Abstract
1. Na and K contents of isolated rat superior cervical ganglia were measured by flame photometry, and intracellular Na and K concentrations ([Na]i and [K]i) calculated using Li and 35SO4 to determine extracellular space (e.c.s.).
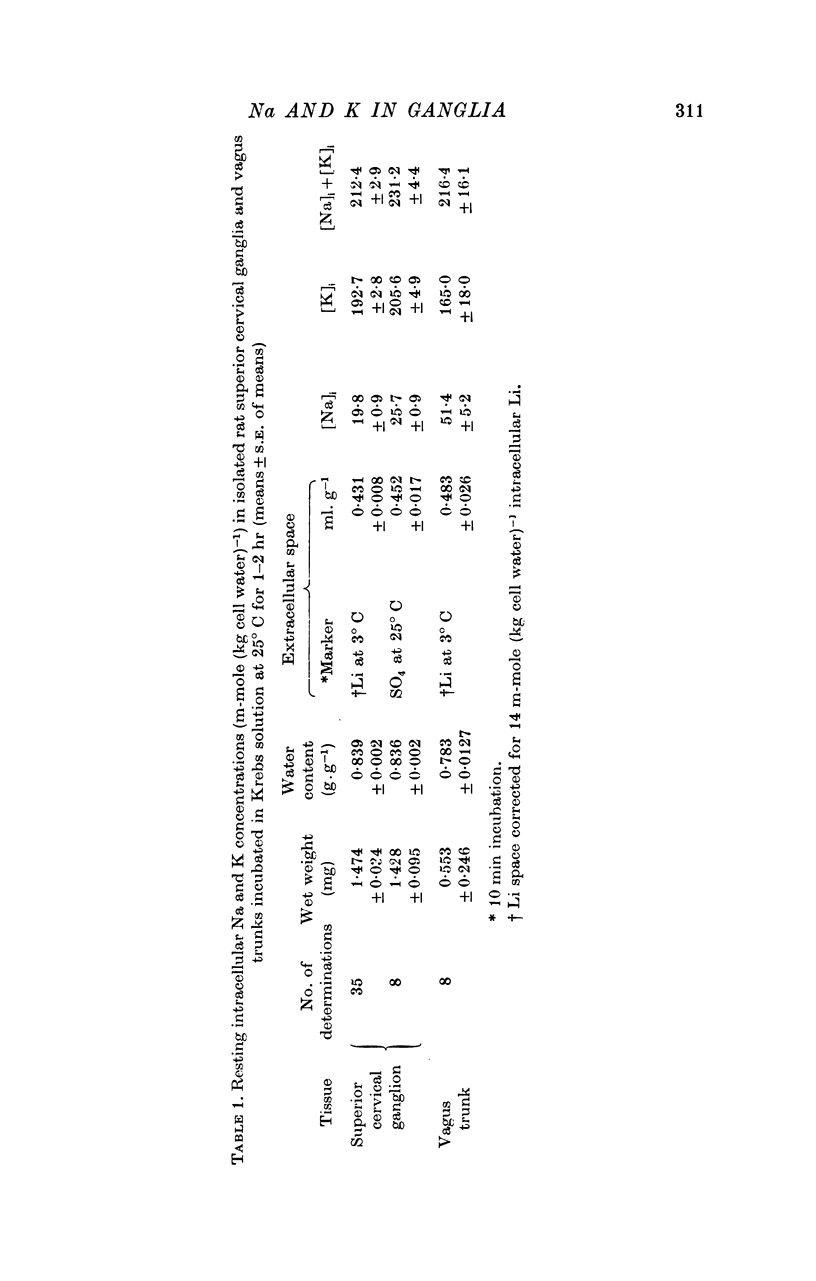
2. Resting concentrations after 1-2 hr incubation at 25° C in normal Krebs solution were: [Na]i, 19·8 ± 0·9 m-mole (kg cell water)-1; [K]i, 192·7 ± 2·8 m-mole (kg cell water)-1 (mean ± S.E. of mean of thirty-five ganglia). Correction for losses during e.c.s. measurement gave 22 mM [Na]i and 207 mM [K]i as probable fresh concentrations.
