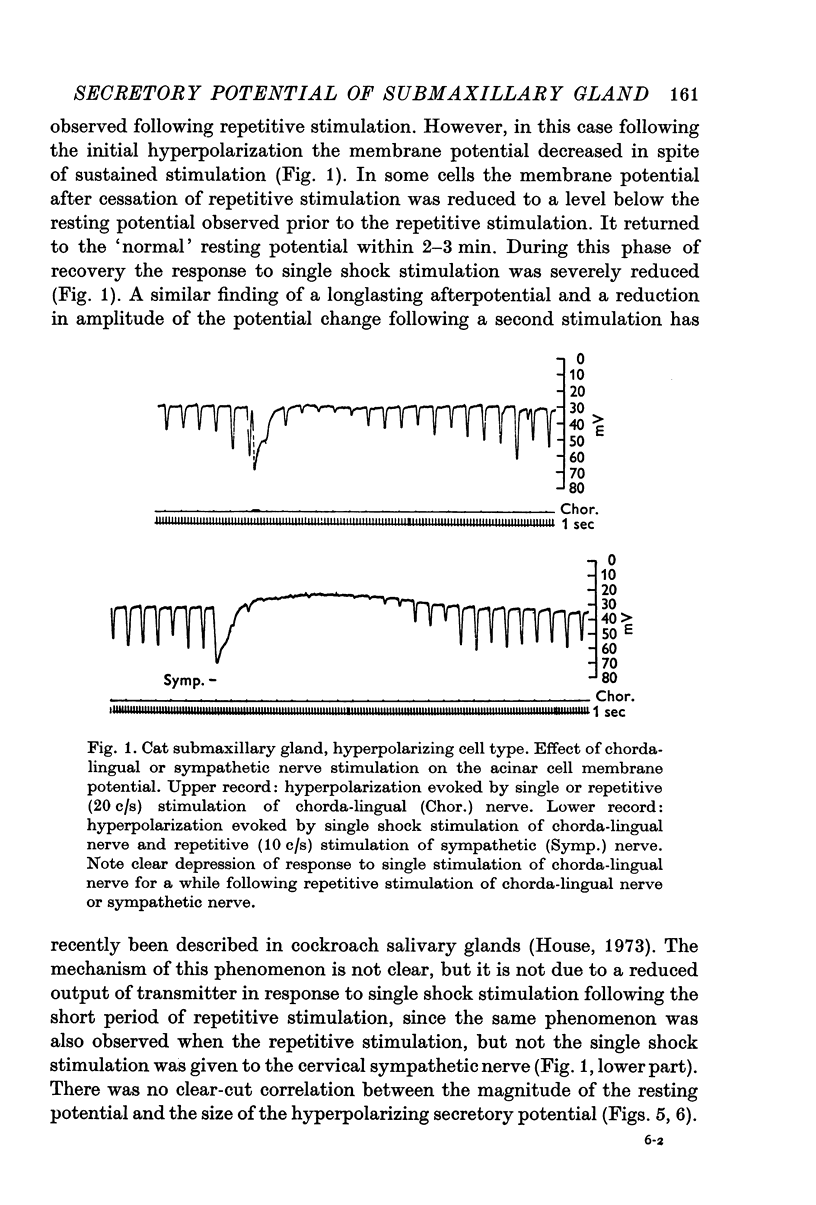
Abstract
1. Membrane potential and input resistance measurements were made from acinar cells of cat and rabbit submaxillary glands in vivo, using intracellular glass micro-electrodes.
2. The mean resting cell membrane potential was higher than previously reported, but ranged widely from -15 to -80 mV.
3. Single shock electrical stimulation of the parasympathetic nerve fibres evoked characteristic potential changes. In some cases monophasic hyperpolarizations, in others biphasic responses (depolarization — hyperpolarization) were observed.
4. The latency of the hyperpolarizing response was considerably longer (300-550 msec) than the latency of the biphasic response (about 150 msec).
