Abstract
1. Cl--sensitive micro-electrodes were used to measure intracellular Cl- in snail neurones. The electrodes consisted of a sharpened and chlorided silver wire mounted inside a glass micropipette.
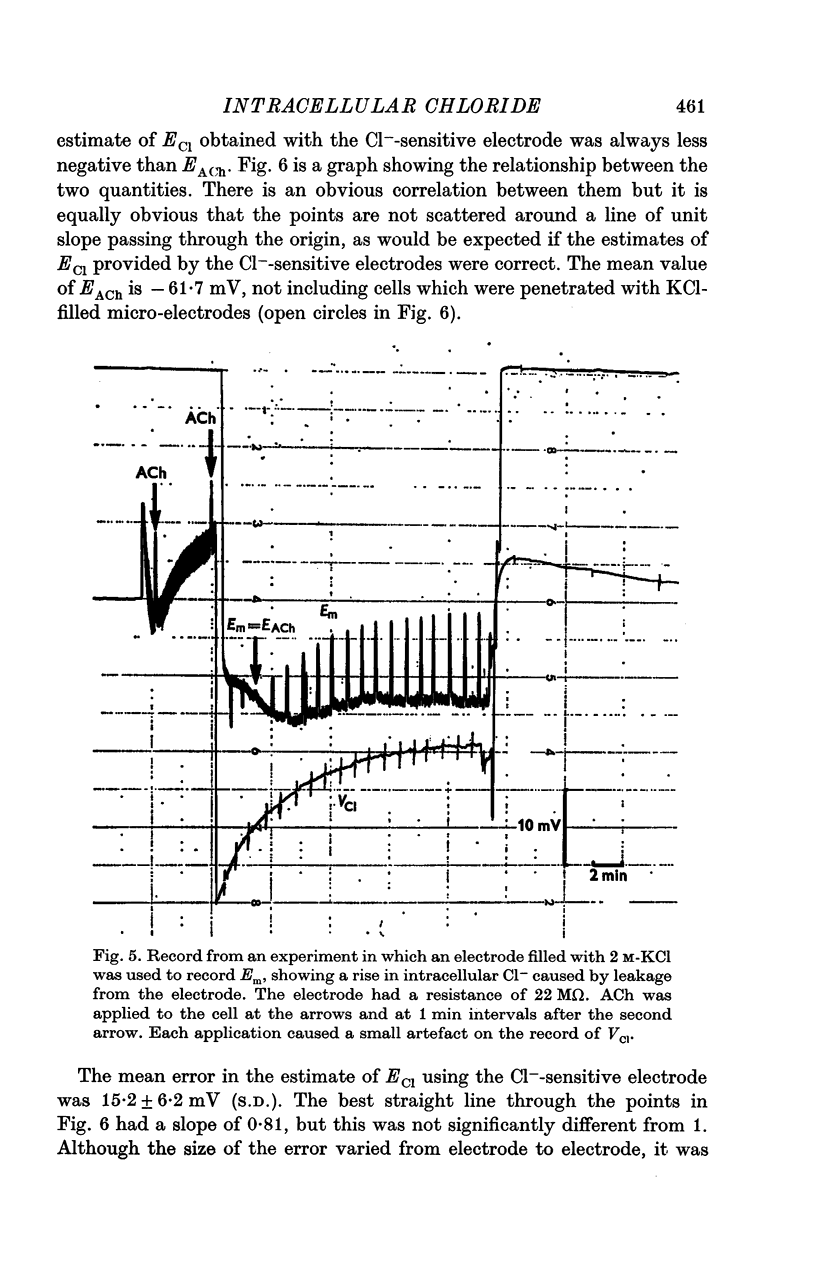
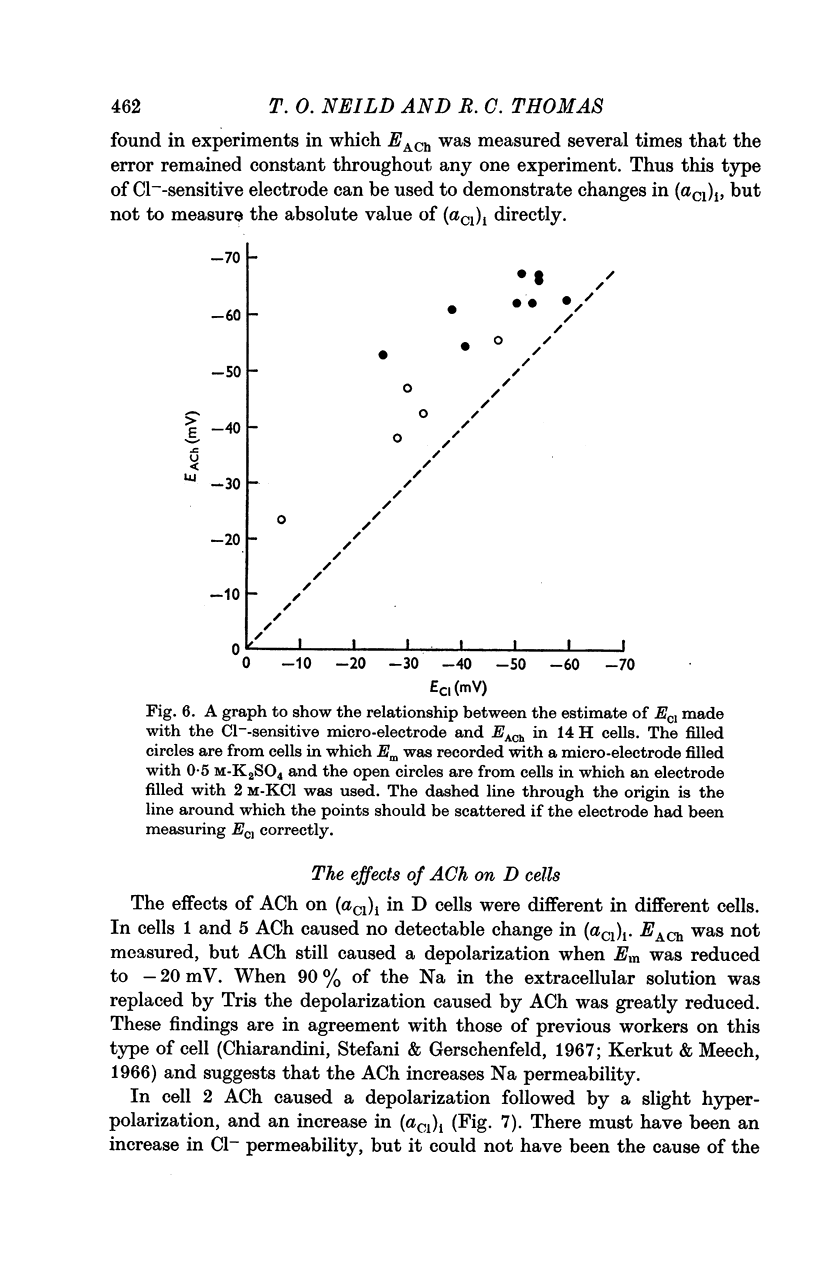
2. The electrodes appeared to record changes in internal Cl- accurately but in H cells the chloride equilibrium potential (ECl) as measured by the Cl--sensitive electrode was always less negative than EACh.
