Abstract
1. The ability of several groups of compounds, related to caffeine, to induce contractures in isolated frog auricular trabeculae has been tested.
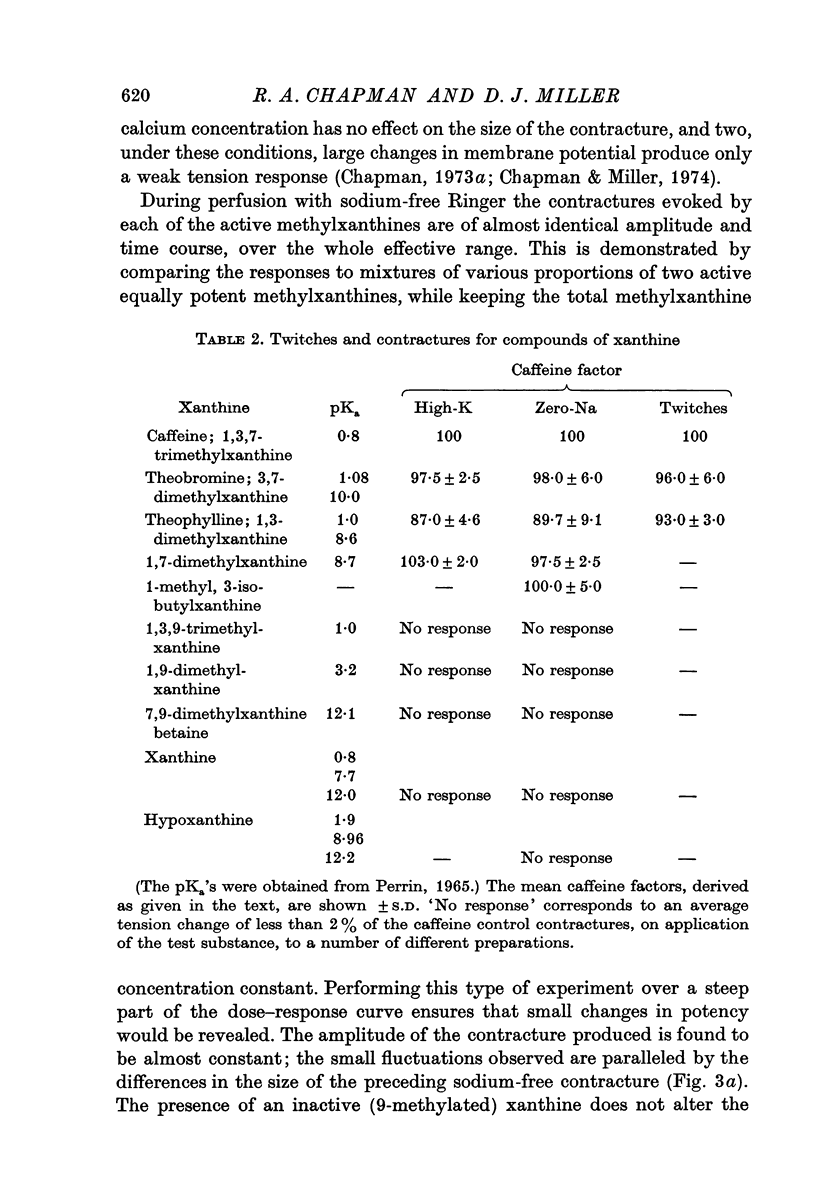
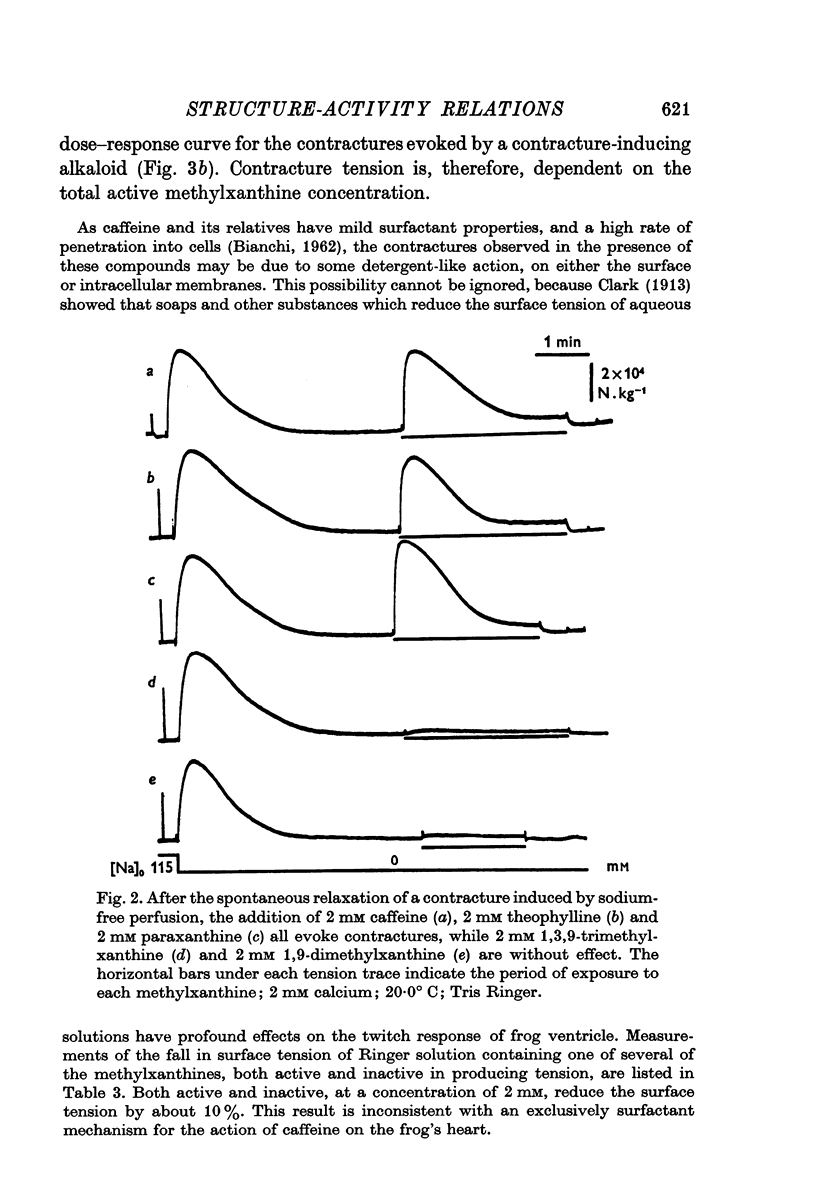