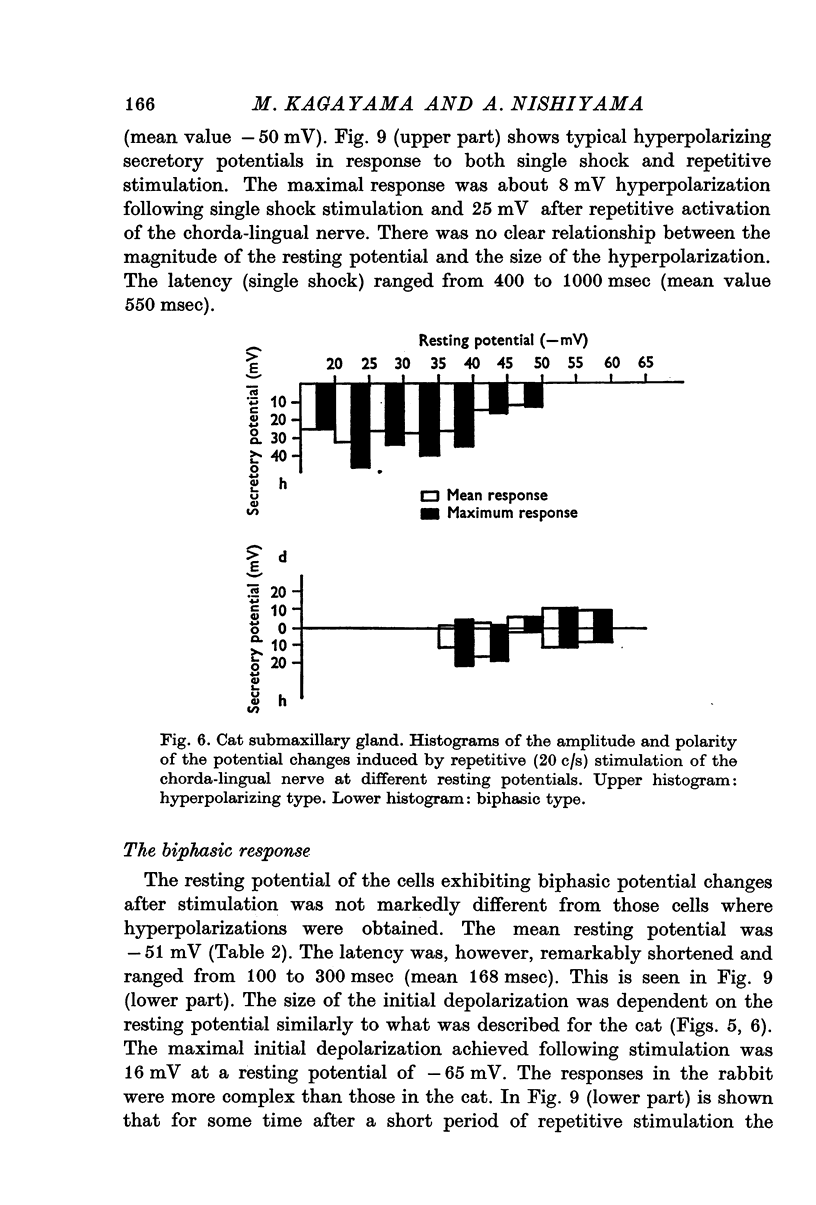
5. Hyperpolarizing and biphasic responses could be observed in the same cell at different levels of membrane potential. The initial depolarization of the biphasic response was dependent on the magnitude of the resting potential in such a manner that it was very small or absent at the lowest potentials and increased gradually with increasing level of the resting potential.
