Abstract
During its interaction with host cells, Salmonella enterica serovar Typhimurium employs a type III secretion system for cytosolic targeting of virulence factors. This protein translocation mechanism is a useful tool for heterologous antigen delivery by attenuated Salmonella vaccine carrier strains. In the present study, we used the Yersinia outer protein E (YopE) as a carrier molecule for Salmonella type III-dependent cytosolic delivery of the immunodominant CD8 T-cell antigens listeriolysin O (LLO) and p60 of Listeria monocytogenes. It is shown that concomitant translocation of hybrid YopE/LLO and YopE/p60 proteins by Salmonella led to antigen presentation and CD8 T-cell priming efficacies comparable to those of translocation of single listerial antigens. However, simultaneous translocation of LLO and p60 significantly surpassed single cytosolic antigen delivery in the ability to protect against Listeria. For the first time, this study demonstrates that concomitant expression of two independent antigens via the same recombinant plasmid leads to superior protection against a challenge with an intracellular bacterial pathogen. In conclusion, these findings emphasize the versatility of Salmonella type III-mediated heterologous antigen delivery for the induction of cytotoxic T-lymphocyte-mediated immunity.
Salmonella enterica serovar Typhimurium employs a type III secretion system (TTSS) encoded in the chromosomal Salmonella pathogenicity island 1 (SPI1) to invade eukaryotic cells (9). After gaining access to the host cell, serovar Typhimurium persists in membrane-bound vacuoles (macropinosomes) during its entire intracellular life cycle (3). In the course of the invasion process and subsequently from the macropinosomal compartment, Salmonella delivers bacterial SPI1 virulence factors in a type III-dependent fashion directly to the cytosol of target cells to modulate host cellular functions (4). This protein translocation process is the most characteristic feature of the TTSS.
Recent progress in the development of bacterial live carrier vaccines has been made by the use of the TTSS for heterologous antigen delivery (29, 30, 31). In a previous study, we and others investigated the possibility of using the Yersinia outer protein E (YopE) as a type III carrier molecule for antigen delivery by the TTSS of Salmonella (31). Defined secretion and translocation domains of YopE located at the N terminus of the molecule were fused to the immunodominant T-cell antigen listeriolysin O (LLO) or p60 of Listeria monocytogenes. T-cell activation assays revealed that Salmonella type III-dependent translocation and cytosolic delivery of YopE/LLO or YopE/p60 chimeric proteins led to efficient major histocompatibility complex (MHC) class I-restricted antigen presentation of listerial nonamer peptides. Oral immunization of mice with a single dose of attenuated serovar Typhimurium expressing either translocated hybrid YopE/LLO or YopE/p60 proteins resulted in prominent LLO- or p60-specific CD8 T-cell priming. However, these recombinant Salmonella strains did not elicit the same protective ability as did an immunizing sublethal dose of wild-type L. monocytogenes, which naturally displays a variety of listerial peptides from different antigens to CD8 T cells of the vaccinated host. Therefore, polyvalent antigen delivery by bacterial live carrier vaccines is a desirable feature for eliciting efficient protection against infectious agents. In this study, we demonstrate that type III-mediated concomitant translocation of YopE/LLO and YopE/p60 by a single Salmonella vaccine strain significantly enhances the ability to protect against listeriosis, emphasizing the versatility of the TTSS for heterologous antigen delivery.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Escherichia coli χ6060 was used as an intermediate host for cloning procedures. The M45 epitope tag (MDRSRDRLPPFETETRIL) is derived from the E4-6/7 protein of adenovirus (24), and its use for chimeric YopE protein tagging has been described previously (31). Translational fusions between the N-terminal 138 amino acids of YopE and LLO or p60 were constructed by PCR cloning procedures. Previously, the construction of plasmids pHR231 and pHR241 (Table 1) was outlined in detail (31). These pWSK29 derivatives (37) bear the genetic information for translocated chimeric YopE/LLO/M45 or YopE/p60/M45 fusion proteins under the expression control of the Plac promoter, which is constitutively active in serovar Typhimurium. Plasmid pHR251 was constructed for vector-derived concomitant expression of translocated YopE/LLO/M45 and YopE/p60/M45. Briefly, the gene fusion coding for YopE/p60/M45 was amplified from pHR241 template DNA (forward primer KpnI 5′-CGGGGTACCATCAATTTGAGCCTA-3′, reverse primer KpnI 5′-TATGGTACCGCTTCTGCGTTCTGA-3′), and the resulting PCR fragment was cloned into the KpnI site of pHR231 downstream of the gene fusion coding for YopE/LLO/M45. All plasmids used in this study carry the genetic information for the YopE-specific chaperone SycE, which is essential for YopE translocation (22). The above-described plasmids were transformed into Salmonella serovar Typhimurium strain SB824 by electroporation (29). Strain SB824 was engineered by introducing the sptP::kan mutant allele from strain SB237 (18) into the ΔaroA strain SL3261 (16) by P22HTint transduction. Serovar Typhimurium was grown in Luria-Bertani medium supplemented with 0.3 M NaCl, pH 7.0, to allow expression of components and targets of the TTSS encoded by SPI1 (5). L. monocytogenes strain sv1/2a EGD (20) was used for challenge experiments with Salmonella-vaccinated mice.