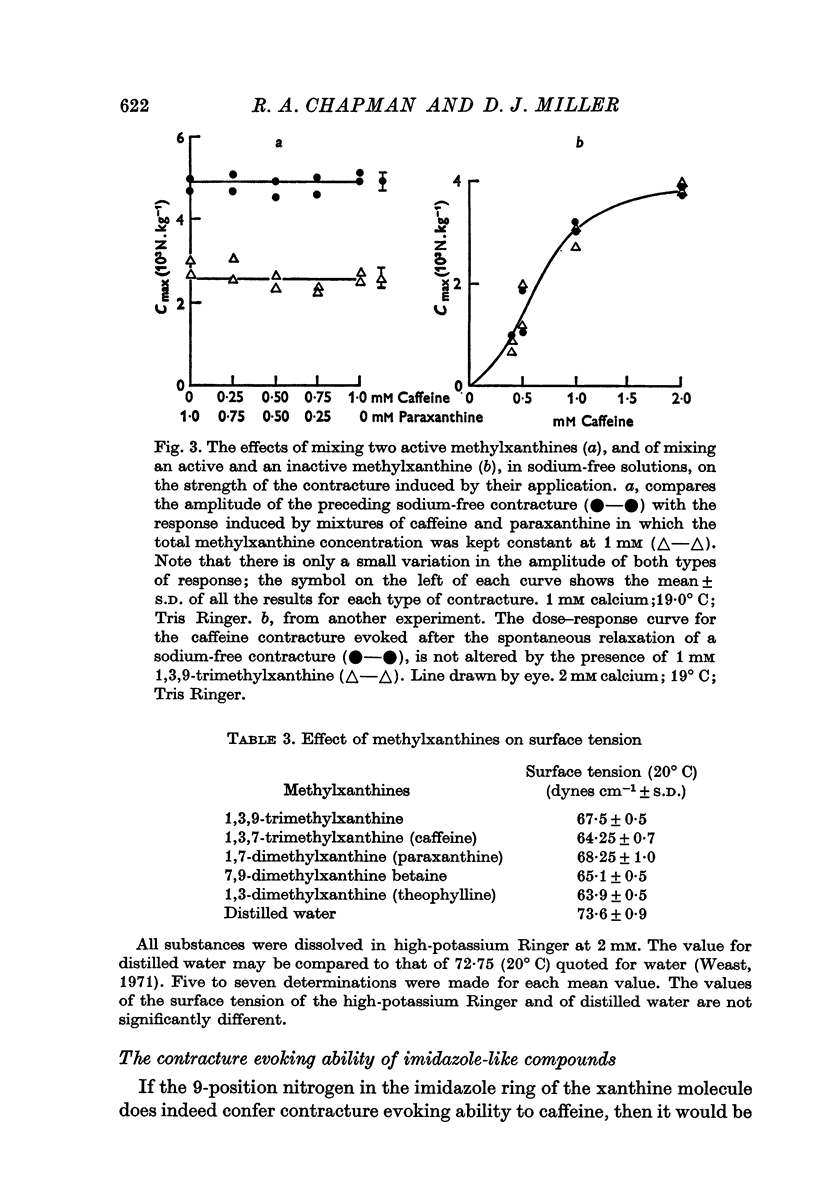
2. Of the methylxanthines, theophylline, theobromine and paraxanthine are of similar potency to caffeine. This applies to contractures produced in either high-potassium or in sodium-free