Abstract
1. The electrical responses of the smooth muscle cells of the rabbit aorta to both extracellular and intracellular stimulation were studied using the partitioned chamber and Wheatstone bridge method.
2. No spontaneous electrical activity was recorded when the tissue was soaked in either isotonic or hypertonic Krebs solutions, and strong depolarizing currents also failed to trigger action potentials in either solution.
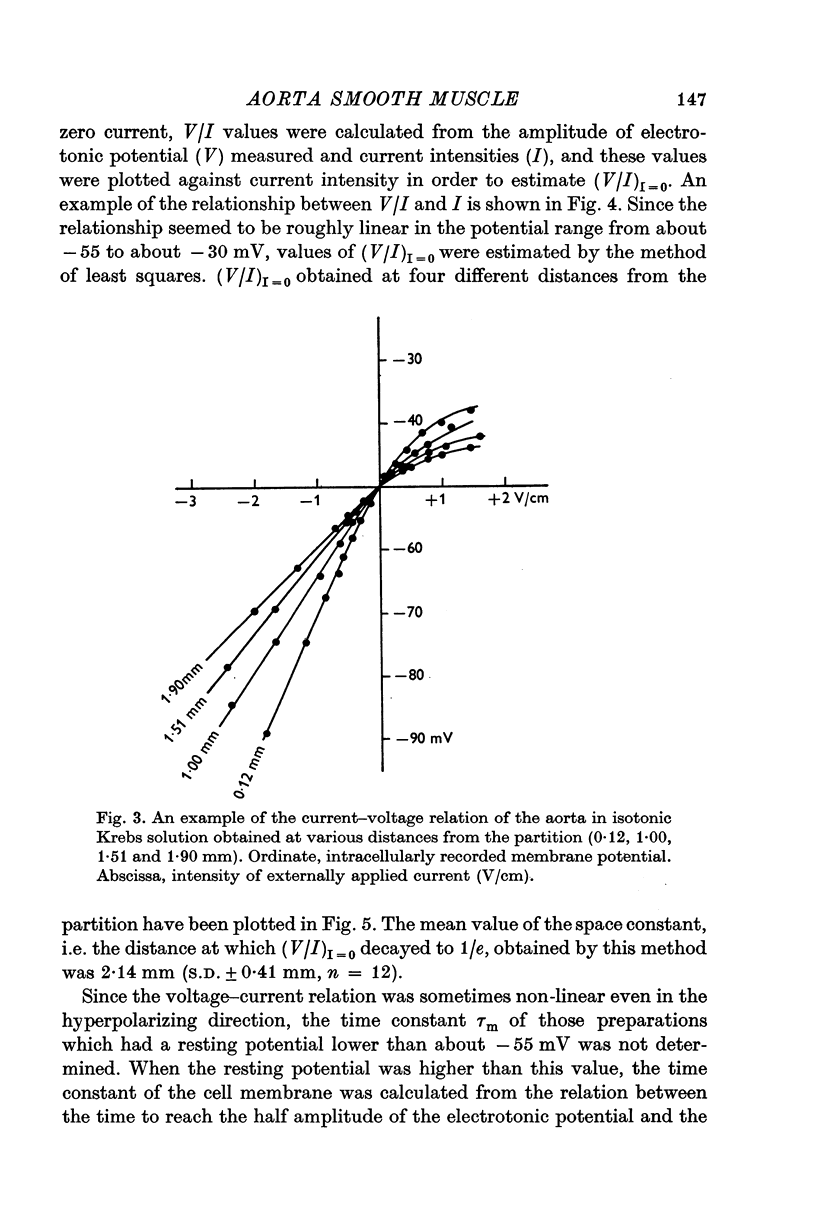
3. The circular muscle of the aorta has cable properties. Mean values in isotonic Krebs solution were 2·1 mm for space constant and 433 msec for time constant.
