Abstract
1. [3H]D,L-5-hydroxytryptophan ([3H]5HTP) was injected under pressure into cell bodies of identified cholinergic and serotonergic neurones in the central nervous system of the marine mollusc, Aplysia californica.
2. Both serotonergic and cholinergic neurones converted [3H]5HTP to [3H]5-hydroxytryptamine ([3H]5HT).
3. The fate of [3H]5HT in the two types of neurones differed. In serotonergic cells, 5HT was present primarily in particulate form; the transmitter readily moved from cell bodies into nerves by selective transport. In contrast, 5HT remained free in the cytoplasm of the cholinergic neurone, and was not transported from the cell body.
4. Treatment of Aplysia with reserpine decreased the proportion of [3H]5HT associated with particulate material, and also decreased the amount of [3H]5HT recovered.
5. Serotonergic neurones possess specific mechanisms for the storage and axonal transport of 5HT which are absent in cholinergic cells.
Full text
PDF




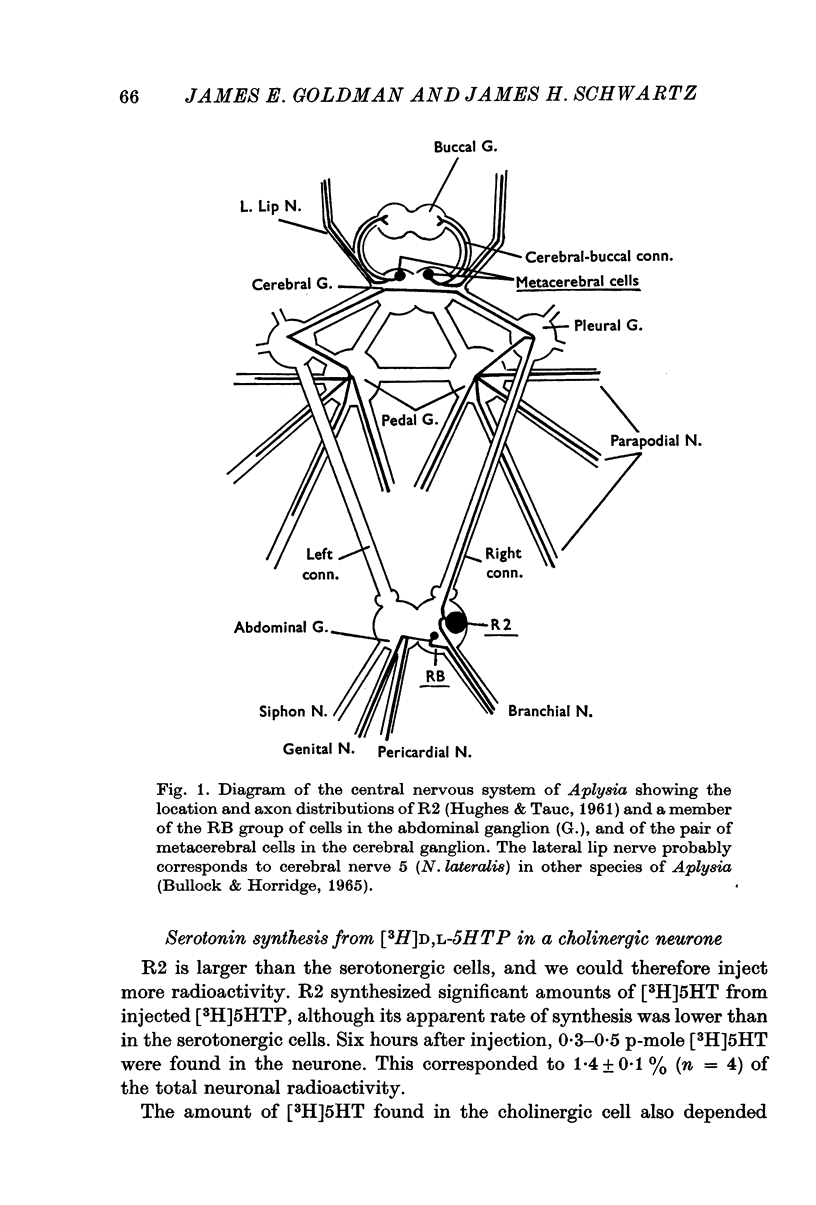
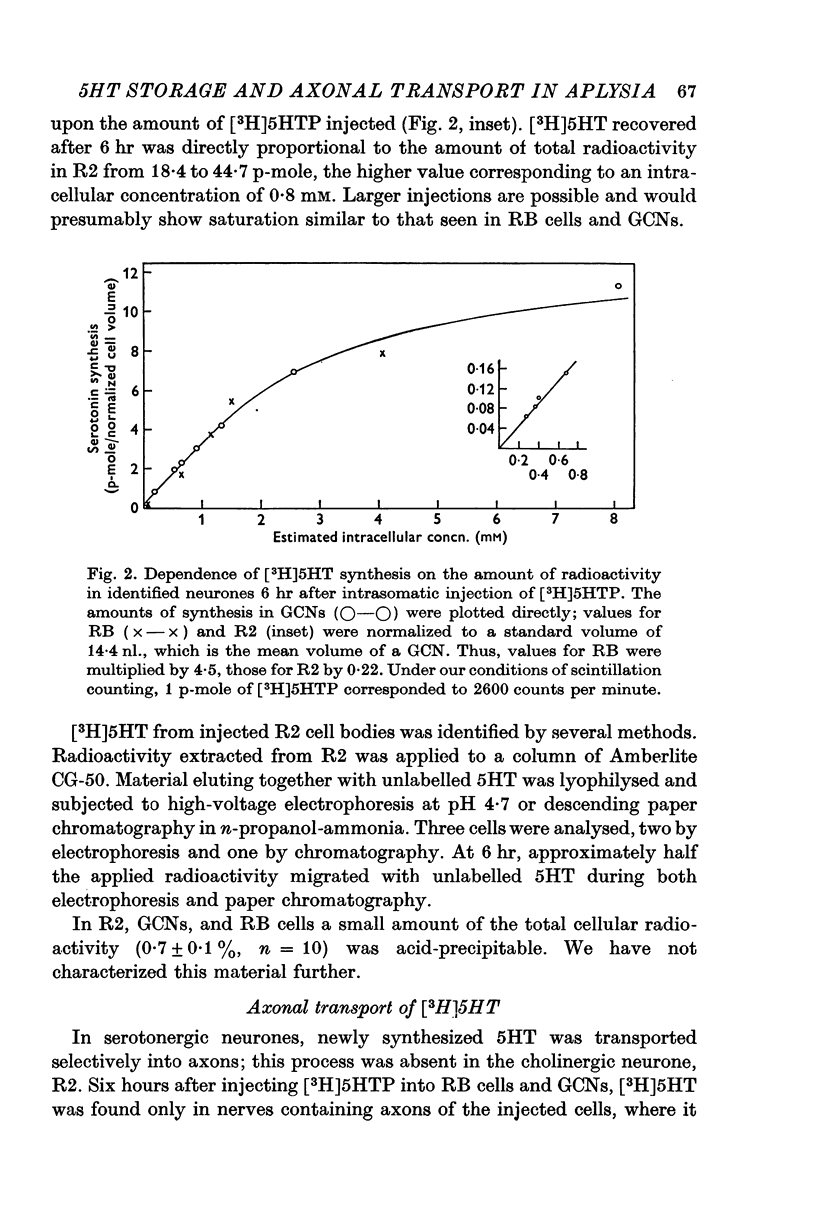
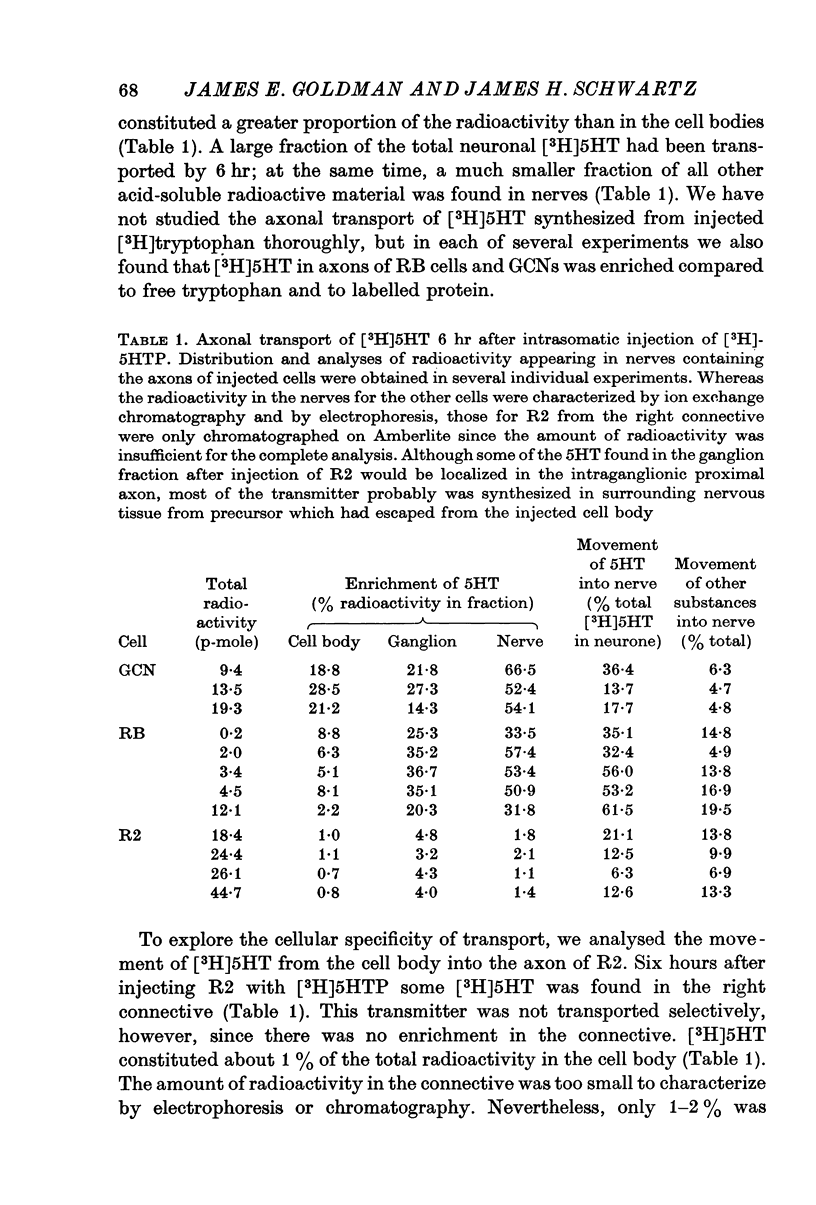








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambron R. T., Goldman J. E., Schwartz J. H. Axonal transport of newly synthesized glycoproteins in a single identified neuron of Aplysia californica. J Cell Biol. 1974 Jun;61(3):665–675. doi: 10.1083/jcb.61.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambron R. T., Goldman J. E., Thompson E. B., Schwartz J. H. Synthesis of glycoproteins in a single identified neuron of Aplysia californica. J Cell Biol. 1974 Jun;61(3):649–664. doi: 10.1083/jcb.61.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldessarini R. J., Fischer J. E. Serotonin metabolism in rat brain after surgical diversion of the portal venous circulation. Nat New Biol. 1973 Sep 5;245(140):25–27. doi: 10.1038/newbio245025a0. [DOI] [PubMed] [Google Scholar]