solution, and to the twitch responses in normal Ringer.
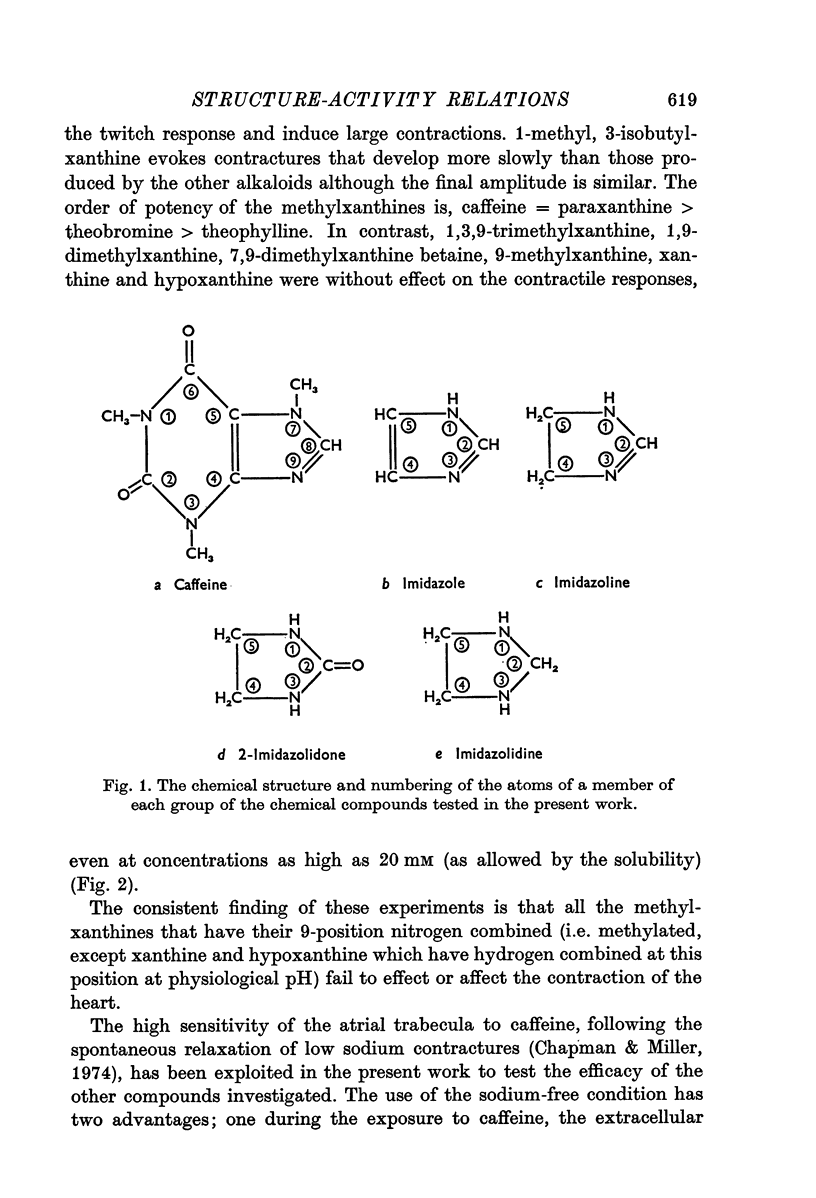
3. Xanthines in which the 9-position nitrogen is combined and is, therefore, without an ethylene bond do not affect contraction.