TABLE 1.
Serovar Typhimurium strains and plasmids used in this study
| Strain | Plasmid-encoded proteinsa | Reference |
|---|---|---|
| SB824(pHR231) | SycE, YopE1-138/LLO51-363/M45 | 31 |
| SB824(pHR241) | SycE, YopE1-138/p60130-477/M45 | 31 |
| SB824(pHR251) | SycE, YopE1-138/LLO51-363/M45, YopE1-138/p60130-477/M45 | This study |
Hybrid yopE gene fusions were cloned into the low-copy-number vector pWSK29. Large C-terminal portions of LLO or p60 were fused to the N-terminal 138 amino acids of YopE, resulting in hybrid proteins that contain the secretion and translocation domain of YopE. All chimeric proteins were M45 tagged at their C terminus.
Western blot analysis of translocated hybrid YopE proteins in Salmonella-infected P388D1 cells.
The detection of translocated hybrid YopE proteins was carried out as described by Collazo and Galán (6). Briefly, P388D1 cells were grown for 2 days in Dulbecco modified Eagle medium supplemented with 5% fetal bovine serum in 100-mm-diameter tissue culture plates to reach 70% confluency. Next, 1 h before the addition of bacteria, the culture medium was replaced with 500 μl of Hanks' balanced salt solution (HBSS). Bacteria were grown overnight for 12 h in Luria-Bertani medium supplemented with 0.3 M NaCl, diluted 1/20 in fresh medium, and grown for another 4 h under mild aeration to reach an optical density at 600 nm of 0.9. P388D1 cells were infected with serovar Typhimurium for 90 min at a multiplicity of infection of 10 bacteria per cell. After infection nonadherent bacteria were removed and cells were washed with HBSS. The infection supernatant was combined with the material from the washes and centrifuged at 8,000 × g for 20 min. The pellet containing nonadherent bacteria was resuspended in 200 μl of phosphate-buffered saline (nonadherent bacterial fraction). The supernatant was filtered through a 0.45-μm-pore-size syringe filter, and proteins were precipitated by addition of 10% trichloroacetic acid (infection medium fraction). Infected P388D1 cells were incubated for 30 min with Dulbecco modified Eagle medium containing 100 μg of gentamicin/ml to kill extracellular bacteria. Cells were then treated with 30 μg of proteinase K/ml in HBSS for 15 min at 37°C to eliminate cell surface-associated hybrid Yop proteins. After proteinase K treatment, 3 ml of chilled HBSS containing 2 mM phenylmethylsulfonyl fluoride was added. Cells detached during the proteinase K treatment and were subsequently collected by low-speed centrifugation (600 × g for 10 min) and lysed in 1 ml of HBSS containing 0.1% Triton X-100 and 1 mM phenylmethylsulfonyl fluoride. Then the cell lysate was centrifuged at 15,000 × g for 10 min. The supernatant was filtered through a 0.45-μm-pore-size syringe filter, and proteins were precipitated in the presence of 10% trichloroacetic acid (Triton X-100-soluble fraction). Samples were separated by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes as described previously (6). Hybrid Yop proteins were detected by immunoblot analysis. Western blots were treated with a monoclonal antibody (MAb) directed against M45 (31) followed by horseradish-labeled anti-mouse antibody. Blots were developed by using a chemiluminescence kit.
T-cell lines and antigen presentation assay.
CD8 T-cell lines specific for LLO91-99 (25) and p60217-225 (26) were derived from L. monocytogenes-infected BALB/c mice and propagated by repeated restimulation in the presence of the appropriate synthetic peptide as described previously (11). The detection limit of the CD8 T-cell lines used was between 10−11 and 10−12 M peptide. CD8 T-cell activation by infected P388D1 macrophage-like antigen-presenting cells (APC) was measured by the detection of gamma interferon (IFN-γ) in culture supernatants (12). Briefly, P388D1 cells were infected with serovar Typhimurium in 96-well flat-bottom microwell plates by 10 min of 200 × g centrifugation. After 2 h at 37°C infected APC were washed and culture medium supplemented with 25 μg of gentamicin/ml was added. CD8 T cells were added to each well, and after 12 to 18 h at 37°C supernatants were harvested and the IFN-γ concentration was measured by means of an IFN-γ-specific enzyme-linked immunosorbent assay that binds and detects IFN-γ with a pair of specific MAbs. Results were corrected for dilution of the sample to yield the sample concentration in nanograms per milliliter.
Oral immunization of mice with recombinant Salmonella and in vivo protection assay.
Female BALB/c mice, 6 to 8 weeks old, were purchased from RCC (Füllinsdorf, Switzerland). All mice were kept under specific-pathogen-free conditions (positive-pressure cabinet) and were provided with food and water ad libitum. Groups of 12 mice were orally immunized with either a single dose of 107 CFU of serovar Typhimurium strain SB824, SB824(pHR231), SB824(pHR241), or SB824(pHR251) or intraperitoneally with 5 × 103 CFU of L. monocytogenes. Eight weeks after immunization four mice per group were sacrificed and spleens were used for further enzyme-linked immunospot (ELISPOT) analysis. The remaining eight mice per group were challenged intravenously with 103 CFU of log-phase L. monocytogenes strain sv1/2a EGD in 0.2 ml of phosphate-buffered saline. Three days after the challenge, CFU were determined by plating serial dilutions of spleen homogenates on PALCAM Listeria selective agar (Merck, Darmstadt, Germany). Colonies were enumerated after 48 h of incubation. Colony counts were corrected for dilution and averaged to yield CFU per organ. The level of protection was calculated as the log10 difference of the bacterial count from immunized mice and that from naive control mice. Each experiment was performed at least twice with similar results.
