Abstract
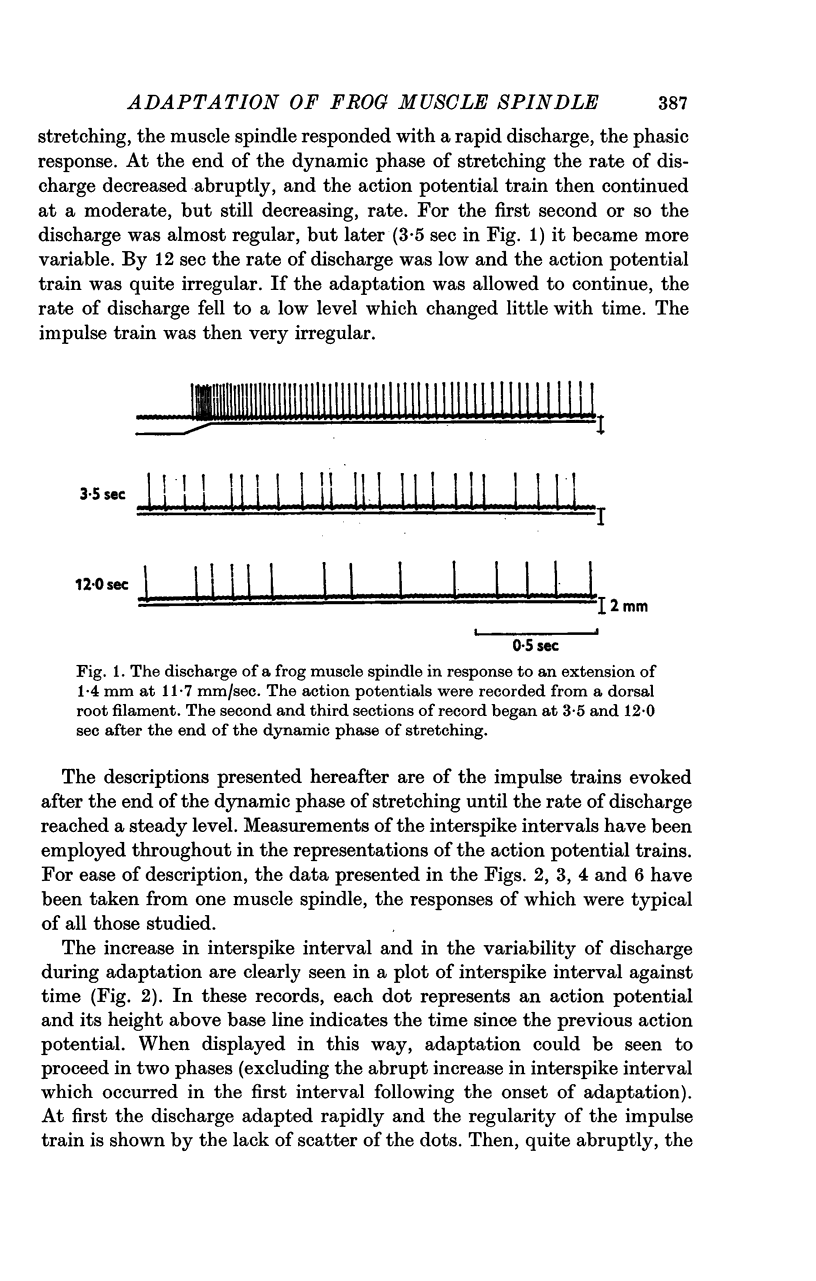
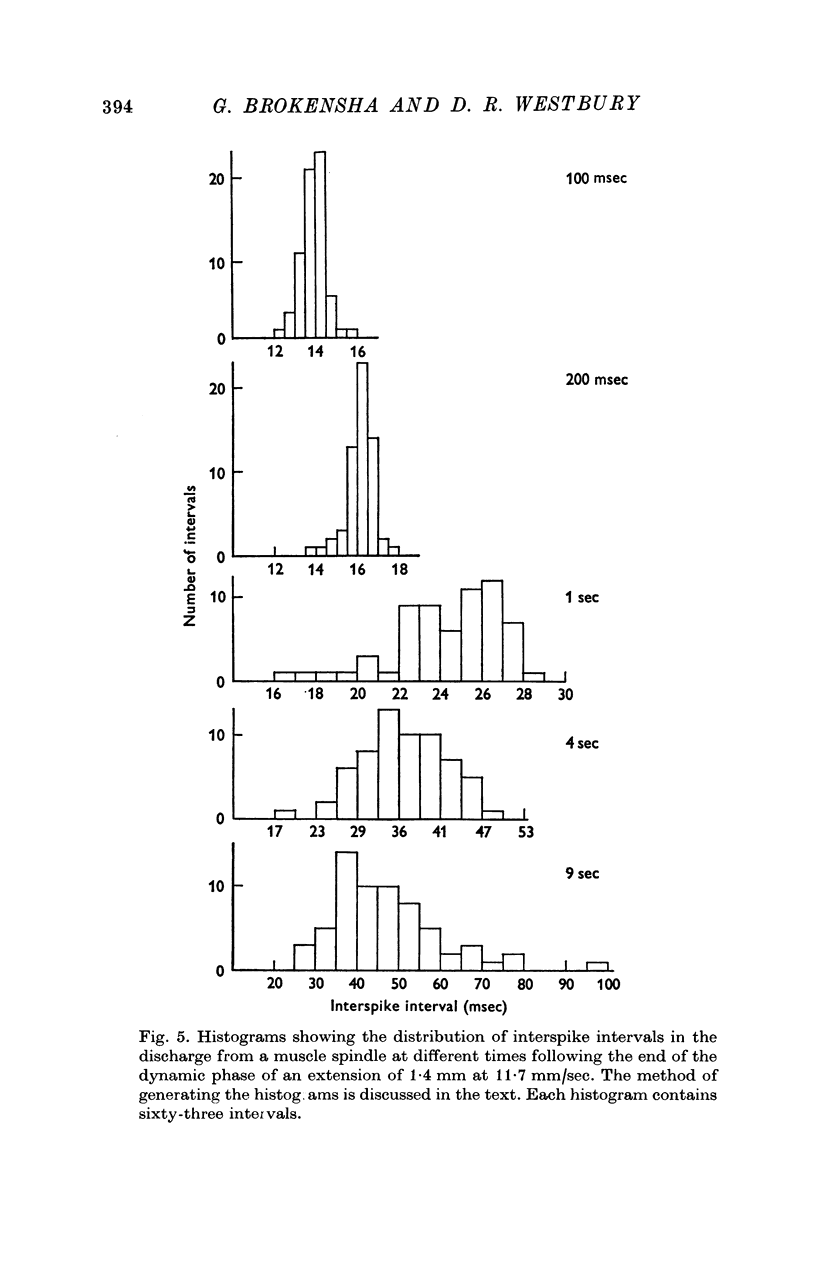
1. Stretching a frog muscle spindle evoked a discharge of action potentials in its sensory axon. As the rate of this discharge decreased during the adaptation that followed the dynamic phase of a stretch, the variability of the interspike intervals of the impulse train increased.