4. The input resistance (mean 12 MΩ) measured with the Wheatstone bridge method was considerably smaller than that calculated from values measured with the partitioned chamber method.
5. Electrotonic potentials could be recorded from the smooth muscle of `injury bundles' although their amplitude was smaller than that from the intact bundle.
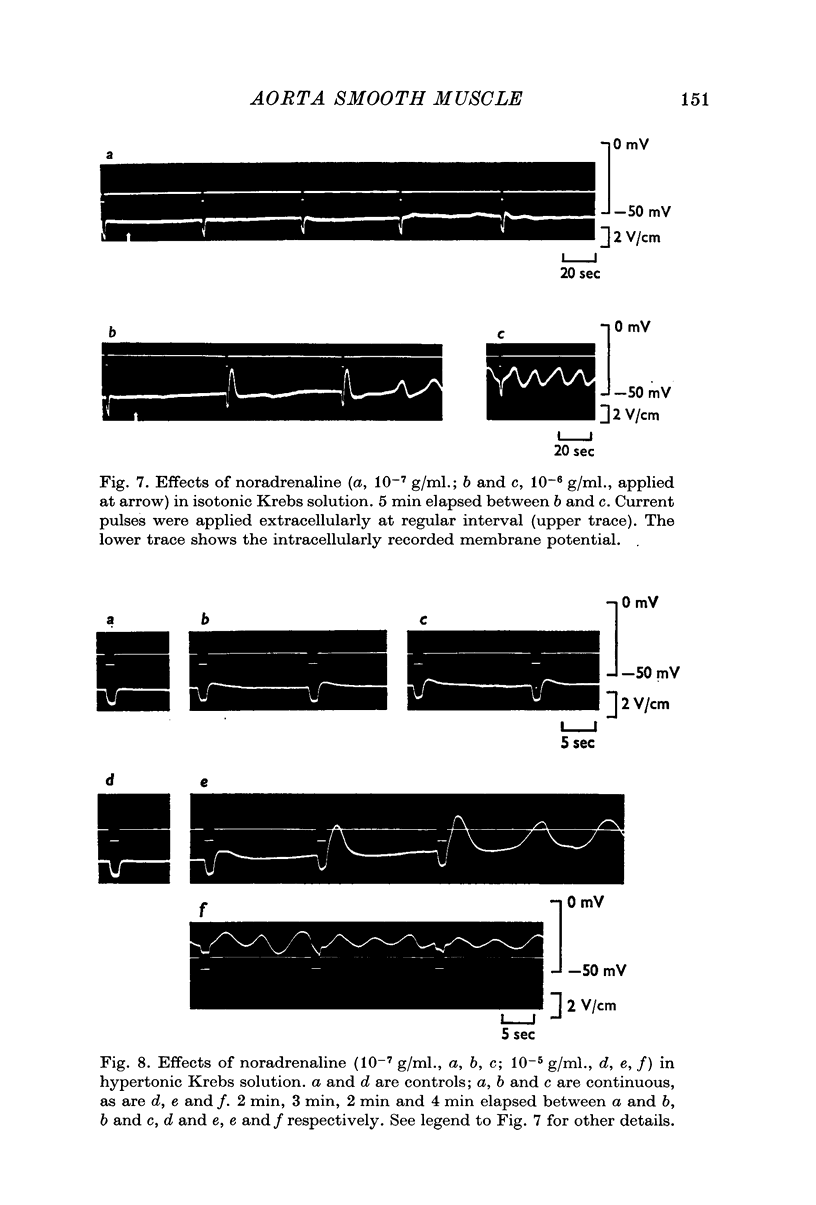
6. High concentrations of noradrenaline readily induce oscillatory potentials from the aorta in both isotonic and hypertonic Krebs solutions. It was estimated by simultaneous recording with two micro-electrodes that noradrenaline-induced oscillatory potential can conduct in both longitudinal and transverse directions of the smooth muscle.
