Abstract
1. The cells of the cortex of the posterior bank of the superior temporal sulcus of the monkey appear to be specialized to signal motion in the visual field. In this paper, cells in this cortical area capable of signalling motion towards or away from the animal are described.
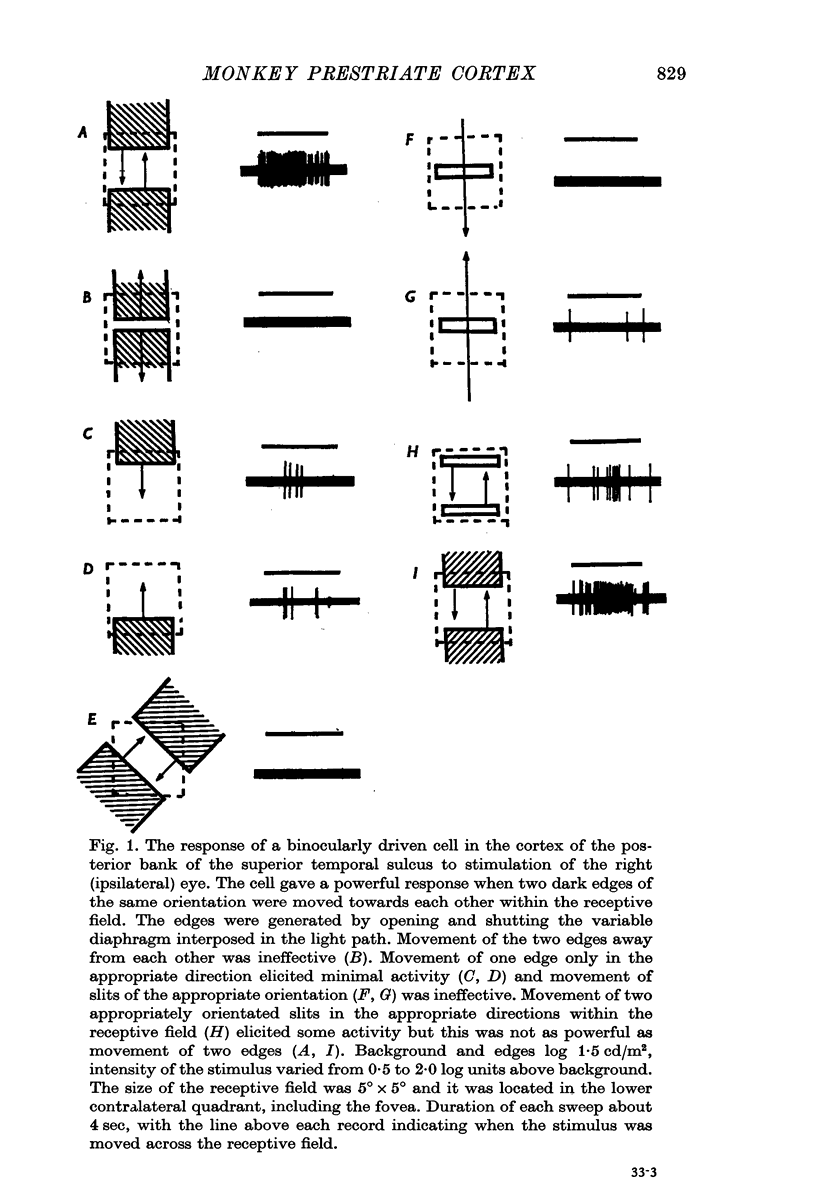
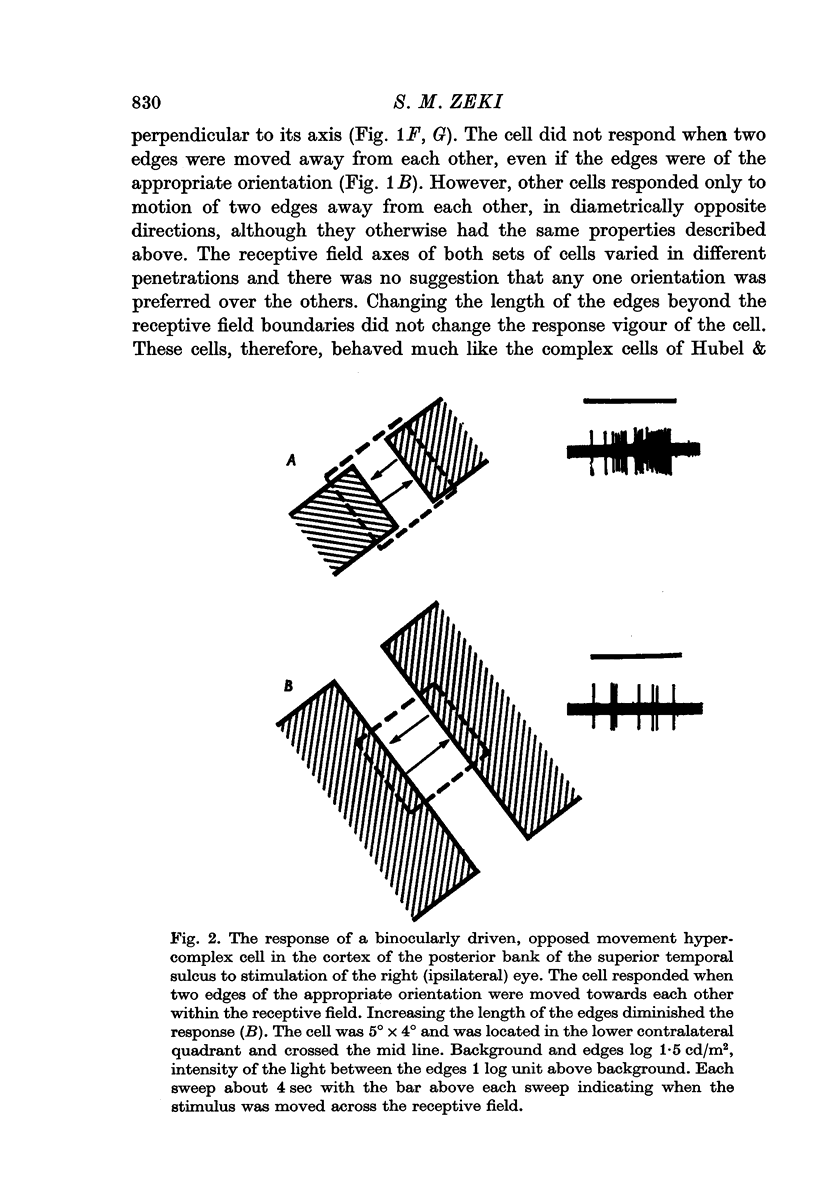
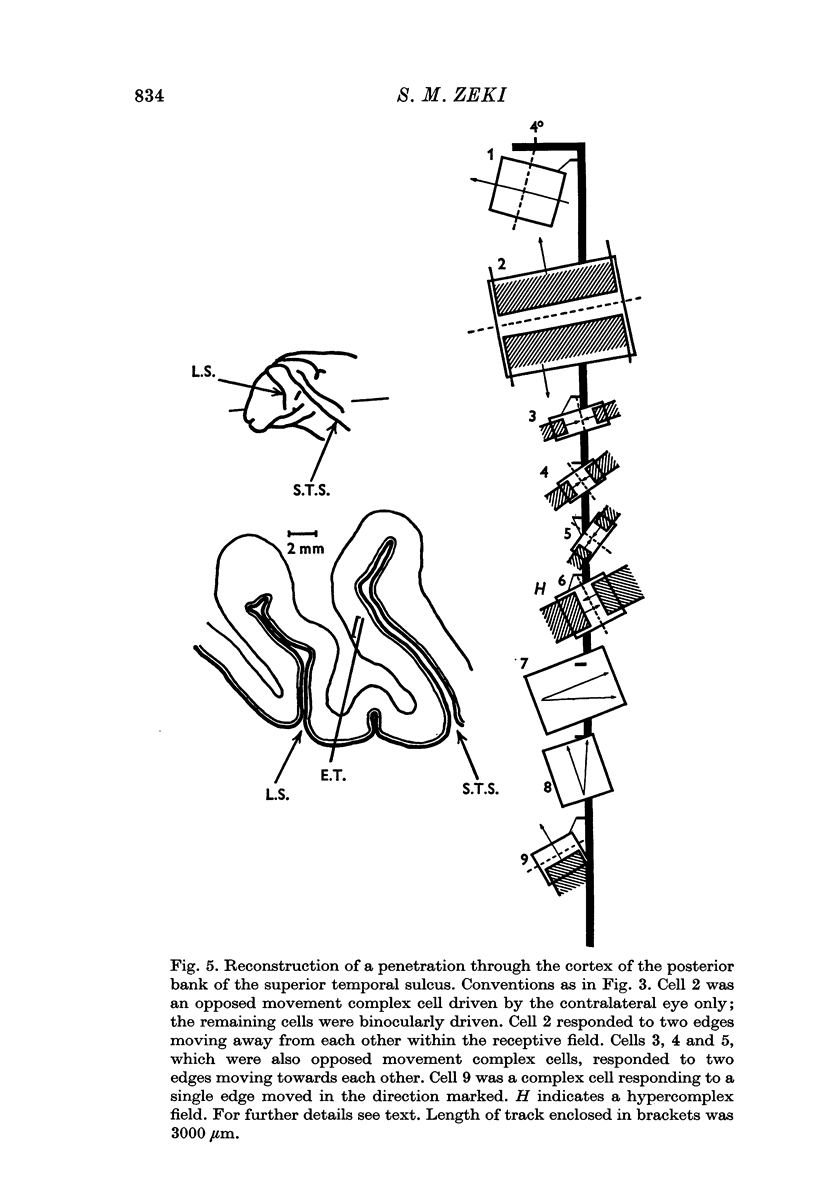
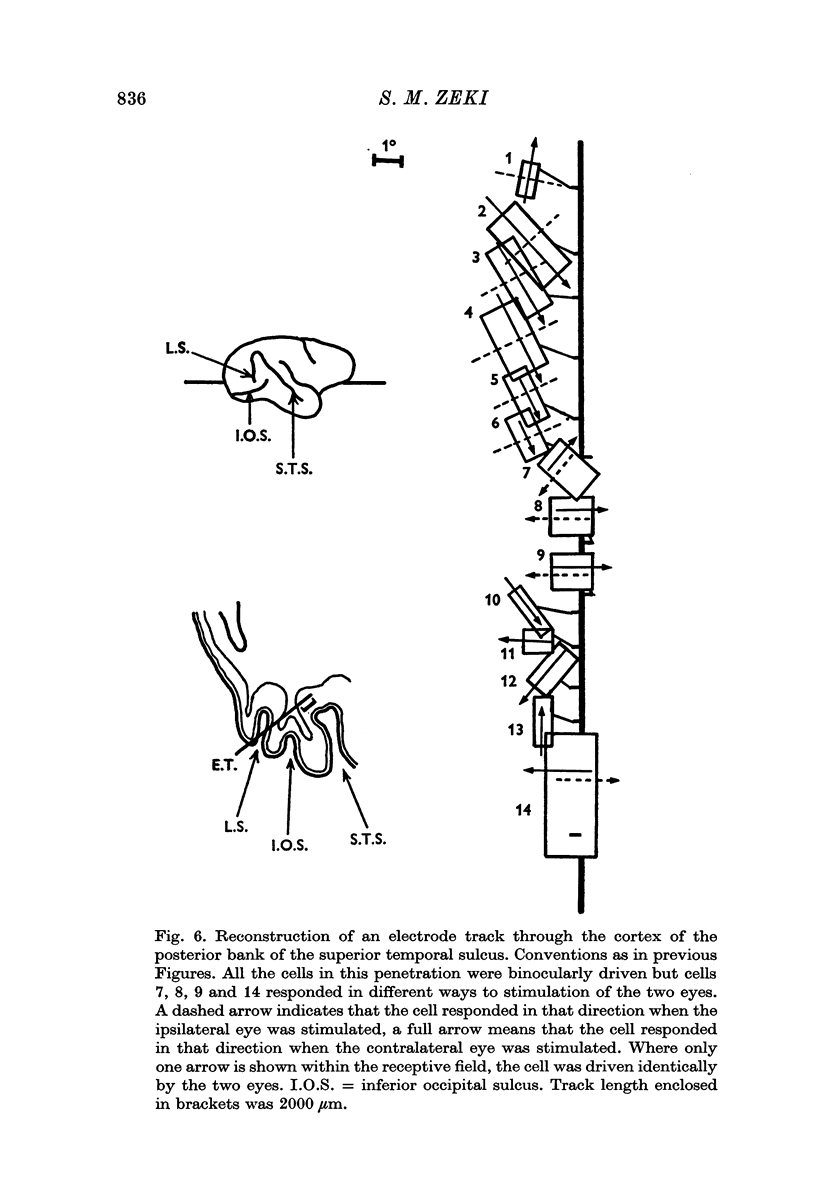
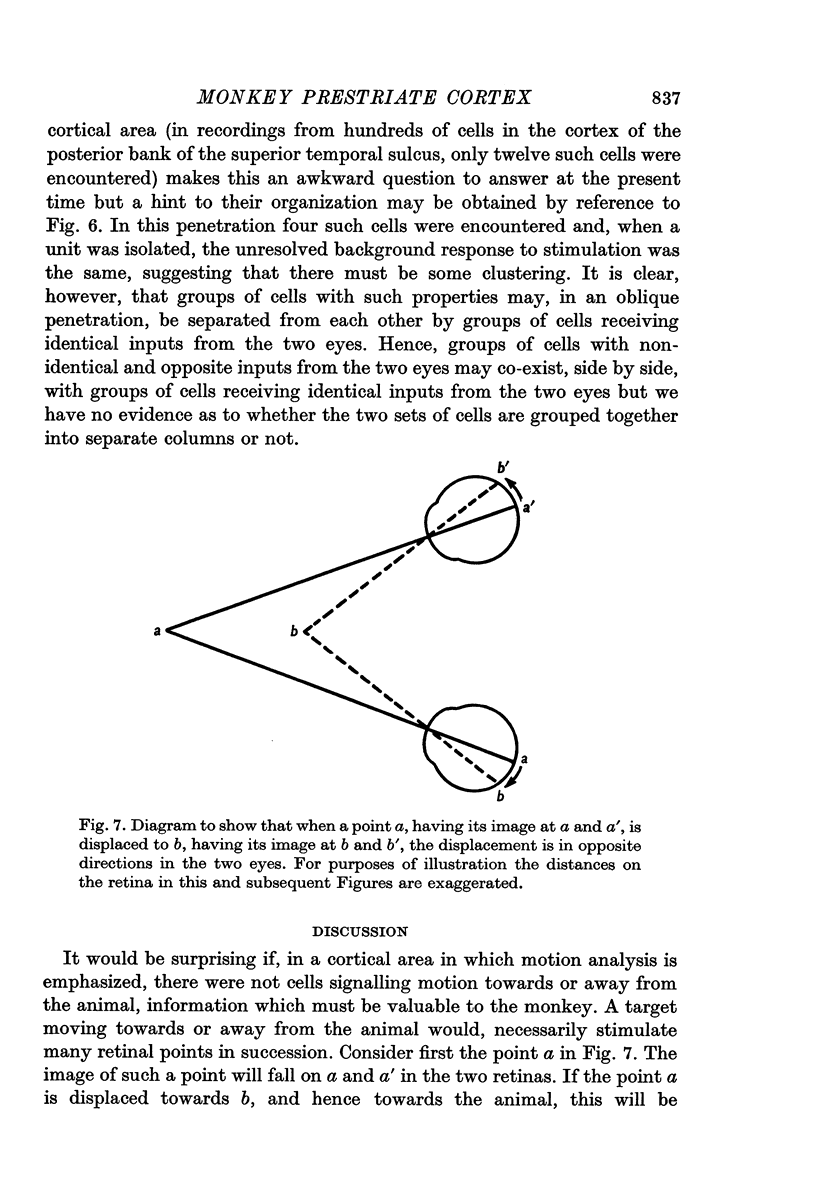
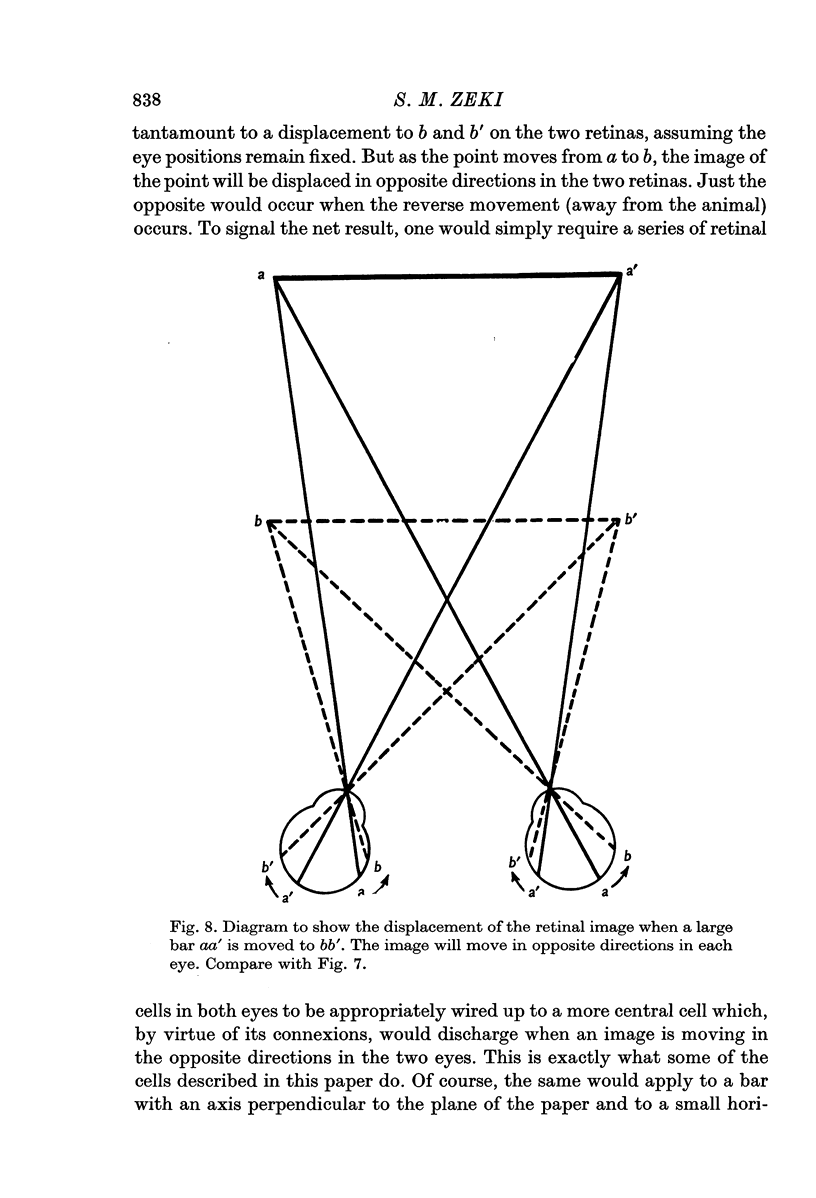
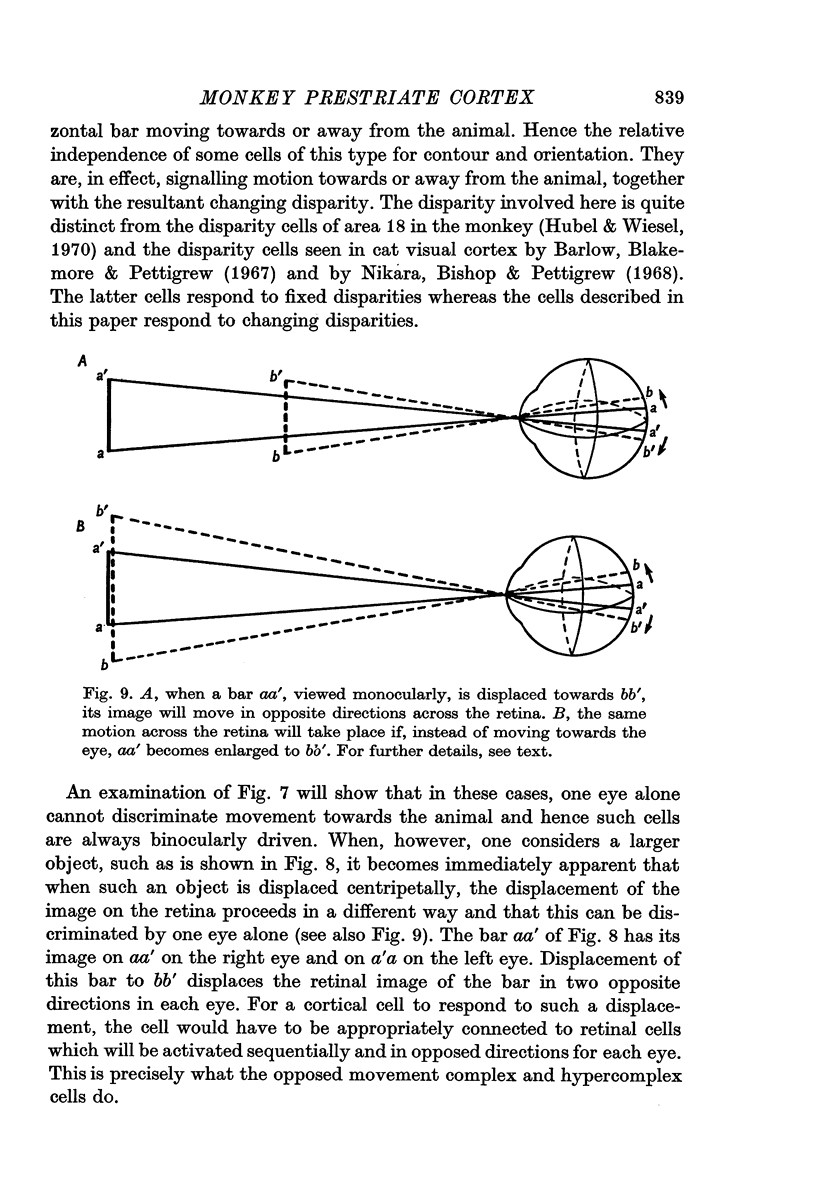