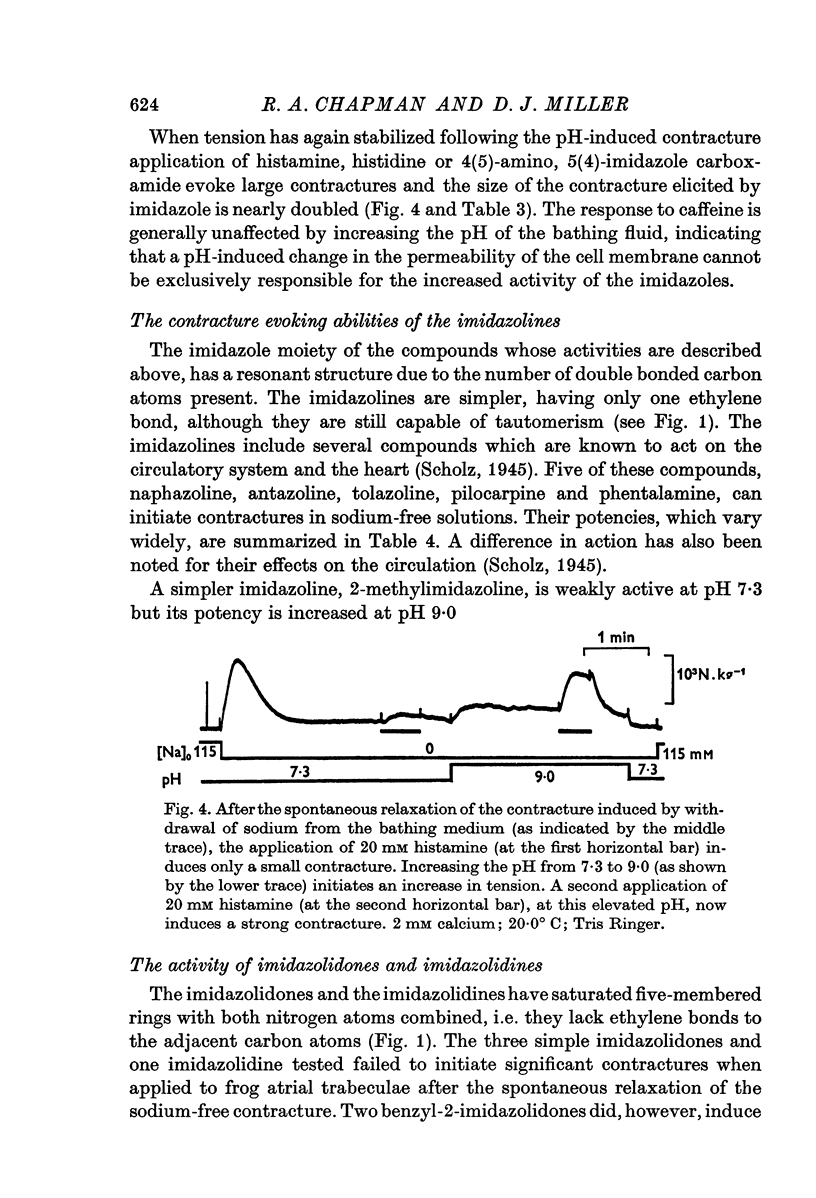
4. The hypothesis is put forward that a double-bonded nitrogen, in an imidazole ring, is required for activity of the methylxanthine. This hypothesis is supported by the ability of imidazole and several close derivatives (e.g. histamine), as well as imidazolines, to evoke contractures. As predicted by the hypothesis, imidazolidines and imidazolidones, in which all the nitrogen atoms have single bonds, fail to initiate tension development.