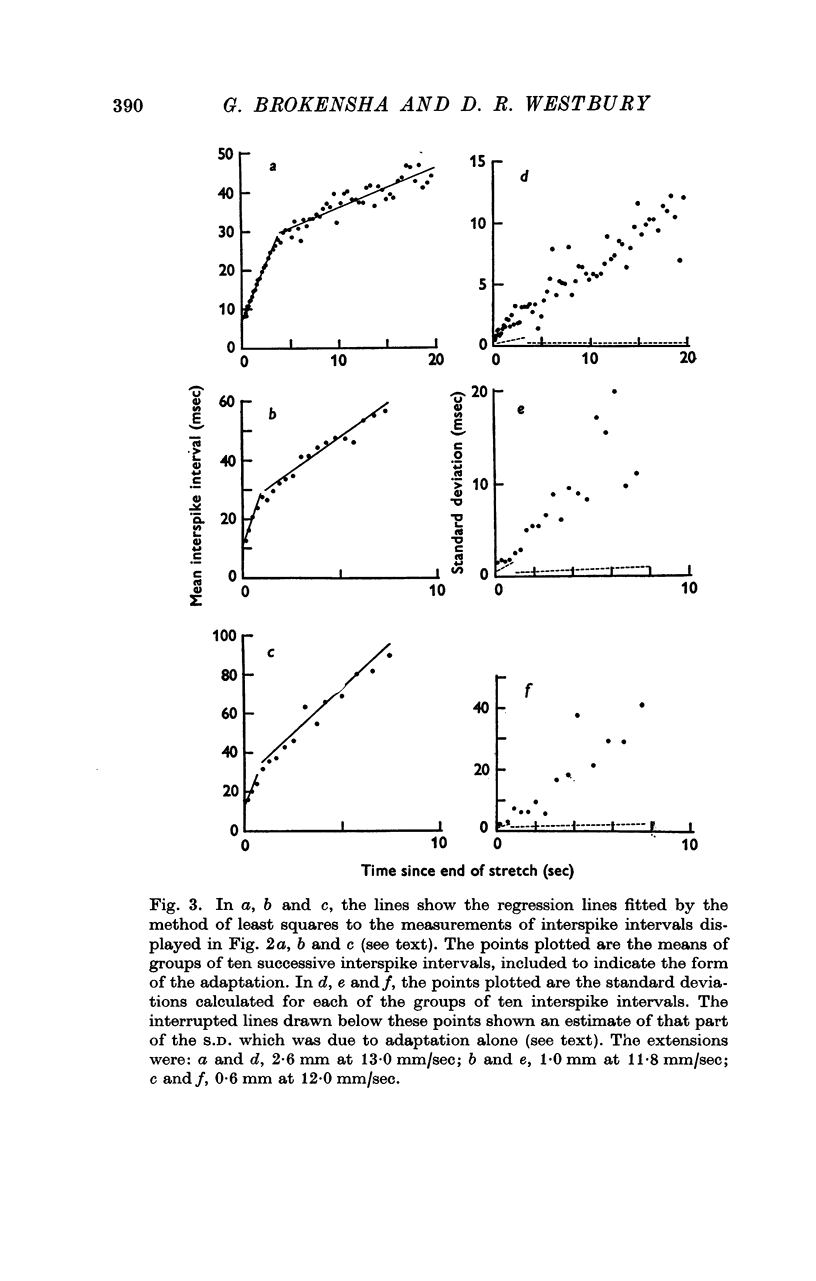
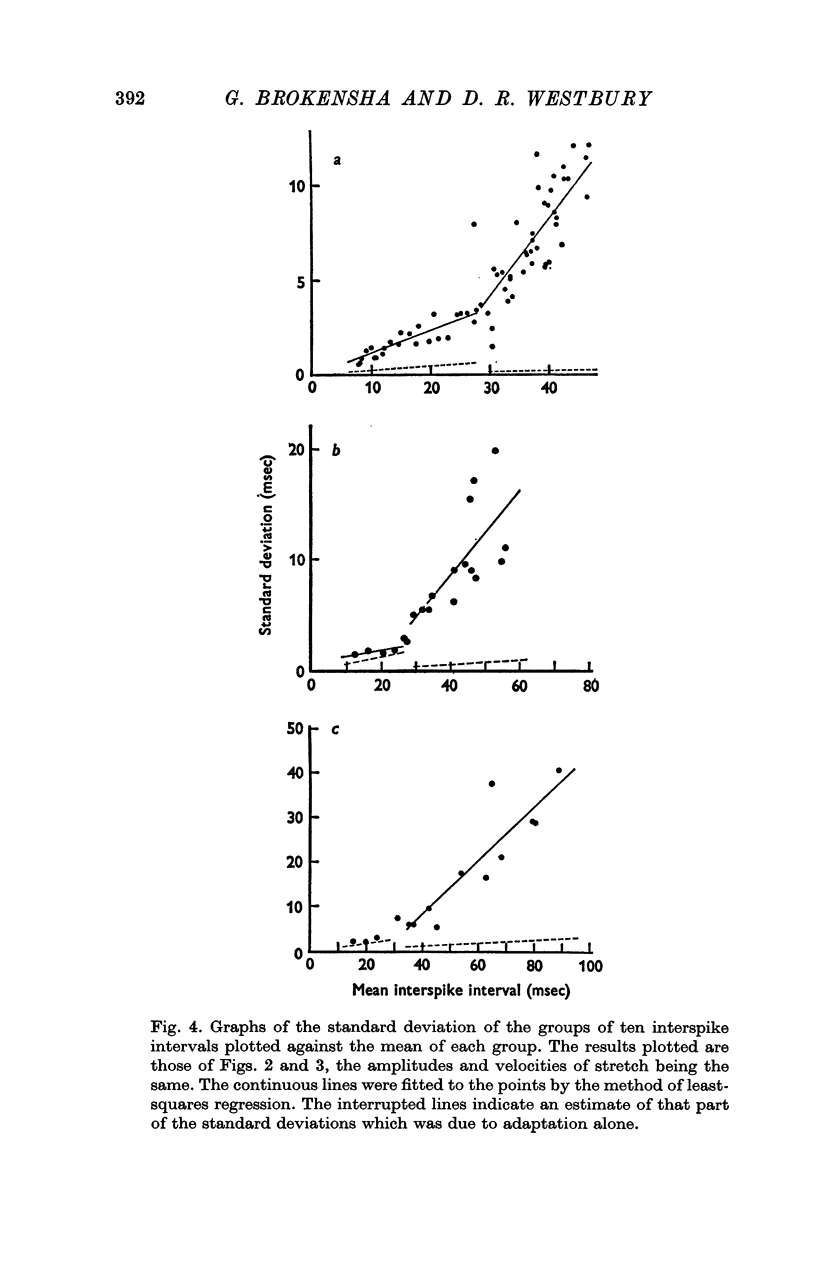
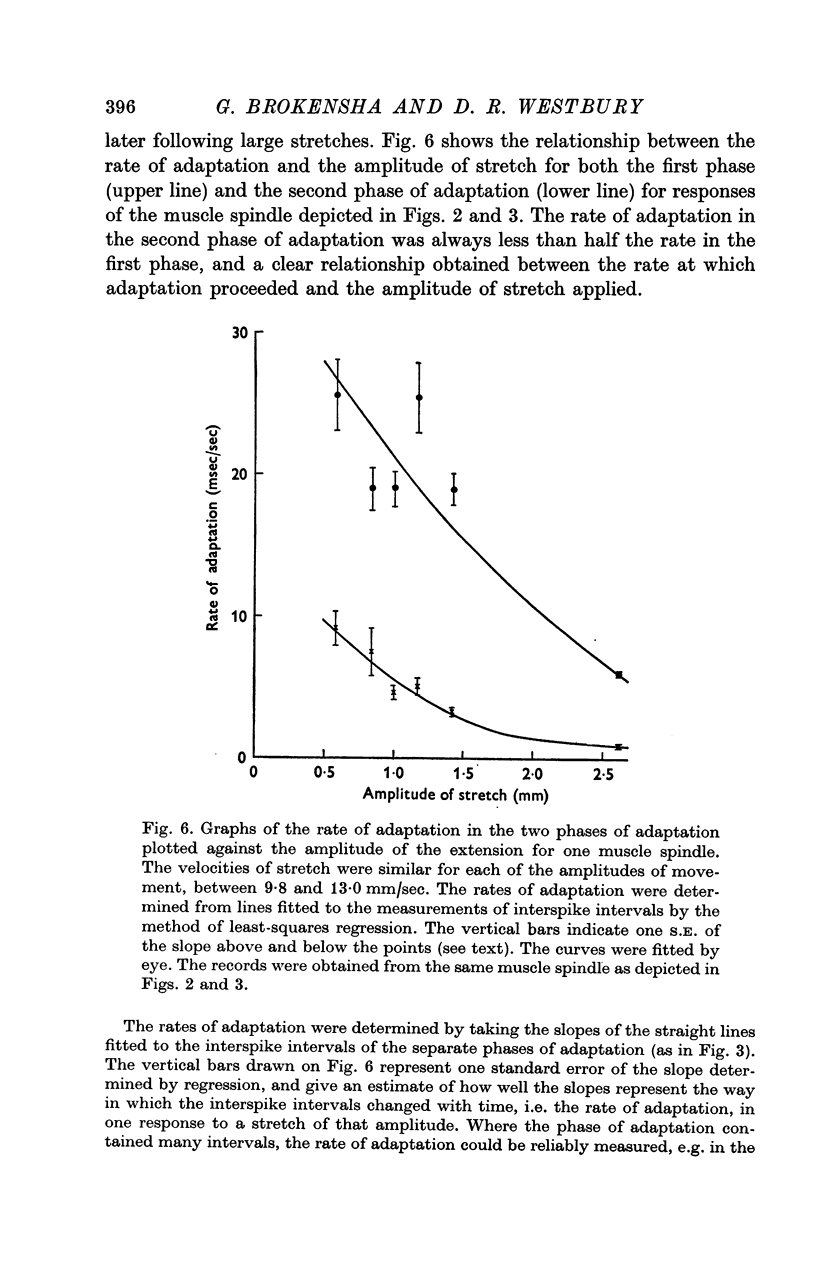
2. Adaptation occurred in two phases. At first the impulse train was almost regular and adapted rapidly, but later this gave way to a phase of slower adaptation where the variability of the discharge was much increased. In the second phase of adaptation the interspike intervals increased in length less than half as quickly as in the first phase.