2. Two such types of cell were encountered. One type, the opposed movement complex and opposed movement hypercomplex cells, responded to two edges at a given orientation moving towards or away from each other within the receptive fields. These cells were driven either monocularly or binocularly, but when binocularly driven the cells responded in an identical manner to stimulation of each eye, thus suggesting that such cells must receive a double, and opposed, input from each eye. The other type of cell, always binocularly driven, responded to movement in opposite directions on the two retinas, thus suggesting that such cells must receive diametrically opposite connexions from the two eyes.
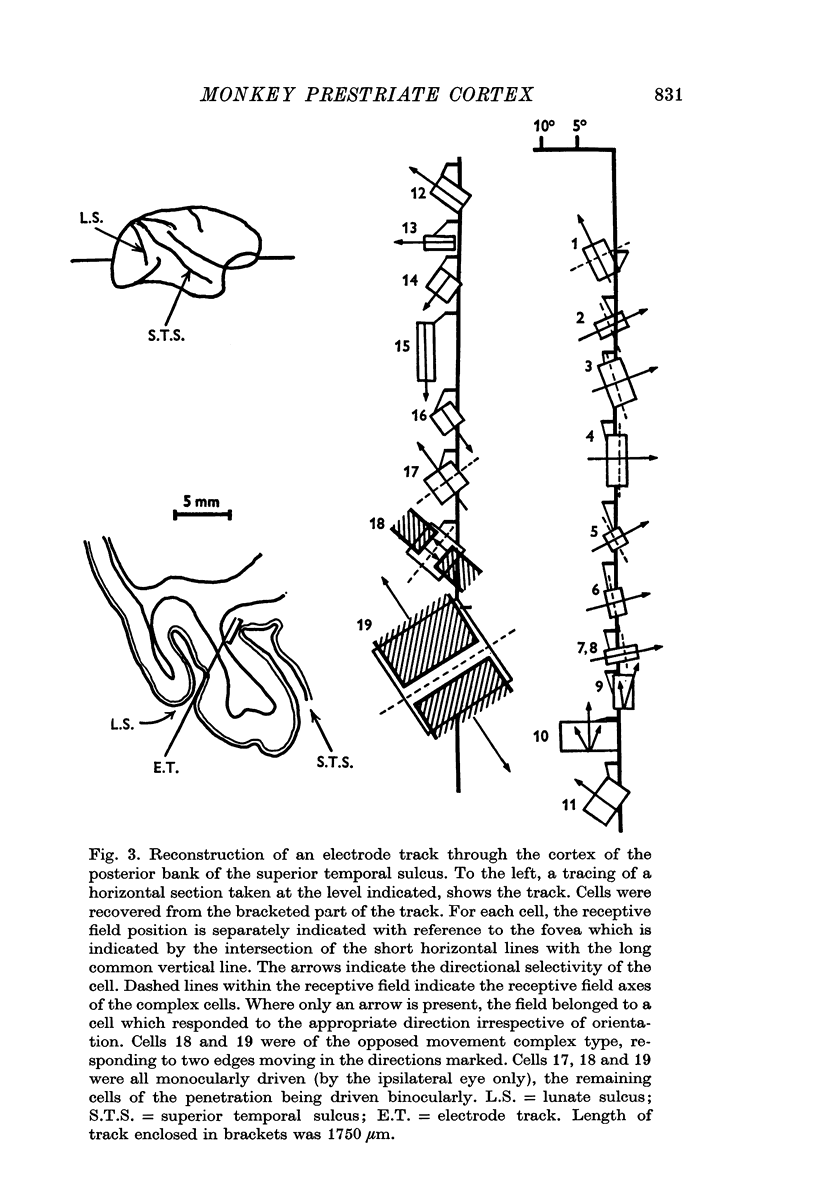
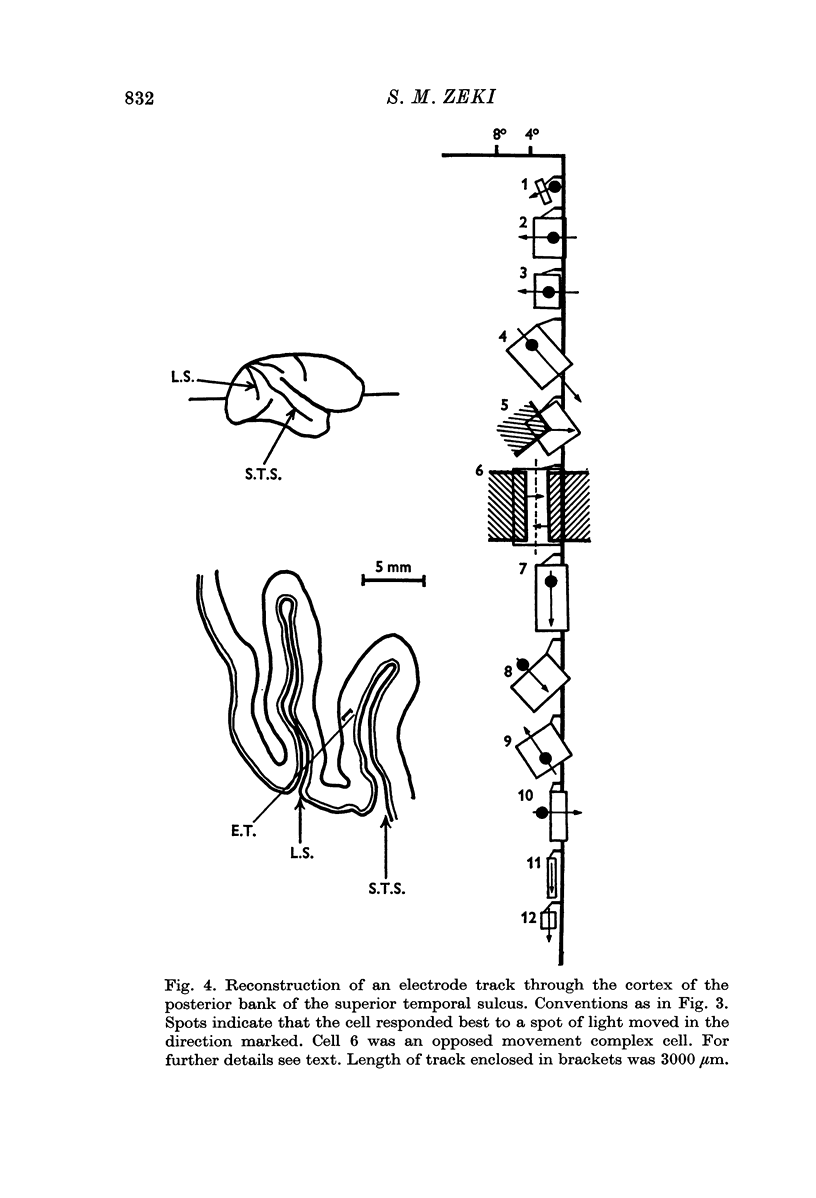
3. Long penetrations made to study the manner in which such cells were grouped together in the cortex revealed that they were arranged in small groups or clusters, separated from each other by the common directionally selective cells so prominently present in this area. Thus, cells with one type of wiring mechanism were separated