5. The activity of histamine and histidine is only demonstrable at high pH (≏ 9·0).
6. Raising the pH in sodium-free solution induces a transient contracture.
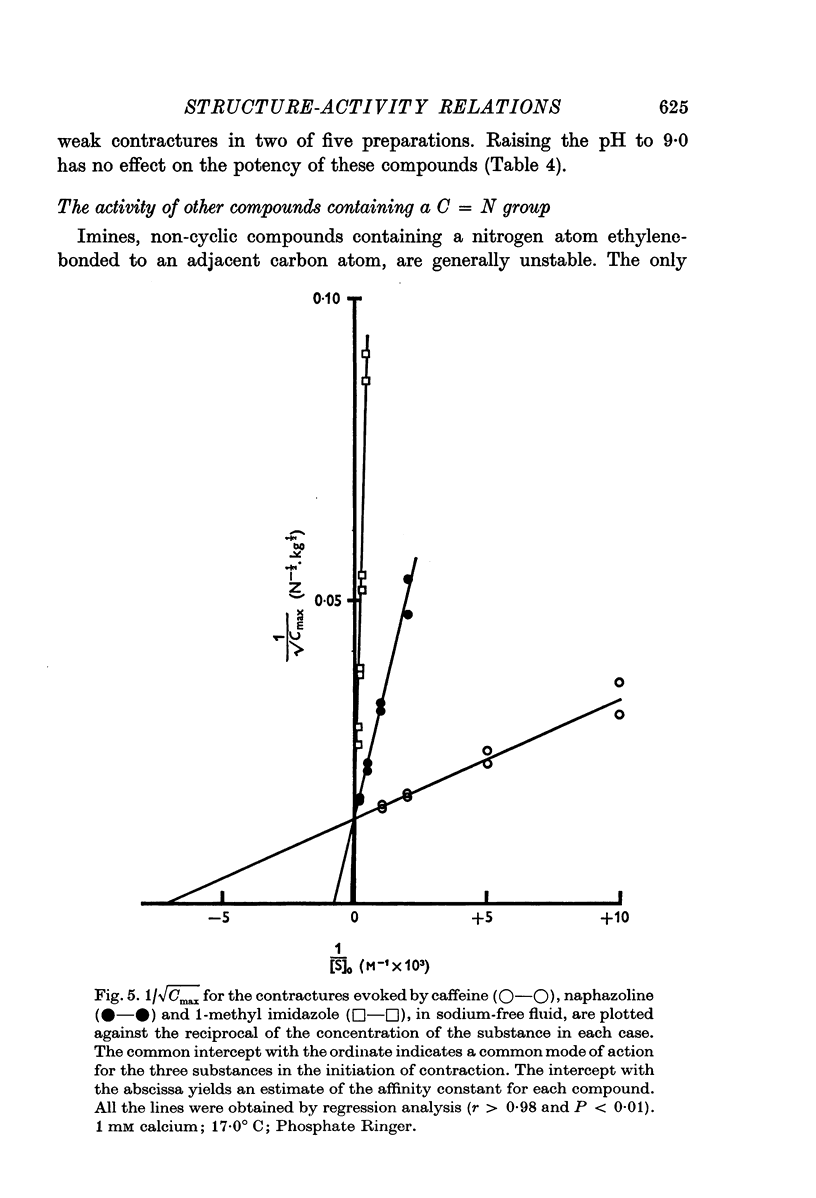
7. Several arguments suggest that cyclic AMP is probably not an intermediate in the response to the methylxanthines. The activity of cyclic AMP (and adenosine) in eliciting contractures is predicted by the hypothesis because they contain an imidazole moiety as part of their molecular structure.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akera T., Brody T. M. Effects of chlorpromazine free radical on brain microsomal enzymes. Biochem Pharmacol. 1972 May 15;21(10):1403–1411. doi: 10.1016/0006-2952(72)90364-4. [DOI] [PubMed] [Google Scholar]
- BUTCHER R. W., SUTHERLAND E. W. Adenosine 3',5'-phosphate in biological materials. I. Purification and properties of cyclic 3',5'-nucleotide phosphodiesterase and use of this enzyme to characterize adenosine 3',5'-phosphate in human urine. J Biol Chem. 1962 Apr;237:1244–1250. [PubMed] [Google Scholar]
- Balzer H., Makinose M., Hasselbach W. The inhibition of the sarcoplasmic calcium pump by prenylamine, reserpine, chlorpromazine and imipramine. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1968;260(5):444–455. doi: 10.1007/BF00537359. [DOI] [PubMed] [Google Scholar]
- Beavo J. A., Rogers N. L., Crofford O. B., Baird C. E., Hardman J. G., Sutherland E. W., Newman E. V. Effects of phosphodiesterase inhibitors on cyclic AMP levels and on lipolysis. Ann N Y Acad Sci. 1971 Dec 30;185:129–136. doi: 10.1111/j.1749-6632.1971.tb45243.x. [DOI] [PubMed] [Google Scholar]
- Beavo J. A., Rogers N. L., Crofford O. B., Hardman J. G., Sutherland E. W., Newman E. V. Effects of xanthine derivatives on lipolysis and on adenosine 3',5'-monophosphate phosphodiesterase activity. Mol Pharmacol. 1970 Nov;6(6):597–603. [PubMed] [Google Scholar]
- Bianchi C. P. The Effect of Caffeine on Radiocalcium Movement in Frog Sartorius. J Gen Physiol. 1961 May 1;44(5):845–858. doi: 10.1085/jgp.44.5.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinks J. R., Olson C. B., Jewell B. R., Bravený P. Influence of caffeine and other methylxanthines on mechanical properties of isolated mammalian heart muscle. Evidence for a dual mechanism of action. Circ Res. 1972 Apr;30(4):367–392. doi: 10.1161/01.res.30.4.367. [DOI] [PubMed] [Google Scholar]
- Bowman W. C., Nott M. W. Actions of sympathomimetic amines and their antagonists on skeletal muscle. Pharmacol Rev. 1969 Mar;21(1):27–72. [PubMed] [Google Scholar]