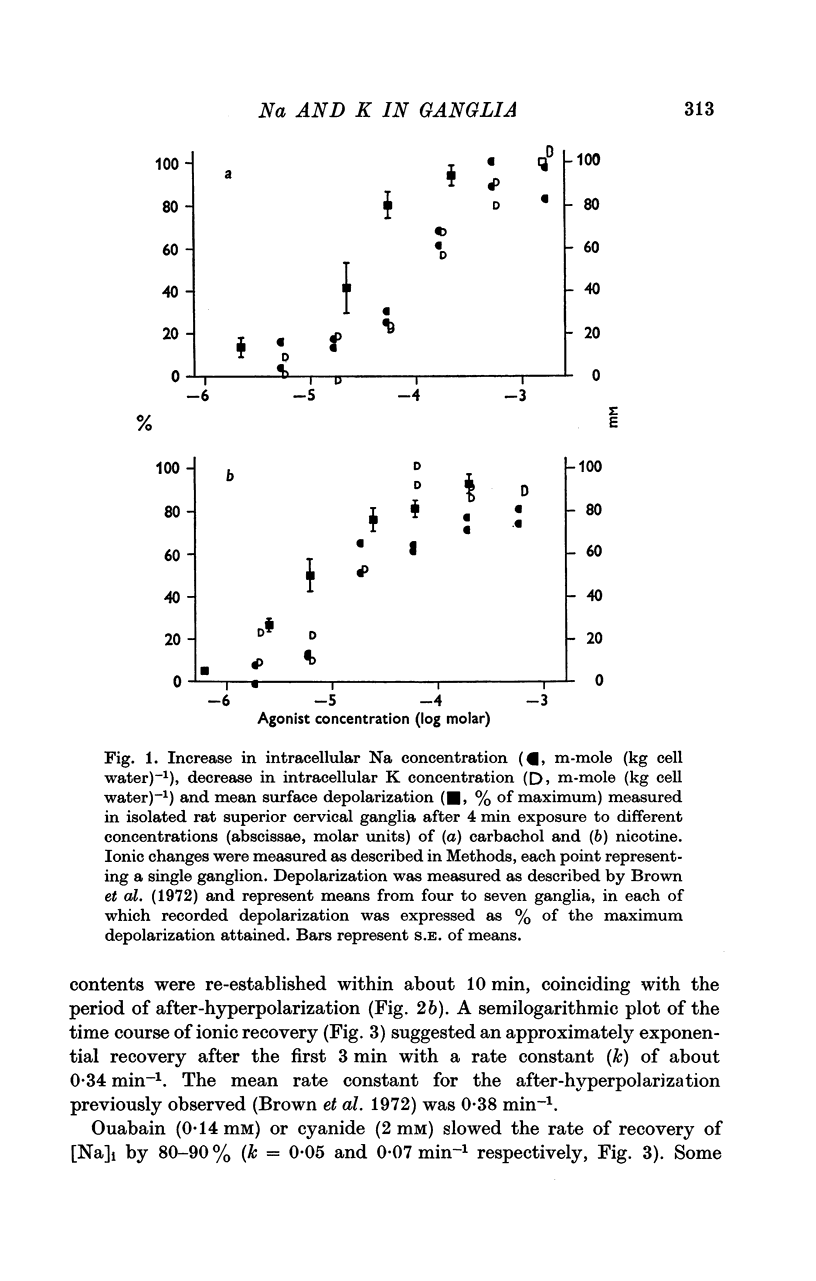
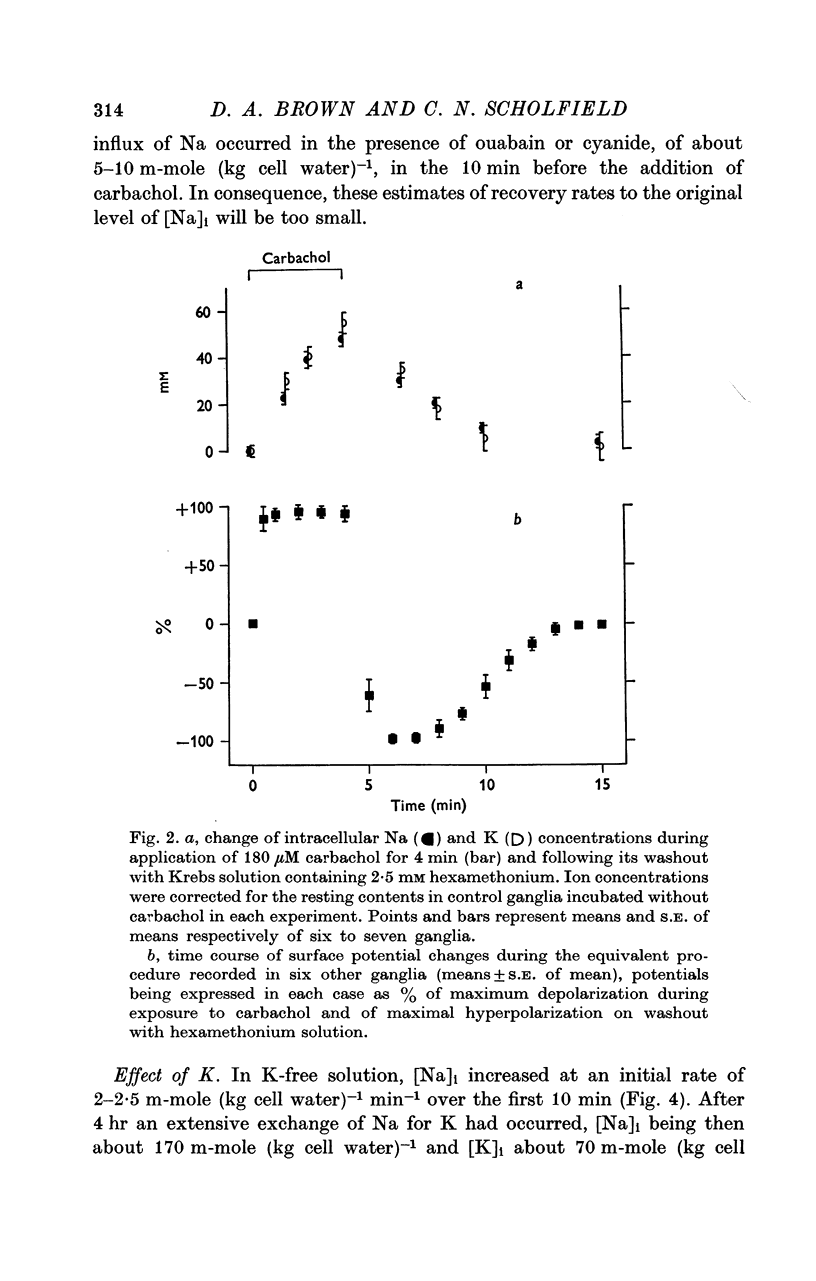
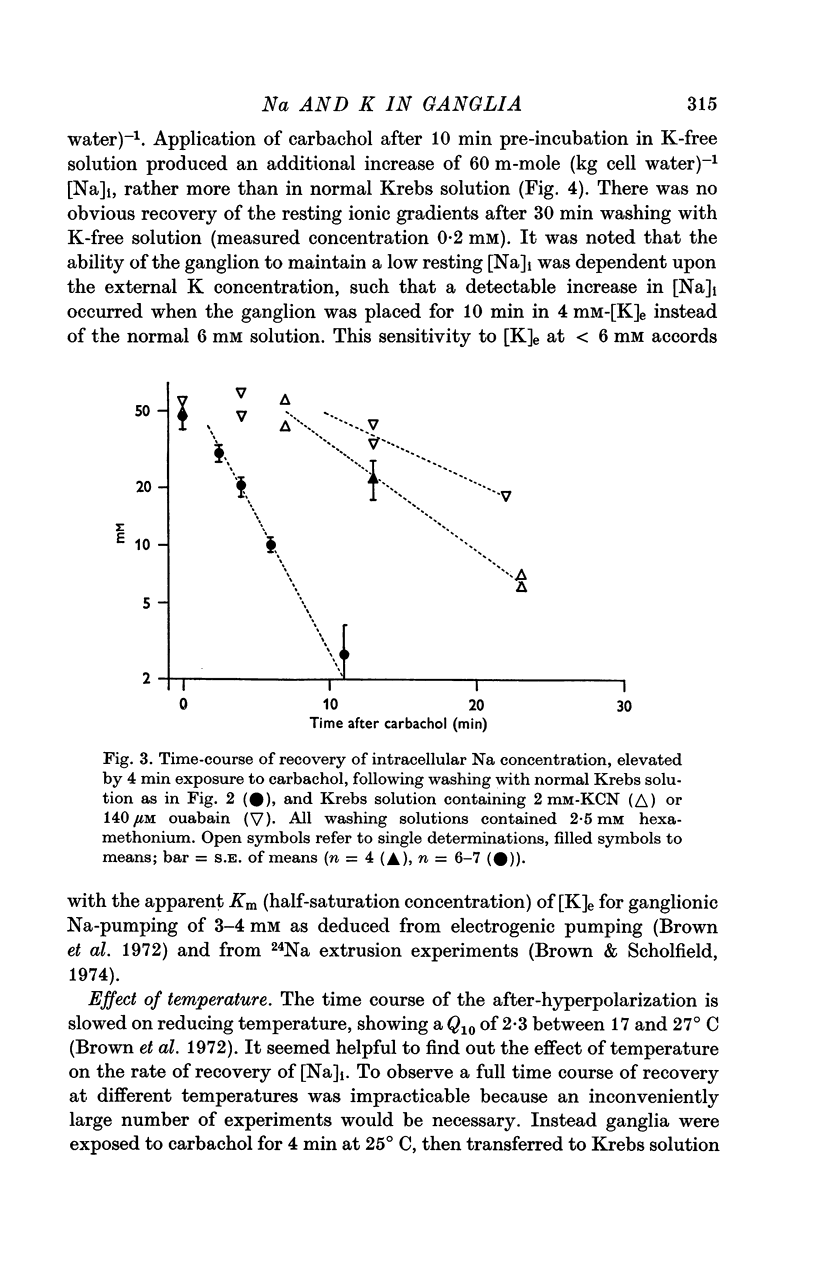
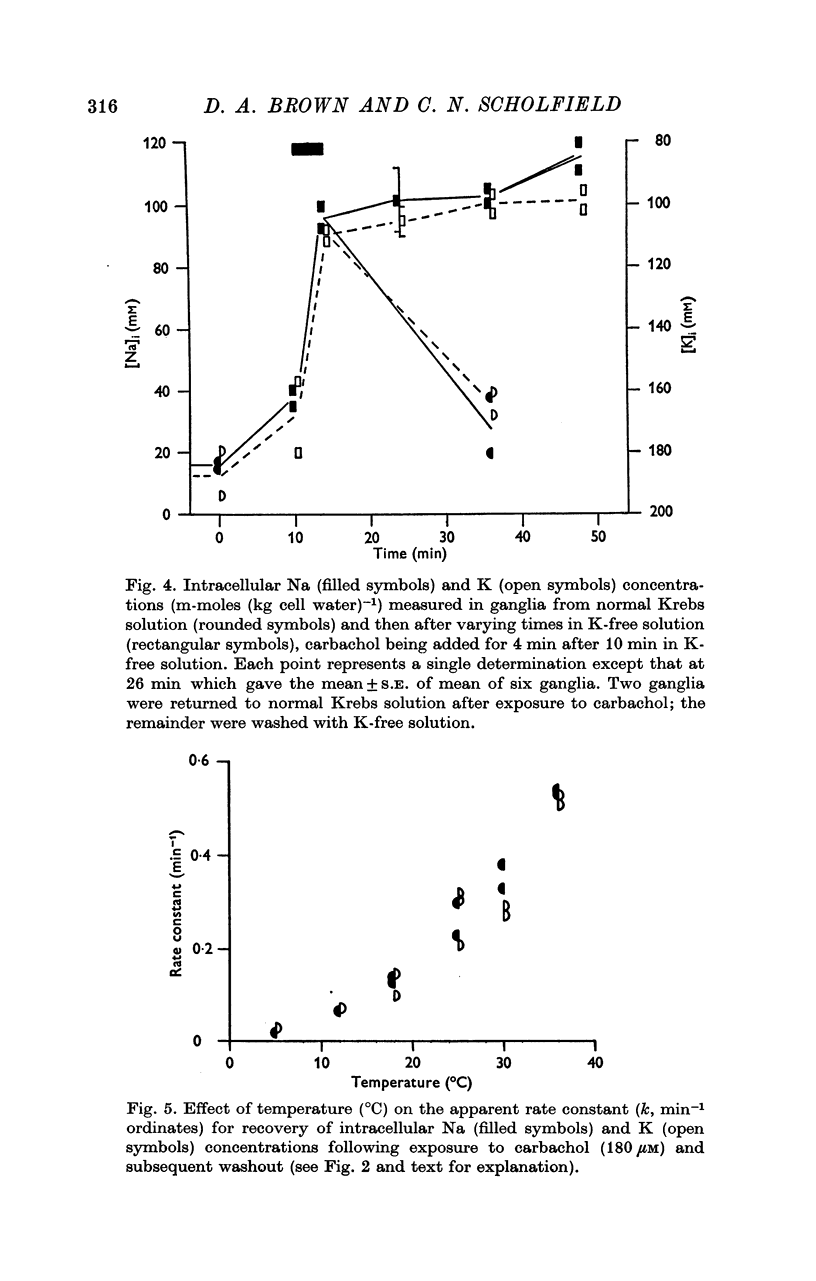
3. Carbachol (180 μM for 4 min) increased [Na]i by 47·8 ± 2·9 m-mole (kg cell water)-1 and decreased [K]i by 54·6 ± 4·3 m-mole (kg cell water)-1. Maximal exchange with carbachol or nicotine (at ∼ 1 mM for 4 min) amounted to 80-100 m-mole (kg cell water)-1. On washing with Krebs solution containing 2·5 mM hexamethonium recovery of ionic concentrations occurred with a rate constant of 0·3-0·4 min-1.