- Barker D. L., Herbert E., Hildebrand J. G., Kravitz E. A. Acetylcholine and lobster sensory neurones. J Physiol. 1972 Oct;226(1):205–229. doi: 10.1113/jphysiol.1972.sp009981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell G. A. Direct postsynaptic responses to stimulation of serotonin-containing neurones. Nature. 1970 Mar 14;225(5237):1060–1062. doi: 10.1038/2251060a0. [DOI] [PubMed] [Google Scholar]
- Cottrell G. A., Osborne N. N. Localization and mode of action of cardioexcitatory agents in molluscan hearts. Experientia Suppl. 1969;15:220–231. doi: 10.1007/978-3-0348-6800-6_20. [DOI] [PubMed] [Google Scholar]
- Cottrell G. A., Osborne N. N. Subcellular localization of serotonin in an identified serotonin-containing neurone. Nature. 1970 Jan 31;225(5231):470–472. doi: 10.1038/225470a0. [DOI] [PubMed] [Google Scholar]
- Dahl E., Falck B., von Mecklenburg C., Myhrberg H., Rosengren E. Neuronal localization of dopamine and 5-hydroxytryptamine in some mollusca. Z Zellforsch Mikrosk Anat. 1966;71(4):489–498. doi: 10.1007/BF00349609. [DOI] [PubMed] [Google Scholar]
- Dahlström A. Axoplasmic transport (with particular respect to adrenergic neurons). Philos Trans R Soc Lond B Biol Sci. 1971 Jun 17;261(839):325–358. doi: 10.1098/rstb.1971.0064. [DOI] [PubMed] [Google Scholar]
- Eisenstadt M., Goldman J. E., Kandel E. R., Koike H., Koester J., Schwartz J. H. Intrasomatic injection of radioactive precursors for studying transmitter synthesis in identified neurons of Aplysia californica. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3371–3375. doi: 10.1073/pnas.70.12.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fibiger H. C., McGeer E. G., Atmadja S. Axoplasmic transport of dopamine in nigro-striatal neurons. J Neurochem. 1973 Aug;21(2):373–385. doi: 10.1111/j.1471-4159.1973.tb04257.x. [DOI] [PubMed] [Google Scholar]
- Geffen L. B., Livett B. G. Synaptic vesicles in sympathetic neurons. Physiol Rev. 1971 Jan;51(1):98–157. doi: 10.1152/physrev.1971.51.1.98. [DOI] [PubMed] [Google Scholar]