from each other by cells receiving another, and more common, type of anatomical wiring.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlow H. B., Blakemore C., Pettigrew J. D. The neural mechanism of binocular depth discrimination. J Physiol. 1967 Nov;193(2):327–342. doi: 10.1113/jphysiol.1967.sp008360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg B. G. The topography of the afferent projections in the circumstriate visual cortex of the monkey studied by the Nauta method. Vision Res. 1969 Jul;9(7):733–747. doi: 10.1016/0042-6989(69)90011-x. [DOI] [PubMed] [Google Scholar]
- Dubner R., Zeki S. M. Response properties and receptive fields of cells in an anatomically defined region of the superior temporal sulcus in the monkey. Brain Res. 1971 Dec 24;35(2):528–532. doi: 10.1016/0006-8993(71)90494-x. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. RECEPTIVE FIELDS AND FUNCTIONAL ARCHITECTURE IN TWO NONSTRIATE VISUAL AREAS (18 AND 19) OF THE CAT. J Neurophysiol. 1965 Mar;28:229–289. doi: 10.1152/jn.1965.28.2.229. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. A re-examination of stereoscopic mechanisms in area 17 of the cat. J Physiol. 1973 Jul;232(1):29P–30P. [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Stereoscopic vision in macaque monkey. Cells sensitive to binocular depth in area 18 of the macaque monkey cortex. Nature. 1970 Jan 3;225(5227):41–42. doi: 10.1038/225041a0. [DOI] [PubMed] [Google Scholar]
- Nikara T., Bishop P. O., Pettigrew J. D. Analysis of retinal correspondence by studying receptive fields of binocular single units in cat striate cortex. Exp Brain Res. 1968;6(4):353–372. doi: 10.1007/BF00233184. [DOI] [PubMed] [Google Scholar]