ELISPOT assay.
The frequency of T lymphocytes in mice immunized with attenuated serovar Typhimurium was determined with an IFN-γ-specific ELISPOT assay (12, 36). Assays were performed in nitrocellulose-backed 96-well microtiter plates (Nunc, Wiesbaden, Germany) coated with rat anti-mouse IFN-γ MAb (RMMG-1; Biosource, Camarillo, Calif.). Unseparated splenocytes (6 × 105/well) were stimulated for 6 h in round-bottomed microtiter plates in the presence of 10−8 M peptide. Subsequently, activated cells (4 × 105/well) were transferred to ELISPOT plates and incubated overnight. ELISPOT plates were developed with biotin-labeled rat anti-mouse IFN-γ MAb (clone XMG1.2; Pharmingen, San Diego, Calif.), horseradish peroxidase-streptavidin conjugate (Dianova, Hamburg, Germany), and aminoethylcarbazole dye solution. The frequency of antigen-specific cells was calculated as the number of spots per splenocyte seeded. The specificity and sensitivity of the ELISPOT assay were controlled with IFN-γ-secreting CD8 T-cell lines specific for p60217-225. Recovery of seeded CD8 T cells was higher than 90%.
Statistical analysis.
The statistical analysis of the results of in vitro experiments was performed with the Newman-Keuls multiple comparison test at the 0.05 significance level. The statistical significance of the results of in vivo experiments was checked with the nonparametric Tukey multiple comparison test at the 0.05 significance level. All tests were performed with WINKS statistical analysis software (Texasoft, Cedar Hill, Tex.).
RESULTS
Plasmid construction for concomitant YopE/LLO and YopE/p60 chimeric protein delivery.
In a recent study, we and others used the LLO and p60 proteins of L. monocytogenes as model antigens for the construction of hybrid YopE proteins to be delivered by the Salmonella TTSS (31). After invasion of eukaryotic cells, Listeria escapes from the phagosome by secreting the pore-forming virulence factor LLO (28). Subsequently, LLO is directed to the MHC class I antigen-processing pathway, leading to presentation of antigen-derived peptides to MHC class I-restricted CD8 T cells (27). In the cytosol of infected cells, L. monocytogenes constitutively secretes the murein hydrolase p60 that enters the MHC class I processing pathway as well (27). Analysis of CD8 T-cell clones from Listeria-infected BALB/c mice revealed that two immunodominant listerial nonamer peptides (LLO91-99 and p60217-225) are presented to cytotoxic T lymphocytes (CTL) in the context of the H2-Kd MHC class I molecule (25, 26, 34). CD8 T cells specific for these epitopes were shown elsewhere to transfer protective immunity against L. monocytogenes to naive mice (36).
Table 1 shows that previously described plasmids pHR231 and pHR241 (31) bear the genetic information for YopE hybrid proteins containing the N-terminal secretion and translocation domains of YopE (30, 31, 32, 35). The N-terminal 138 amino acids of YopE were fused to LLO51-363 or to p60130-477. The resulting chimeric proteins are both tagged at their C terminus with an adenoviral M45 epitope and were shown previously to be translocated into the cytosol of macrophages by serovar Typhimurium in a type III-dependent fashion (31). For this study, we decided to construct a plasmid to be used for concomitant antigen delivery of YopE/LLO/M45 and YopE/p60/M45. Therefore, the gene fusion coding for YopE/p60/M45 was amplified from pHR241 and cloned into pHR231 immediately downstream of the gene fusion coding for YopE/LLO/M45. Thus, the resulting plasmid, pHR251, carries an operon-like gene cluster coding for (i) the YopE-specific chaperone, SycE, necessary for YopE translocation (22), (ii) YopE/LLO/M45, and (iii) YopE/p60/M45 under the control of the Plac promoter.
Concomitant translocation of YopE/LLO and YopE/p60 chimeric proteins into the cytosol of P388D1 cells.
