from the lungs of mice genetically susceptible, resistant, and hyperresistant to Mycobacterium tuberculosis-triggered disease. J. Immunol. 165:5921-5931. [DOI] [PubMed] [Google Scholar]
- 30.Molloy, A., P. Laochumroonvorapong, and G. Kaplan. 1994. Apoptosis, but not necrosis, of infected monocytes is coupled with killing of intracellular bacillus Calmette-Guerin. J. Exp. Med. 180:1499-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moore, K. W., R. de Waal Malefyt, R. L. Coffman, and A. O'Garra. 2001. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19:683-765. [DOI] [PubMed] [Google Scholar]
- 32.Muller, F., P. Aukrust, E. Lien, C. J. Haug, and S. S. Froland. 1998. Enhanced interleukin-10 production in response to Mycobacterium avium products in mononuclear cells from patients with human immunodeficiency virus infection. J. Infect. Dis. 177:586-594. [DOI] [PubMed] [Google Scholar]
- 33.Murray, P. J. 1999. Defining the requirements for immunological control of mycobacterial infections. Trends Microbiol. 7:366-372. [DOI] [PubMed] [Google Scholar]
- 34.Murray, P. J., L. Wang, C. Onufryk, R. I. Tepper, and R. A. Young. 1997. T cell-derived IL-10 antagonizes macrophage function in mycobacterial infection. J. Immunol. 158:315-321. [PubMed] [Google Scholar]
- 35.Murray, P. J., and R. A. Young. 1999. Increased antimycobacterial immunity in interleukin-10-deficient mice. Infect. Immun. 67:3087-3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.North, R. J. 1998. Mice incapable of making IL-4 or IL-10 display normal resistance to infection with Mycobacterium tuberculosis. Clin. Exp Immunol. 113:55-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Othieno, C., C. S. Hirsch, B. D. Hamilton, K. Wilkinson, J. J. Ellner, and Z. Toossi. 1999. Interaction of Mycobacterium tuberculosis-induced transforming growth factor beta1 and interleukin-10. Infect. Immun. 67:5730-5735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Redpath, S., P. Ghazal, and N. R. Gascoigne. 2001. Hijacking and exploitation of IL-10 by intracellular pathogens. Trends Microbiol. 9:86-92. [DOI] [PubMed] [Google Scholar]
- 39.Roach, D. R., E. Martin, A. G. Bean, D. M. Rennick, H. Briscoe, and W. J. Britton. 2001. Endogenous inhibition of antimycobacterial immunity by IL-10 varies between mycobacterial species. Scand. J. Immunol. 54:163-170. [DOI] [PubMed] [Google Scholar]
- 40.Trinchieri, G. 2001. Regulatory role of T cells producing both interferon gamma and interleukin 10 in persistent infection. J. Exp. Med. 194:F53-F57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vankayalapati, R., B. Wizel, B. Samten, D. E. Griffith, H. Shams, M. R. Galland, C. F. Von Reyn, W. M. Girard, R. J. Wallace, Jr., and P. F. Barnes. 2001. Cytokine profiles in immunocompetent persons infected with Mycobacterium avium complex. J. Infect. Dis. 183:478-484. [DOI] [PubMed] [Google Scholar]