- CALDWELL P. C. Studies on the internal pH of large muscle and nerve fibres. J Physiol. 1958 Jun 18;142(1):22–62. doi: 10.1113/jphysiol.1958.sp005998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman R. A., Ellis D. Synergistic effects of cooling and caffeine on the contraction of the frog's heart. J Physiol. 1973 Jul;232(2):101P–102P. [PubMed] [Google Scholar]
- Chapman R. A., Miller D. J. The effects of caffeine on the contraction of the frog heart. J Physiol. 1974 Nov;242(3):589–613. doi: 10.1113/jphysiol.1974.sp010725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman R. A. The effects of temperature and metabolic inhibitors on the spontaneous relaxation of the potassium contracture of the heart of the frog Rana pipiens. J Physiol. 1973 Jun;231(2):233–249. doi: 10.1113/jphysiol.1973.sp010230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman R. A. The onic dependence of the strength and spontaneous relations of the potassium contracture induced in the heart of the frog Rana pipiens. J Physiol. 1973 Jun;231(2):209–232. doi: 10.1113/jphysiol.1973.sp010229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarandini D. J., Reuben J. P., Brandt P. W., Grundfest H. Effects of caffeine on crayfish muscle fibers. I. Activation of contraction and induction of Ca spike electrogenesis. J Gen Physiol. 1970 May;55(5):640–664. doi: 10.1085/jgp.55.5.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. J. The action of ions and lipoids upon the frog's heart. J Physiol. 1913 Oct 17;47(1-2):66–107. doi: 10.1113/jphysiol.1913.sp001614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967 Nov;193(2):419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entman M. L., Levey G. S., Epstein S. E. Mechanism of action of epinephrine and glucagon on the canine heart. Evidence for increase in sarcotubular calcium stores mediated by cyclic 3',5'-AMP. Circ Res. 1969 Oct;25(4):429–438. doi: 10.1161/01.res.25.4.429. [DOI] [PubMed] [Google Scholar]
- GOFFART M., GOUTIER R. Les méthylxanthines dans le groupe des sensibilisateurs au potassium. Arch Int Physiol. 1950 Apr;57(3):297–308. doi: 10.3109/13813455009144913. [DOI] [PubMed] [Google Scholar]
- Johnson P. N., Inesi G. The effect of methylxanthines and local anesthetics on fragmented sarcoplasmic reticulum. J Pharmacol Exp Ther. 1969 Oct;169(2):308–314. [PubMed] [Google Scholar]
- Kukovetz W. R., Pöch G. The action of imidazole on the effects of methyl-xanthines and catecholamines on cardiac contraction and phosphorylase activity. J Pharmacol Exp Ther. 1967 Jun;156(3):514–521. [PubMed] [Google Scholar]
- Kukovetz W. R., Pöch G. The positive inotropic effect of cyclic AMP. Adv Cyclic Nucleotide Res. 1972;1:261–290. [PubMed] [Google Scholar]