- Gerschenfeld H. M. Chemical transmission in invertebrate central nervous systems and neuromuscular junctions. Physiol Rev. 1973 Jan;53(1):1–119. doi: 10.1152/physrev.1973.53.1.1. [DOI] [PubMed] [Google Scholar]
- Giller E., Jr, Schwartz J. H. Choline acetyltransferase in identified neurons of abdominal ganglion of Aplysia californica. J Neurophysiol. 1971 Jan;34(1):93–107. doi: 10.1152/jn.1971.34.1.93. [DOI] [PubMed] [Google Scholar]
- HUGHES G. M., TAUC L. The path of the giant cell axons in Aplysia depilans. Nature. 1961 Jul 22;191:404–405. doi: 10.1038/191404a0. [DOI] [PubMed] [Google Scholar]
- Hiripi L., Sálanki J., Zs-Nagy I., Muskó I. Subcellular distribution of biogenic monoamines in the central nervous system of Anodonta cygnea L. as revealed by density gradient centrifugation. J Neurochem. 1973 Oct;21(4):791–797. doi: 10.1111/j.1471-4159.1973.tb07523.x. [DOI] [PubMed] [Google Scholar]
- Holtzman E., Teichberg S., Abrahams S. J., Citkowitz E., Crain S. M., Kawai N., Peterson E. R. Notes on synaptic vesicles and related structures, endoplasmic reticulum, lysosomes and peroxisomes in nervous tissue and the adrenal medulla. J Histochem Cytochem. 1973 Apr;21(4):349–385. doi: 10.1177/21.4.349. [DOI] [PubMed] [Google Scholar]
- Israël M., Gautron J., Lesbats B. Fractionnement de l'organe electrique de la torpille: localisation subcellulaire de l'acetylcholine. J Neurochem. 1970 Oct;17(10):1441–1450. doi: 10.1111/j.1471-4159.1970.tb00511.x. [DOI] [PubMed] [Google Scholar]
- Koike H., Eisenstadt M., Schwartz J. H. Axonal transport of newly synthesized acetylcholine in an identified neuron of Aplysia. Brain Res. 1972 Feb 11;37(1):152–159. doi: 10.1016/0006-8993(72)90359-9. [DOI] [PubMed] [Google Scholar]
- Kopin I. J. False adrenergic transmitters. Annu Rev Pharmacol. 1968;8:377–394. doi: 10.1146/annurev.pa.08.040168.002113. [DOI] [PubMed] [Google Scholar]