3. When the rate of adaptation changed from the more rapid to the slower phase there was often an abrupt change in the character of the discharge and the relationship between the mean interspike interval and the variability changed. The interspike interval at which this change-over occurred was relatively constant in records of the discharge from one afferent fibre even though stretches of different amplitude were employed, though it differed from one afferent fibre to another.
4. These features of the discharge during adaptation suggest that the two sections of the impulse trains were derived from different spike generators by a process of probabilistic mixing.
Full text
PDF















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADRIAN R. H. The effect of internal and external potassium concentration on the membrane potential of frog muscle. J Physiol. 1956 Sep 27;133(3):631–658. doi: 10.1113/jphysiol.1956.sp005615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULLER A. J., NICHOLLS J. G., STROM G. Spontaneous fluctuations of excitability in the muscle spindle of the frog. J Physiol. 1953 Nov 28;122(2):409–418. doi: 10.1113/jphysiol.1953.sp005011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D., Cope M. Tandem muscle-spindles in the frog. J Anat. 1962 Jan;96(Pt 1):49–57. [PMC free article] [PubMed] [Google Scholar]
- Brown M. C. The responses of frog muscle spindles and fast and slow muscle fibres to a variety of mechanical inputs. J Physiol. 1971 Oct;218(1):1–17. doi: 10.1113/jphysiol.1971.sp009601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller A. J. A model illustrating some aspects of muscle spindle physiology. J Physiol. 1965 Aug;179(3):402–416. doi: 10.1113/jphysiol.1965.sp007669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROWE A., MATTHEWS P. B. THE EFFECTS OF STIMULATION OF STATIC AND DYNAMIC FUSIMOTOR FIBRES ON THE RESPONSE TO STRETCHING OF THE PRIMARY ENDINGS OF MUSCLE SPINDLES. J Physiol. 1964 Oct;174:109–131. doi: 10.1113/jphysiol.1964.sp007476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duclaux R., Kenshalo D. R. Cutaneous receptive fields of primate cold fibers. Brain Res. 1973 Jun 15;55(2):437–442. doi: 10.1016/0006-8993(73)90309-0. [DOI] [PubMed] [Google Scholar]
- GRAY E. G. The spindle and extrafusal innervation of a frog muscle. Proc R Soc Lond B Biol Sci. 1957 May 7;146(924):416–430. doi: 10.1098/rspb.1957.0021. [DOI] [PubMed] [Google Scholar]
- Gottschaldt K. M., Iggo A., Young D. W. Functional characteristics of mechanoreceptors in sinus hair follicles of the cat. J Physiol. 1973 Dec;235(2):287–315. doi: 10.1113/jphysiol.1973.sp010388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houk J. C., Cornew R. W., Stark L. A model of adaptation in amphibian spindle receptors. J Theor Biol. 1966 Nov;12(2):196–215. doi: 10.1016/0022-5193(66)90113-5. [DOI] [PubMed] [Google Scholar]
- Ito F. Functional properties of tandem muscle spindles in comparison with those of single muscle spindles in the frog. Jpn J Physiol. 1969 Oct 15;19(5):641–651. doi: 10.2170/jjphysiol.19.641. [DOI] [PubMed] [Google Scholar]
- Ito F., Kuroda H. The positive after-potential following the orthodromic and antidromic propagated impulses in the frog muscle spindle. Jpn J Physiol. 1972 Aug;22(4):441–452. doi: 10.2170/jjphysiol.22.441. [DOI] [PubMed] [Google Scholar]