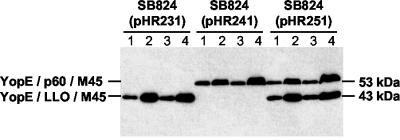
We investigated whether infection of macrophage-like P388D1 cells with attenuated serovar Typhimurium strain SB824(pHR251) expressing YopE/LLO/M45 and YopE/p60/M45 would result in concomitant translocation of both chimeric proteins. Two hours after infection, a biochemical fractionation of P388D1 cells was carried out. Four different fractions were examined by immunoblotting for the presence of hybrid YopE proteins (Fig. 1): (i) nonadherent bacteria that were free from infection medium, (ii) bacterium-free infection medium, (ii) Triton X-100-insoluble fraction containing internalized bacteria, and (iv) Triton X-100-soluble cell lysate containing cytosolic proteins. A proteinase treatment step was performed before lysis in Triton X-100 to ensure the removal of YopE chimeric proteins that were nonspecifically associated with the surface of the host cell. Consistent with previously published immunofluorescence data (31), it is demonstrated in Fig. 1 that YopE/LLO/M45 or YopE/p60/M45 was found in Triton X-100-soluble lysates (containing translocated proteins) of cells infected with SB824(pHR231) or SB824(pHR241), respectively. SB824(pHR251) revealed concomitant expression (lanes 1 and 3) and secretion (lane 2) of YopE/LLO/M45 and YopE/p60/M45 at concentrations indistinguishable from those of SB824(pHR231) or SB824(pHR241). Moreover, SB824(pHR251) efficiently delivered both chimeric proteins into the cytosol of infected P388D1 cells (lane 4). These results demonstrate the versatility of the Salmonella TTSS for cytosolic delivery of heterologous proteins.
FIG. 1.
Fractionation of P388D1 cells infected with serovar Typhimurium strains expressing plasmid-encoded hybrid YopE proteins. Lanes 1, whole-cell lysate of non-cell-associated bacteria; lanes 2, bacterium-free infection medium; lanes 3, Triton X-100-insoluble fraction containing internalized bacteria; lanes 4, Triton X-100-soluble P388D1 cell lysate containing translocated proteins. The total protein amounts obtained from all four fractions were loaded. Chimeric YopE proteins were detected by protein immunoblotting with a MAb to M45.
Concomitant cytosolic delivery of YopE/LLO and YopE/p60 results in efficient antigen presentation of LLO- and p60-derived CD8 T-cell epitopes.
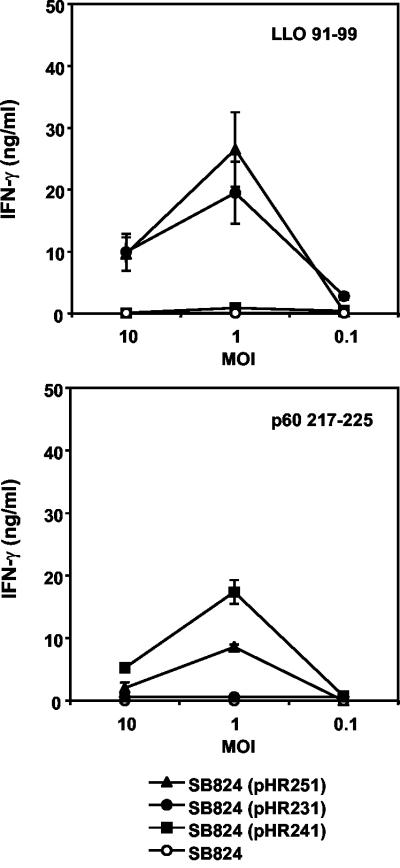
In a subsequent set of experiments, we wanted to answer the question of whether concomitant translocation of YopE/LLO/M45 and YopE/p60/M45 by Salmonella leads to efficient delivery of both listerial nonamer peptides to the MHC class I-restricted antigen presentation pathway. Murine P388D1 cells were infected with recombinant Salmonella strains and used as APC. The ability of P388D1 cells to present the immunodominant epitopes LLO91-99 and p60217-225 to CD8 T cells with corresponding specificities was assessed in an in vitro antigen presentation assay by measuring secreted IFN-γ in the culture supernatant (Fig. 2).
FIG. 2.
Antigen presentation of LLO and p60 nonamer peptides by P388D1 cells. APC were infected with nontransfected serovar Typhimurium strain SB824 or SB824 bearing the indicated plasmid at a multiplicity of infection (MOI) of ∼10, ∼1, or ∼0.1. Activation of T cells (upper panel, anti-LLO91-99; lower panel, anti-p60217-225) was measured as described in Materials and Methods. Standard deviations of duplicate cultures are indicated. The limit of the IFN-γ enzyme-linked immunosorbent assay was 0.5 ng/ml.
P388D1 cells infected with SB824(pHR251) concomitantly translocating YopE/LLO/M45 and YopE/p60/M45 were efficiently recognized by both LLO91-99- and p60217-225-specific T cells (Fig. 2). Furthermore, the direct comparison of Salmonella strains that selectively express the YopE/LLO or the YopE/p60 fusion protein revealed that the T-cell recognition of APC infected with these strains was in the same order of magnitude as the recognition of APC infected with SB824(pHR251). Thus, concomitant Salmonella type III-mediated delivery of YopE/LLO/M45 and YopE/p60/M45 into the cytosol of macrophage-like cells resulted in efficient antigen presentation of CD8 T-cell epitopes derived from both recombinant YopE proteins.
Efficient in vivo induction of LLO- and p60-specific T-cell responses.