- Kravitz E. A., Molinoff P. B., Hall Z. W. A comparison of the enzymes and substrates of gamma-aminobutyric acid metabolism in lobster excitatory and inhibitory axons. Proc Natl Acad Sci U S A. 1965 Sep;54(3):778–782. doi: 10.1073/pnas.54.3.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhar M. J., Roth R. H., Aghajanian G. K. Synaptosomes from forebrains of rats with midbrain raphe lesions: selective reduction of serotonin uptake. J Pharmacol Exp Ther. 1972 Apr;181(1):36–45. [PubMed] [Google Scholar]
- Livett B. G., Geffen L. B., Austin L. Proximo-distal transport of [14C]noradrenaline and protein in sympathetic nerves. J Neurochem. 1968 Sep;15(9):931–939. doi: 10.1111/j.1471-4159.1968.tb11635.x. [DOI] [PubMed] [Google Scholar]
- Morales T., Chalazonitis N. Ondes lentes du potentiel initiées par le cycloheximide au niveau du neurone géant d'Aplysia. C R Seances Soc Biol Fil. 1970;164(8):1792–1797. [PubMed] [Google Scholar]
- Pare C. M. 5-hydroxyindoles in phenylketonuric and nonphenylketonuric mental defectives. Adv Pharmacol. 1968;6(Pt B):159–165. doi: 10.1016/s1054-3589(08)60308-7. [DOI] [PubMed] [Google Scholar]
- Paupardin-Tritsch D., Gerschenfeld H. M. Transmitter role of serotonin in identified synapses in Aplysia nervous system. Brain Res. 1973 Aug 30;58(2):529–534. doi: 10.1016/0006-8993(73)90027-9. [DOI] [PubMed] [Google Scholar]
- Pitman R. M., Tweedle C. D., Cohen M. J. Branching of central neurons: intracellular cobalt injection for light and electron microscopy. Science. 1972 Apr 28;176(4033):412–414. doi: 10.1126/science.176.4033.412. [DOI] [PubMed] [Google Scholar]
- Rude S., Coggeshall E., Van Orden L. S., 3rd Chemical and ultrastructural identification of 5-hydroxytryptamine in an identified neuron. J Cell Biol. 1969 Jun;41(3):832–854. doi: 10.1083/jcb.41.3.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler M. The role of 5-hydroxyindoles in the carcinoid syndrome. Adv Pharmacol. 1968;6(Pt B):127–142. doi: 10.1016/s1054-3589(08)60303-8. [DOI] [PubMed] [Google Scholar]