7. These results suggest that the smooth muscle of the aorta behaves like a syncytium or single unit muscle and activation of cells on the inner surface of the media can be induced both by electrotonic current spread and by propagation of oscillatory potentials from the outer cells directly activated by the transmitter.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARAKI T., OTANI T. Response of single motoneurons to direct stimulation in toad's spinal cord. J Neurophysiol. 1955 Sep;18(5):472–485. doi: 10.1152/jn.1955.18.5.472. [DOI] [PubMed] [Google Scholar]
- Abe Y., Tomita T. Cable properties of smooth muscle. J Physiol. 1968 May;196(1):87–100. doi: 10.1113/jphysiol.1968.sp008496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliff W. J. The aortic tunica media in growing rats studied with the electron microscope. Lab Invest. 1967 Dec;17(6):599–615. [PubMed] [Google Scholar]
- Hashimoto Y., Holman M. E., Tille J. Electrical properties of the smooth muscle membrane of the guinea-pig vas deferens. J Physiol. 1966 Sep;186(1):27–41. doi: 10.1113/jphysiol.1966.sp008018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KURIYAMA H., TOMITA T. THE RESPONSES OF SINGLE SMOOTH MUSCLE CELLS OF GUINEA-PIG TAENIA COLI TO INTRACELLULARLY APPLIED CURRENTS, AND THEIR EFFECT ON THE SPONTANEOUS ELECTRICAL ACTIVITY. J Physiol. 1965 May;178:270–289. doi: 10.1113/jphysiol.1965.sp007627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keatinge W. R. Ionic requirements for arterial action potential. J Physiol. 1968 Jan;194(1):169–182. doi: 10.1113/jphysiol.1968.sp008400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama H., Mekata F. Biophysical properties of the longitudinal smooth muscle of the guinea-pig rectum. J Physiol. 1971 Feb;212(3):667–683. doi: 10.1113/jphysiol.1971.sp009349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama H., Osa T., Tasaki H. Electrophysiological studies of the antrum muscle fibers of the guinea pig stomach. J Gen Physiol. 1970 Jan;55(1):48–62. doi: 10.1085/jgp.55.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekata F. Electrophysiological studies of the smooth muscle cell membrane of the rabbit common carotid artery. J Gen Physiol. 1971 Jun;57(6):738–751. doi: 10.1085/jgp.57.6.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekata F., Niu H. Biophysical effects of adrenaline on the smooth muscle of the rabbit common carotid artery. J Gen Physiol. 1972 Jan;59(1):92–102. doi: 10.1085/jgp.59.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PROSSER C. L., BURNSTOCK G., KAHN J. Conduction in smooth muscle: comparative structural properties. Am J Physiol. 1960 Sep;199:545–552. doi: 10.1152/ajplegacy.1960.199.3.545. [DOI] [PubMed] [Google Scholar]
- SU C., BEVAN J. A., URSILLO R. C. ELECTRICAL QUIESCENCE OF PULMONARY ARTERY SMOOTH MUSCLE DURING SYMPATHOMIMETIC STIMULATION. Circ Res. 1964 Jul;15:26–27. doi: 10.1161/01.res.15.1.20. [DOI] [PubMed] [Google Scholar]
- Tomita T. Current spread in the smooth muscle of the guinea-pig vas deferens. J Physiol. 1967 Mar;189(1):163–176. doi: 10.1113/jphysiol.1967.sp008161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T. Electrical responses of smooth muscle to external stimulation in hypertonic solution. J Physiol. 1966 Mar;183(2):450–468. doi: 10.1113/jphysiol.1966.sp007876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T. The longitudinal tissue impedance of the smooth muscle of guinea-pig taenia coli. J Physiol. 1969 Mar;201(1):145–159. doi: 10.1113/jphysiol.1969.sp008748. [DOI] [PMC free article] [PubMed] [Google Scholar]