4. Restitution of ganglionic Na and K after carbachol was inhibited by washing with K-free solution, and slowed by ouabain (0·14 mM), cyanide (2 mM) or cooling (Q10 2·7 between 17 and 27° C).
5. Equilibrium potentials for Na and K (ENa, EK) at rest were calculated to be +49 and -88 mV. At a membrane potential (Em) of -70 mV, the permeability ratio PNa:PK was calculated at 0·04:1 (assuming PCl:PK < 0·1).
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armett C. J., Ritchie J. M. On the permeability of mammalian non-myelinated fibres to sodium and to lithium ions. J Physiol. 1963 Jan;165(1):130–140. doi: 10.1113/jphysiol.1963.sp007047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLACKMAN J. G., GINSBORG B. L., RAY C. Synaptic transmission in the sympathetic ganglion of the frog. J Physiol. 1963 Jul;167:355–373. doi: 10.1113/jphysiol.1963.sp007155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Brownstein M. J., Scholfield C. N. On the nature of the drug-induced after-hyperpolarization in isolated rat ganglia. Br J Pharmacol. 1969 Oct;37(2):511P–513P. [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Brownstein M. J., Scholfield C. N. Origin of the after-hyperpolarization that follows removal of depolarizing agents from the isolated superior cervical ganglion of the rat. Br J Pharmacol. 1972 Apr;44(4):651–671. doi: 10.1111/j.1476-5381.1972.tb07305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Halliwell J. V., Scholfield C. N. Uptake of nicotine and extracellular space markers by isolated rat ganglia in relation to receptor activation. Br J Pharmacol. 1971 May;42(1):100–113. doi: 10.1111/j.1476-5381.1971.tb07090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Scholfield C. N. Movements of labelled sodium ions in isolated rat superior cervical ganglia. J Physiol. 1974 Oct;242(2):321–351. doi: 10.1113/jphysiol.1974.sp010710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRIS E. J., McLENNAN H. Cation exchanges in sympathetic ganglia. J Physiol. 1953 Sep;121(3):629–637. doi: 10.1113/jphysiol.1953.sp004970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynes R. D., Ritchie J. M. The movements of labelled ions in mammalian non-myelinated nerve fibres. J Physiol. 1965 Jul;179(2):333–367. doi: 10.1113/jphysiol.1965.sp007666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosterlitz H. W., Lees G. M., Wallis D. I. Resting and action potentials recorded by the sucrose-gap method in the superior cervical ganglion of the rabbit. J Physiol. 1968 Mar;195(1):39–53. doi: 10.1113/jphysiol.1968.sp008445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perri V., Sacchi O., Caella C. Electrical properties and synaptic connections of the sympathetic neurons in the rat and guinea-pig superior cervical ganglion. Pflugers Arch. 1970;314(1):40–54. doi: 10.1007/BF00587045. [DOI] [PubMed] [Google Scholar]
- Rang H. P., Ritchie J. M. The ionic content of mammalian non-myelinated nerve fibres and its alteration as a result of electrical activity. J Physiol. 1968 May;196(1):223–236. doi: 10.1113/jphysiol.1968.sp008503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward J. K., Bianchi C. P., Erulkar S. D. Electrolyte distribution in rabbit superior cervical ganglion. J Neurochem. 1969 Mar;16(3):289–299. doi: 10.1111/j.1471-4159.1969.tb10367.x. [DOI] [PubMed] [Google Scholar]