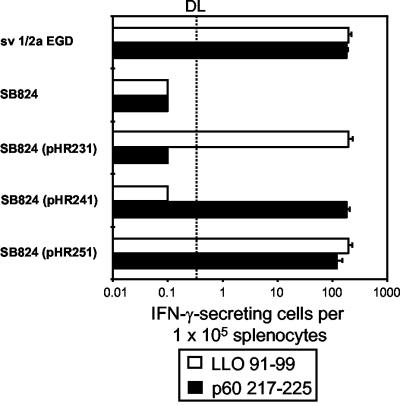
The ability of SB824(pHR251) concomitantly translocating YopE/LLO and YopE/p60 to induce cytotoxic CD8 T cells in vivo was investigated. BALB/c mice were orally inoculated with a single dose of 107 CFU of serovar Typhimurium strain SB824 harboring the indicated plasmid (Fig. 3). Control groups received a sublethal intraperitoneal dose of 5 × 103 CFU of L. monocytogenes. Eight weeks after inoculation, ELISPOT assays were performed to determine the frequency of LLO- and p60-specific T cells in vivo. The frequency of LLO91-99- and p60217-225-specific CD8 T cells was calculated as the number of IFN-γ spots generated per 105 spleen cells in the presence of the corresponding synthetic peptide. Supporting the observed in vitro data, mice immunized with SB824(pHR251) translocating YopE/LLO/M45 and YopE/p60/M45 revealed no significant differences in the numbers of IFN-γ-producing cells reactive with LLO91-99 and p60217-225 compared to mice infected with SB824(pHR231) or SB824(pHR241) (Fig. 3). The efficiency of type III-mediated concomitant foreign antigen delivery is also emphasized by the fact that the frequency of antigen-specific T cells in mice immunized with SB824(pHR251) is as high as that in mice infected with L. monocytogenes.
FIG. 3.
Frequency of LLO- and p60-specific CD8 T cells in spleens of mice immunized with nontransfected serovar Typhimurium strain SB824 or SB824 bearing the indicated plasmid. Spleens from mice infected with L. monocytogenes sv1/2a EGD were used as controls. The frequency of LLO- and p60-specific T cells was determined by ELISPOT assay as described in Materials and Methods. The frequency of cells reactive with LLO91-99 (open bars) or p60217-225 (filled bars) is shown as the number of reactive cells per 105 splenocytes. The dotted line at 0.2 × 10−5 indicates the detection limit (DL) of the ELISPOT assay. The standard deviation of triplicate cultures is indicated.
Superior protection against murine listeriosis by concomitant translocation of two antigens.
To compare the contribution of concomitantly translocated LLO and p60 antigens versus a single listerial antigen on vaccine-induced protection, mice were intravenously challenged with 103 CFU of L. monocytogenes 8 weeks after oral immunization with Salmonella expressing hybrid YopE proteins. CFU were determined in spleens 3 days after the challenge. Spleens of animals infected with the nontransfected vaccine strain SB824 were colonized with (2.3 ± 3.6) × 105 CFU of Listeria (Table 2). In contrast, no bacteria were detected in spleens of mice which had received a sublethal intraperitoneal dose of 5 × 103 CFU of Listeria 8 weeks prior to the challenge infection. Mice immunized with SB824(pHR231) or SB824(pHR241) translocating LLO or p60, respectively, showed a pronounced decrease of the bacterial load in spleens ([2.6 ± 1.5] × 102 and [2.6 ± 2.9] × 102 CFU) compared to nonimmunized mice. However, animals orally inoculated with SB824(pHR251) translocating both listerial antigens, p60 and LLO, revealed significantly (P < 0.05) superior protection against the challenge with Listeria ([1.4 ± 1.5] × 101 CFU).
TABLE 2.
Protective immunity induced by recombinant Salmonella
| Immunizing strain | CFU (mean ± SD) of Listeria in spleens of 8 mice/group | No. of mice containing Listeria in spleens/total no. of mice |
|---|---|---|
| sv1/2a EGD | 0a | 0/8 |
| SB824 | (2.3 ± 3.6) × 105 | 8/8 |
| SB824(pHR231) | (2.6 ± 1.5) × 102 | 8/8 |
| SB824(pHR241) | (2.6 ± 2.9) × 102 | 8/8 |
| SB824(pHR251) | (1.4 ± 1.5) × 101b | 5/8 |
No bacteria were detected in spleens of eight mice that had received a sublethal intraperitoneal dose of 5 × 103 CFU of Listeria 8 weeks before the challenge infection.
Value differs significantly from those of mice immunized with SB824(pHR231) or SB824(pHR241) (P < 0.05).
DISCUSSION
Many viruses and other intracellular pathogens induce strong antigen-specific CD8 T-cell responses that might contribute to rapid clearance of the infectious agent (14). For vaccination purposes, a variety of bacterial live carrier strains, such as Salmonella, Listeria, and Mycobacterium species, have been used and were reported previously to present heterologous antigens to the immune system of vaccinated mice for proper CD8 T-cell priming (2, 7, 8). Probably the most promising viable carrier system is represented by attenuated serovar Typhimurium strains (33). These bacteria invade eukaryotic host cells and persist in endosomes (9). The confinement of Salmonella to these membrane-bound compartments complicates delivery of secreted foreign proteins to the cytosol of infected cells and subsequent MHC class I antigen presentation (29). In an attempt to circumvent this problem, we have reported the use of the serovar Typhimurium TTSS to target heterologous antigens directly to the cytosol of eukaryotic cells by translocation (29, 31). Defined secretion and translocation domains of the Yersinia type III effector protein YopE were fused to large antigenic protein fragments of L. monocytogenes (31). The resulting chimeric YopE/LLO and YopE/p60 proteins were engaged by the Salmonella TTSS, leading to the induction of antigen-specific CD8 T-cell responses in orally vaccinated mice (31). In these animals, translocation of either single YopE/LLO or YopE/p60 by Salmonella resulted in significant protection against a challenge with wild-type Listeria.