- Lorković H. The effect of pH on the mechanical activity of the frog toe muscle. J Gen Physiol. 1967 Mar;50(4):863–882. [PubMed] [Google Scholar]
- Lüttgau H. C., Oetliker H. The action of caffeine on the activation of the contractile mechanism in straited muscle fibres. J Physiol. 1968 Jan;194(1):51–74. doi: 10.1113/jphysiol.1968.sp008394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus M. L., Skelton C. L., Prindle K. H., Jr, Epstein S. E. Potentiation of the inotropic effects of glucagon by theophylline. J Pharmacol Exp Ther. 1971 Nov;179(2):331–337. [PubMed] [Google Scholar]
- Mircevová L., Simonová A. The effect of nervous active drugs on MG ++ -dependent ATPase in erythrocyte membrane. Arch Int Physiol Biochim. 1971 Dec;79(5):903–916. doi: 10.3109/13813457109104849. [DOI] [PubMed] [Google Scholar]
- Nakamaru Y., Schwartz A. The influence of hydrogen ion concentration on calcium binding and release by skeletal muscle sarcoplasmic reticulum. J Gen Physiol. 1972 Jan;59(1):22–32. doi: 10.1085/jgp.59.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering J. W. Observations on the Physiology of the Embryonic Heart. J Physiol. 1893 Jun;14(6):382.2–38466. doi: 10.1113/jphysiol.1893.sp000459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison G. A., Butcher R. W., Sutherland E. W. Cyclic AMP. Annu Rev Biochem. 1968;37:149–174. doi: 10.1146/annurev.bi.37.070168.001053. [DOI] [PubMed] [Google Scholar]
- Sandow A. Excitation-contraction coupling in skeletal muscle. Pharmacol Rev. 1965 Sep;17(3):265–320. [PubMed] [Google Scholar]
- Sandow A. Skeletal muscle. Annu Rev Physiol. 1970;32:87–138. doi: 10.1146/annurev.ph.32.030170.000511. [DOI] [PubMed] [Google Scholar]
- Skelton C. L., Karch F. E., Hougen T. J., Marcus M. L., Epstein S. E. Potentiation of the inotropic effects of norepinephrine and dibutyryl cyclic AMP by theophylline. J Mol Cell Cardiol. 1971 Dec;3(3):243–253. doi: 10.1016/0022-2828(71)90044-7. [DOI] [PubMed] [Google Scholar]
- Weber A., Herz R. The relationship between caffeine contracture of intact muscle and the effect of caffeine on reticulum. J Gen Physiol. 1968 Nov;52(5):750–759. doi: 10.1085/jgp.52.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A. The mechanism of the action of caffeine on sarcoplasmic reticulum. J Gen Physiol. 1968 Nov;52(5):760–772. doi: 10.1085/jgp.52.5.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. F. Effects of caffeine on neuromuscular transmission in the rat. Am J Physiol. 1973 Oct;225(4):862–865. doi: 10.1152/ajplegacy.1973.225.4.862. [DOI] [PubMed] [Google Scholar]