- Schwartz J. H., Castellucci V. F., Kandel E. R. Functioning of identified neurons and synapses in abdominal ganglion of Aplysia in absence of protein synthesis. J Neurophysiol. 1971 Nov;34(6):939–953. doi: 10.1152/jn.1971.34.6.939. [DOI] [PubMed] [Google Scholar]
- Shaskan E. G., Snyder S. H. Kinetics of serotonin accumulation into slices from rat brain: relationship to catecholamine uptake. J Pharmacol Exp Ther. 1970 Nov;175(2):404–418. [PubMed] [Google Scholar]
- Sims K. L., Davis G. A., Bloom F. E. Activities of 3,4-dihydroxy-L-phenylalanine and 5-hydroxy-L-tryptophan decarboxylases in rat brain: assay characteristics and distribution. J Neurochem. 1973 Feb;20(2):449–464. doi: 10.1111/j.1471-4159.1973.tb12144.x. [DOI] [PubMed] [Google Scholar]
- Snyder S. H., Young A. B., Bennett J. P., Mulder A. H. Synaptic biochemistry of amino acids. Fed Proc. 1973 Oct;32(10):2039–2047. [PubMed] [Google Scholar]
- Walker J. L., Jr, Brown A. M. Unified account of the variable effects of carbon dioxide on nerve cells. Science. 1970 Mar 13;167(3924):1502–1504. doi: 10.1126/science.167.3924.1502. [DOI] [PubMed] [Google Scholar]
- Weinreich D., Dewhurst S. A., McCaman R. E. Metabolism of putative transmitters in individual neurons of Aplysia californica: aromatic amino acid decarboxylase. J Neurochem. 1972 Apr;19(4):1125–1130. doi: 10.1111/j.1471-4159.1972.tb01432.x. [DOI] [PubMed] [Google Scholar]
- Weinreich D., McCaman M. W., McCaman R. E., Vaughn J. E. Chemical, enzymatic and ultrastructural characterization of 5-hydroxytryptamine-containing neurons from the ganglia of Aplysia californica and Tritionia diomedia. J Neurochem. 1973 Apr;20(4):969–976. doi: 10.1111/j.1471-4159.1973.tb00067.x. [DOI] [PubMed] [Google Scholar]
- Whittaker V. P. The application of subcellular fractionation techniques to the study of brain function. Prog Biophys Mol Biol. 1965;15:39–96. doi: 10.1016/0079-6107(65)90004-0. [DOI] [PubMed] [Google Scholar]