However, for two reasons, a versatile antigen delivery system used by attenuated serovar Typhimurium strains should be capable of targeting more than one antigen at the same time: (i) polyvalent vaccines displaying antigenic fragments derived from diverse microorganisms are powerful tools with which to immunize against several pathogens in a single application step, and (ii) sufficient vaccine-induced protection against particular infectious agents might require the stimulation of immune responses against several pathogen-derived antigens. Thus, the aim of the present study was to investigate whether the TTSS encoded by Salmonella is able to engage two independent listerial antigens fused to YopE for translocation purposes. The murine model of listeriosis served as a model system to investigate the impact of concomitant antigen delivery on the efficacy of protection.
L. monocytogenes is a gram-positive bacterium that causes life-threatening infections during pregnancy and in immunocompromised patients (13). Preferentially macrophages and hepatocytes are infected by Listeria. After invasion of the host cell, the pathogen secretes pore-forming LLO to gain access to the eukaryotic cytoplasm and the murein hydrolase p60, resulting in the induction of vigorous dominant MHC class I-restricted CD8 T-cell responses that lead to rapid clearance of bacteria (19). In the present study, mice were orally inoculated with a single dose of 107 CFU of attenuated serovar Typhimurium expressing either translocated YopE/LLO or YopE/p60. Vaccinated mice revealed high numbers of IFN-γ-producing cells reactive with LLO91-99 or p60217-225. These CD8 T-cell responses led to a pronounced decrease of the bacterial load in spleens of mice challenged with Listeria. Recombinant Salmonella expressing both listerial antigens was able to concomitantly translocate YopE/LLO and YopE/p60 to the cytosol of macrophages at similar concentrations as Salmonella strains translocating a single YopE hybrid protein. Further in vitro and in vivo assays revealed no significant differences in antigen presentation and CD8 T-cell priming efficacies between the dual translocated LLO and p60 or single translocated listerial antigens. Thus, the translocation process mediated by the Salmonella TTSS appeared to be a versatile mechanism for cytosolic delivery of heterologous antigens.
In the past few years, knowledge about the molecular bases of type III secretion and translocation has increased (17). Composed of up to 40 proteins, TTSSs are among the most complex protein secretion systems known. An essential part of such complexity is the fact that proteins are not only secreted from the bacterial cytoplasm but also delivered directly to the inside of the eukaryotic host cell, therefore effectively working as a molecular syringe (10, 21). The first step of this sophisticated type III export mechanism is the secretion process: effector proteins cross the inner and outer membranes of gram-negative bacteria without making an intermediate stop in the periplasm. Careful analysis of a number of type III proteins revealed that they span both the inner and outer membranes of the bacterial envelope (21). These components assemble into an organelle, termed the “needle complex” (21). The architecture of the needle complex resembles that of the flagellar hook-basal body (1), and this molecular structure provides some understanding of how type III secretion works. However, this model cannot elucidate the second step of the type III export mechanism, by which the system mediates the translocation of secreted proteins into the host cell. To explain translocation, it has been proposed previously that some type III proteins form a pore or channel through which effector proteins cross the eukaryotic cell membrane (15, 23). Obviously, for cytosolic delivery purposes of heterologous antigens this putative pore appears to be very permissive.
Most importantly, the present study revealed a significant difference in the efficacies of protection of vaccinated mice against a Listeria challenge. Spleens of animals immunized with recombinant Salmonella bacteria that concomitantly translocate YopE/LLO and YopE/p60 revealed a reduction of their bacterial load by 1 log step compared to that for mice vaccinated with serovar Typhimurium translocating a single listerial antigen. In fact, in the former group spleens from three out of eight mice did not show any colonization with Listeria, thus emphasizing the impact of dual immunodominant antigen translocation. Although the focus of this study was to determine the efficacy of a Salmonella vaccine that simultaneously translocates LLO and p60 antigens, we performed immunization experiments where the effect of two single Salmonella vaccines (SB824 with pHR231 and SB824 with pHR241) given to animals as a cocktail (107 CFU of each strain) was investigated (data not shown). In comparison to mice immunized with recombinant SB824(pHR251), animals inoculated with a mixture of SB824(pHR231) and SB824(pHR241) revealed no significant differences in LLO- and p60-specific CD8 T-cell priming efficacies, leading to superior protection against murine listeriosis as well. Interestingly, by applying a DNA vaccination strategy, Yamada et al. demonstrated that simultaneous inoculation of plasmids coding for LLO91-99 and p60217-225 failed to surpass a single plasmid expressing one dominant nonamer peptide in the efficacy of protection (38). Unlike a DNA vaccine, live recombinant Salmonella is capable of cytosolic listerial antigen targeting during its intracellular replication, thus mimicking a wild-type L. monocytogenes infection. In summary, we demonstrate for the first time that type III-mediated concomitant translocation of two immunodominant antigens results in superior protection against an intracellular pathogen. The versatile application of the TTSS for heterologous antigen delivery might be attractive for the development of vaccines designed to emphasize CD8 T-cell-mediated immunity.