- Pettigrew J. D. Binocular neurons which signal change of disparity in area 18 of cat visual cortex. Nat New Biol. 1973 Jan 24;241(108):123–124. doi: 10.1038/newbio241123a0. [DOI] [PubMed] [Google Scholar]
- Regan D., Beverley K. I. Electrophysiological evidence for existence of neurones sensitive to direction of depth movement. Nature. 1973 Dec 21;246(5434):504–506. doi: 10.1038/246504a0. [DOI] [PubMed] [Google Scholar]
- Zeki S. M. Convergent input from the striate cortex (area 17) to the cortex of the superior temporal sulcus in the rhesus monkey. Brain Res. 1971 May 7;28(2):338–340. doi: 10.1016/0006-8993(71)90665-2. [DOI] [PubMed] [Google Scholar]
- Zeki S. M. Cortical projections from two prestriate areas in the monkey. Brain Res. 1971 Nov;34(1):19–35. doi: 10.1016/0006-8993(71)90348-9. [DOI] [PubMed] [Google Scholar]
- Zeki S. M. Functional organization of a visual area in the posterior bank of the superior temporal sulcus of the rhesus monkey. J Physiol. 1974 Feb;236(3):549–573. doi: 10.1113/jphysiol.1974.sp010452. [DOI] [PMC free article] [PubMed] [Google Scholar]