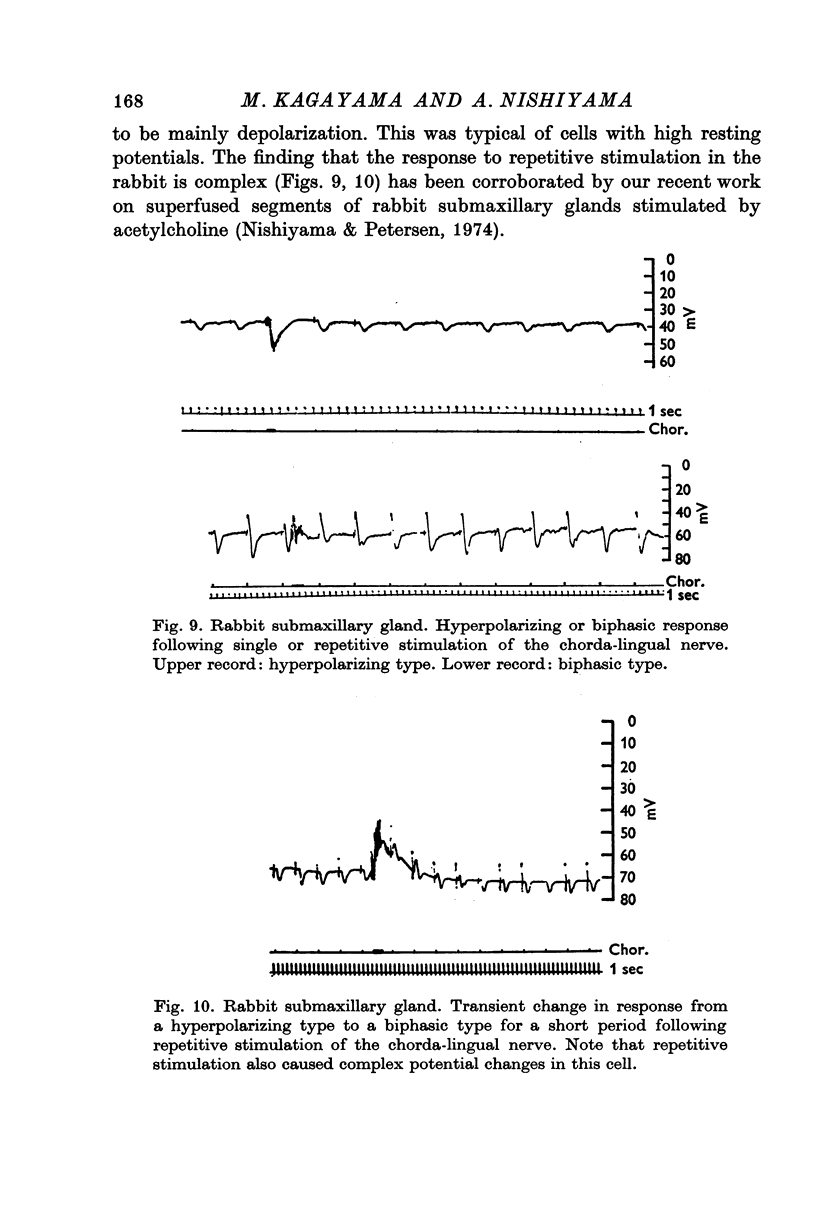
6. Single-shock stimulation of the sympathetic nerve fibres to the gland did not evoke any response. In the cat, repetitive stimulation evoked hyperpolarizing or biphasic responses similar to those seen after repetitive stimulation of the parasympathetic nerve fibres. In the rabbit small hyperpolarizations were seen in a few cells only; mostly there was no response. Repetitive stimulation of the parasympathetic nerve fibres to the rabbit submaxillary gland evoked complex potential changes mostly of the depolarization—hyperpolarization type.
7. Both single shock and repetitive stimulation of the parasympathetic nerve fibres evoked marked reductions in cell input resistance. In the hyperpolarizing cell type the conductance change sometimes preceded the potential change whereas they always occurred simultaneously in the biphasic cell type.