Acknowledgments
We thank Jeannette Sauer and Simone Schenk for expert technical assistance.
H.R. was supported by the “AIDS-Stipendienprogramm” from the Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie/Germany, and by the Deutsche Forschungsgemeinschaft (Schwerpunktprogramm “Neue Vakzinierungsstrategien”).
Editor: J. T. Barbieri
REFERENCES
- 1.Aizawa, S. I. 1996. Flagellar assembly in Salmonella typhimurium. Mol. Microbiol. 19:1-5. [DOI] [PubMed] [Google Scholar]
- 2.Aldovini, A., and R. A. Young. 1991. Humoral and cell-mediated immune responses to live recombinant BCG-HIV vaccines. Nature 351:479-482. [DOI] [PubMed] [Google Scholar]
- 3.Alpuche-Aranda, C. M., E. L. Racoosin, J. A. Swanson, and S. I. Miller. 1994. Salmonella stimulate macrophage macropinocytosis and persist within spacious phagosomes. J. Exp. Med. 179:601-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson, D. M., and O. Schneewind. 1999. Type III machines of Gram-negative pathogens: injecting virulence factors into host cells and more. Curr. Opin. Microbiol. 2:18-24. [DOI] [PubMed] [Google Scholar]
- 5.Collazo, C. M., and J. E. Galán. 1996. Requirement for exported proteins in secretion through the invasion-associated type III system of Salmonella typhimurium. Infect. Immun. 64:3524-3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collazo, C. M., and J. E. Galán. 1997. The invasion-associated type III system of Salmonella typhimurium directs the translocation of Sip proteins into the host cell. Mol. Microbiol. 24:747-756. [DOI] [PubMed] [Google Scholar]
- 7.Dietrich, G., A. Bubert, I. Gentchev, Z. Sokolovic, A. Simm, A. Catic, S. H. Kaufmann, J. Hess, A. A. Szalay, and W. Goebel. 1998. Delivery of antigen-encoding plasmid DNA into the cytosol of macrophages by attenuated suicide Listeria monocytogenes. Nat. Biotechnol. 16:181-185. [DOI] [PubMed] [Google Scholar]
- 8.Everest, P., P. Griffiths, and G. Dougan. 1995. Live Salmonella vaccines as a route towards oral immunisation. Biologicals 23:119-124. [DOI] [PubMed] [Google Scholar]
- 9.Galán, J. E. 1996. Molecular genetic bases of Salmonella entry into host cells. Mol. Microbiol. 20:263-271. [DOI] [PubMed] [Google Scholar]
- 10.Galán, J. E. 1998. Interactions of Salmonella with host cells: encounters of the closest kind. Proc. Natl. Acad. Sci. USA 95:14006-14008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geginat, G., M. Kretschmar, S. Walter, D. Junker, H. Hof, and T. Nichterlein. 1999. Suppression of acquired immunity against Listeria monocytogenes by amphotericin B-induced mediated inhibition of CD8 T cell function. J. Infect. Dis. 180:1186-1194. [DOI] [PubMed] [Google Scholar]
- 12.Geginat, G., T. Nichterlein, M. Kretschmar, S. Schenk, H. Hof, M. Lalic-Multhaler, W. Goebel, and A. Bubert. 1999. Enhancement of the Listeria monocytogenes p60-specific CD4 and CD8 T cell memory by nonpathogenic Listeria innocua. J. Immunol. 162:4781-4789. [PubMed] [Google Scholar]
- 13.Gellin, B. G., and C. V. Broome. 1989. Listeriosis. JAMA 261:1313-1320. [PubMed] [Google Scholar]
- 14.Germain, R. N. 1995. The biochemistry and cell biology of antigen presentation by MHC class I and class II molecules. Implications for development of combination vaccines. Ann. N. Y. Acad. Sci. 754:114-125. [DOI] [PubMed] [Google Scholar]
- 15.Hakansson, S., K. Schesser, C. Persson, E. E. Galyov, R. Rosqvist, F. Homble, and H. Wolf-Watz. 1996. The YopB protein of Yersinia pseudotuberculosis is essential for the translocation of Yop effector proteins across the target cell membrane and displays a contact-dependent membrane disrupting activity. EMBO J. 15:5812-5823. [PMC free article] [PubMed] [Google Scholar]
- 16.Hoiseth, S. K., and B. A. Stocker. 1981. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature 291:238-239. [DOI] [PubMed] [Google Scholar]
- 17.Hueck, C. J. 1998. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev. 62:379-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaniga, K., J. Uralil, J. B. Bliska, and J. E. Galán. 1996. A secreted protein tyrosine phosphatase with modular effector domains in the bacterial pathogen Salmonella typhimurium. Mol. Microbiol. 21:633-641. [DOI] [PubMed] [Google Scholar]
- 19.Kaufmann, S. H. E., H. R. Rodewald, E. Hug, and G. De Libro. 1988. Cloned Listeria monocytogenes specific non-MHC-restricted Lyt-2+ T cells with cytolytic and protective activity. J. Immunol. 140:3173-3179. [PubMed] [Google Scholar]