- KATZ B. Action potentials from a sensory nerve ending. J Physiol. 1950 Oct 16;111(3-4):248–260. doi: 10.1113/jphysiol.1950.sp004478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B. Depolarization of sensory terminals and the initiation of impulses in the muscle spindle. J Physiol. 1950 Oct 16;111(3-4):261–282. doi: 10.1113/jphysiol.1950.sp004479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennerstrand G. Position and velocity sensitivity of muscle spindles in the cat. IV. Interaction between two fusimotor fibres converging on the same spindle ending. Acta Physiol Scand. 1968 Nov;74(3):257–273. doi: 10.1111/j.1748-1716.1968.tb04235.x. [DOI] [PubMed] [Google Scholar]
- Lindblom Y., Tapper D. N. Integration of impulse activity in a peripheral sensory unit. Exp Neurol. 1966 May;15(1):63–69. doi: 10.1016/0014-4886(66)90034-3. [DOI] [PubMed] [Google Scholar]
- MATTHEWS P. B. The differentiation of two types of fusimotor fibre by their effects on the dynamic response of muscle spindle primary endings. Q J Exp Physiol Cogn Med Sci. 1962 Oct;47:324–333. doi: 10.1113/expphysiol.1962.sp001616. [DOI] [PubMed] [Google Scholar]
- MATTHEWS P. B., WESTBURY D. R. SOME EFFECTS OF FAST AND SLOW MOTOR FIBRES ON MUSCLE SPINDLES OF THE FROG. J Physiol. 1965 May;178:178–192. doi: 10.1113/jphysiol.1965.sp007622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews B. H. The response of a muscle spindle during active contraction of a muscle. J Physiol. 1931 Jun 26;72(2):153–174. doi: 10.1113/jphysiol.1931.sp002768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews B. H. The response of a single end organ. J Physiol. 1931 Jan 21;71(1):64–110. doi: 10.1113/jphysiol.1931.sp002718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKAJIMA S. ADAPTATION IN STRETCH RECEPTOR NEURONS OF CRAYFISH. Science. 1964 Nov 27;146(3648):1168–1170. doi: 10.1126/science.146.3648.1168. [DOI] [PubMed] [Google Scholar]
- Ottoson D., McReynolds J. S., Shepherd G. M. Sensitivity of isolated frog muscle spondle during and after stretching. J Neurophysiol. 1969 Jan;32(1):24–34. doi: 10.1152/jn.1969.32.1.24. [DOI] [PubMed] [Google Scholar]
- Ottoson D., Shepherd G. M. Receptor potentials and impulse generation in the isolated spindle during controlled extension. Cold Spring Harb Symp Quant Biol. 1965;30:105–114. doi: 10.1101/sqb.1965.030.01.014. [DOI] [PubMed] [Google Scholar]
- Ottoson D., Shepherd G. M. Synchronization of activity in afferent nerve branches within the frog's muscle spindle. Acta Physiol Scand. 1970 Dec;80(4):492–501. doi: 10.1111/j.1748-1716.1970.tb04816.x. [DOI] [PubMed] [Google Scholar]
- STEIN R. B. A THEORETICAL ANALYSIS OF NEURONAL VARIABILITY. Biophys J. 1965 Mar;5:173–194. doi: 10.1016/s0006-3495(65)86709-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein R. B., Mattews P. B. Differences in variability of discharge frequency between primary and secondary muscle spindle afferent endings of the cat. Nature. 1965 Dec 18;208(5016):1217–1218. doi: 10.1038/2081217a0. [DOI] [PubMed] [Google Scholar]
- VERVEEN A. A. Axon diameter and fluctuation in excitability. Acta Morphol Neerl Scand. 1962;5:79–85. [PubMed] [Google Scholar]