8. It is concluded that both the hyperpolarizing and the biphasic secretory potentials are derived from the same type of acinar cells. The neurotransmitter released by the parasympathetic nerve endings (ACh) acts on the acinar cell membrane by increasing the ion permeability.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARAKI T., OTANI T. Response of single motoneurons to direct stimulation in toad's spinal cord. J Neurophysiol. 1955 Sep;18(5):472–485. doi: 10.1152/jn.1955.18.5.472. [DOI] [PubMed] [Google Scholar]
- Creed K. E., Wilson J. A. The latency of response of secretory acinar cells to nerve stimulation in the submandibular gland of the cat. Aust J Exp Biol Med Sci. 1969 Feb;47(1):135–144. doi: 10.1038/icb.1969.13. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. On the localization of acetylcholine receptors. J Physiol. 1955 Apr 28;128(1):157–181. doi: 10.1113/jphysiol.1955.sp005297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. THE MEASUREMENT OF SYNAPTIC DELAY, AND THE TIME COURSE OF ACETYLCHOLINE RELEASE AT THE NEUROMUSCULAR JUNCTION. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:483–495. doi: 10.1098/rspb.1965.0016. [DOI] [PubMed] [Google Scholar]
- Kagayama M., Nishiyama A. Comparative aspect on the innervation of submandibular glands in cat and rabbit: an electron microscopic study. Tohoku J Exp Med. 1972 Oct;108(2):179–183. doi: 10.1620/tjem.108.179. [DOI] [PubMed] [Google Scholar]
- LUNDBERG A. Anionic dependence of secretion and secretory potentials in the perfused sublingual gland. Acta Physiol Scand. 1957 Oct 10;40(2-3):101–112. doi: 10.1111/j.1748-1716.1957.tb01480.x. [DOI] [PubMed] [Google Scholar]
- LUNDBERG A. Electrophysiology of salivary glands. Physiol Rev. 1958 Jan;38(1):21–40. doi: 10.1152/physrev.1958.38.1.21. [DOI] [PubMed] [Google Scholar]
- LUNDBERG A. Secretory potentials in the sublingual gland of the cat. Acta Physiol Scand. 1957 Sep 17;40(1):21–34. doi: 10.1111/j.1748-1716.1957.tb01475.x. [DOI] [PubMed] [Google Scholar]
- LUNDBERG A. The electrophysiology of the submaxillary gland of the cat. Acta Physiol Scand. 1955 Dec 22;35(1):1–25. doi: 10.1111/j.1748-1716.1955.tb01258.x. [DOI] [PubMed] [Google Scholar]
- LUNDBERG A. The mechanism of establishment of secretory potentials in sublingual gland cells. Acta Physiol Scand. 1957 Sep 17;40(1):35–58. doi: 10.1111/j.1748-1716.1957.tb01476.x. [DOI] [PubMed] [Google Scholar]
- Nishiyama A., Kagayama M. Biphasic secretory potentials in cat and rabbit submaxillary glands. Experientia. 1973 Feb 15;29(2):161–163. doi: 10.1007/BF01945449. [DOI] [PubMed] [Google Scholar]
- Nishiyama A., Petersen O. H. Membrane potential and resistance measurement in acinar cells from salivary glands in vitro: effect of acetylcholine. J Physiol. 1974 Oct;242(1):173–188. doi: 10.1113/jphysiol.1974.sp010700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen G. L., Petersen O. H. Membrane potential measurement in parotid acinar cells. J Physiol. 1973 Oct;234(1):217–227. doi: 10.1113/jphysiol.1973.sp010342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen O. H. The dependence of the transmembrane salivary secretory potential on the external potassium and sodium concentration. J Physiol. 1970 Sep;210(1):205–215. doi: 10.1113/jphysiol.1970.sp009204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneyer L. H., Yoshida Y. Secretory potentials in rat submaxillary gland. Proc Soc Exp Biol Med. 1969 Jan;130(1):192–196. doi: 10.3181/00379727-130-33519. [DOI] [PubMed] [Google Scholar]
- Yoshimura H., Imai Y. Studies on the secretory potential of acinal cells of the dog submaxillary gland and its ionic dependency. Jpn J Physiol. 1967 Jun;17(3):280–293. doi: 10.2170/jjphysiol.17.280. [DOI] [PubMed] [Google Scholar]