- 20.Köhler, S. A., A. Bubert, M. Vogel, and W. Goebel. 1991. Expression of the iap gene coding for protein p60 of Listeria monocytogenes is controlled on the posttranscriptional level. J. Bacteriol. 173:4668-4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kubori, T., Y. Matsushima, D. Nakamura, J. Uralil, M. Lara-Tejero, A. Sukhan, J. E. Galán, and S. I. Aizawa. 1998. Supramolecular structure of the Salmonella typhimurium type III protein secretion system. Science 280:602-605. [DOI] [PubMed] [Google Scholar]
- 22.Lee, V. T., D. M. Anderson, and O. Schneewind. 1998. Targeting of Yersinia Yop proteins into the cytosol of HeLa cells: one-step translocation of YopE across bacterial and eukaryotic membranes is dependent on SycE chaperone. Mol. Microbiol. 28:593-601. [DOI] [PubMed] [Google Scholar]
- 23.Neyt, C., and G. R. Cornelis. 1999. Insertion of a Yop translocation pore into the macrophage plasma membrane by Yersinia enterocolitica: requirement for translocators YopB and YopD, but not LcrG. Mol. Microbiol. 33:971-981. [DOI] [PubMed] [Google Scholar]
- 24.Obert, S., R. J. O'Connor, S. Schmid, and P. Hearing. 1994. The adenovirus E4-6/7 protein transactivates the E2 promoter by inducing dimerization of a heteromeric E2F complex. Mol. Cell. Biol. 14:1333-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pamer, E. G., J. T. Harty, and M. J. Bevan. 1991. Precise prediction of a dominant class I MHC-restricted epitope of Listeria monocytogenes. Nature 353:852-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pamer, E. G. 1994. Direct sequence identification and kinetic analysis of an MHC class I-restricted Listeria monocytogenes CTL epitope. J. Immunol. 152:686-694. [PubMed] [Google Scholar]
- 27.Pamer, E. G., A. J. Sijts, M. S. Villanueva, D. H. Busch, and S. Vijh. 1997. MHC class I antigen processing of Listeria monocytogenes proteins: implications for dominant and subdominant CTL responses. Immunol. Rev. 158:129-136. [DOI] [PubMed] [Google Scholar]
- 28.Portnoy, D. A., P. S. Jacks, and D. J. Hinrichs. 1988. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J. Exp. Med. 167:1459-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rüssmann, H., H. Shams, F. Poblete, Y. Fu, J. E. Galán, and R. O. Donis. 1998. Delivery of epitopes by the Salmonella type III secretion system for vaccine development. Science 281:565-568. [DOI] [PubMed] [Google Scholar]
- 30.Rüssmann, H., A. Weissmüller, G. Geginat, E. I. Igwe, A. Roggenkamp, A. Bubert, W. Goebel, H. Hof, and J. Heesemann. 2000. Yersinia enterocolitica-mediated translocation of defined fusion proteins to the cytosol of mammalian cells results in peptide-specific MHC class I-restricted antigen presentation. Eur. J. Immunol. 30:1375-1384. [DOI] [PubMed] [Google Scholar]
- 31.Rüssmann, H., E. I. Igwe, J. Sauer, W.-D. Hardt, A. Bubert, and G. Geginat. 2001. Protection against murine listeriosis by oral vaccination with recombinant Salmonella expressing hybrid Yersinia type III proteins. J. Immunol. 167:357-365. [DOI] [PubMed] [Google Scholar]
- 32.Schesser, K., E. Frithz-Lindsten, and H. Wolf-Watz. 1996. Delineation and mutational analysis of the Yersinia pseudotuberculosis YopE domains which mediate translocation across bacterial and eukaryotic cellular membranes. J. Bacteriol. 178:7227-7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schodel, F., and R. Curtiss III. 1995. Salmonellae as oral vaccine carriers. Dev. Biol. Stand. 84:245-253. [PubMed] [Google Scholar]
- 34.Sijts, A. J. A. M., A. Neisig, J. Neefjes, and E. G. Pamer. 1996. Two Listeria monocytogenes CTL epitopes are processed from the same antigen with different efficiencies. J. Immunol. 156:683-692. [PubMed] [Google Scholar]
- 35.Sory, M.-P., A. Boland, I. Lambermont, and G. R. Cornelis. 1995. Identification of the YopE and YopH domains required for secretion and internalization into the cytosol of macrophages, using the cyaA gene fusion approach. Proc. Natl. Acad. Sci. USA 92:1998-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vijh, S., and E. G. Pamer. 1997. Immunodominant and subdominant CTL responses to Listeria monocytogenes infection. J. Immunol. 158:3366-3371. [PubMed] [Google Scholar]
- 37.Wang, R. F., and S. R. Kushner. 1991. Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene 100:195-199. [PubMed] [Google Scholar]
- 38.Yamada, T., H. Uchiyama, T. Nagata, M. Uchijima, T. Suda, K. Chida, H. Nakamura, and Y. Koide. 2001. Protective cytotoxic T-lymphocyte responses induced by DNA immunization against immunodominant and subdominant epitopes of Listeria monocytogenes are noncompetitive. Infect. Immun. 69:3427-3430. [DOI] [PMC free article] [PubMed] [Google Scholar]