3. In some H cells ACh caused a measurable increase in internal Cl- when the cell was at its resting potential. In voltage-clamped cells there was a close correlation between the change in internal Cl- and the extra clamp current caused by a brief application of ACh. This confirmed that ACh increases the cell's membrane permeability only to Cl- ions, and that EACh was equal to ECl.
4. There was good agreement between the measured change in internal Cl- and that calculated from the cell size and clamp charge only when it was assumed that a constant voltage offset was added to the potential of the Cl--sensitive electrode while it was inside the nerve cell.
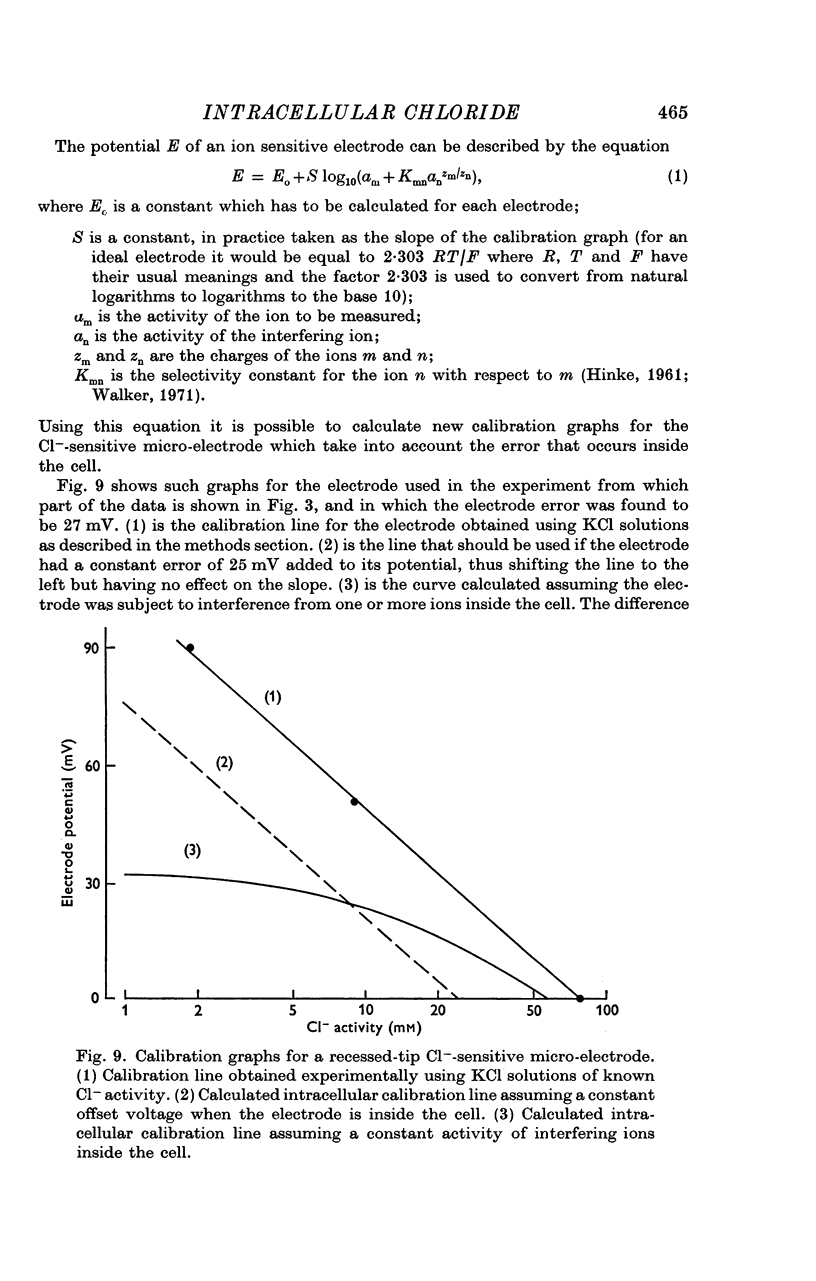
5. Cl--sensitive electrodes with AgCl as the sensitive material appear to be unsuitable for intracellular measurement of Cl-, although they might be suitable for following changes in ECl.
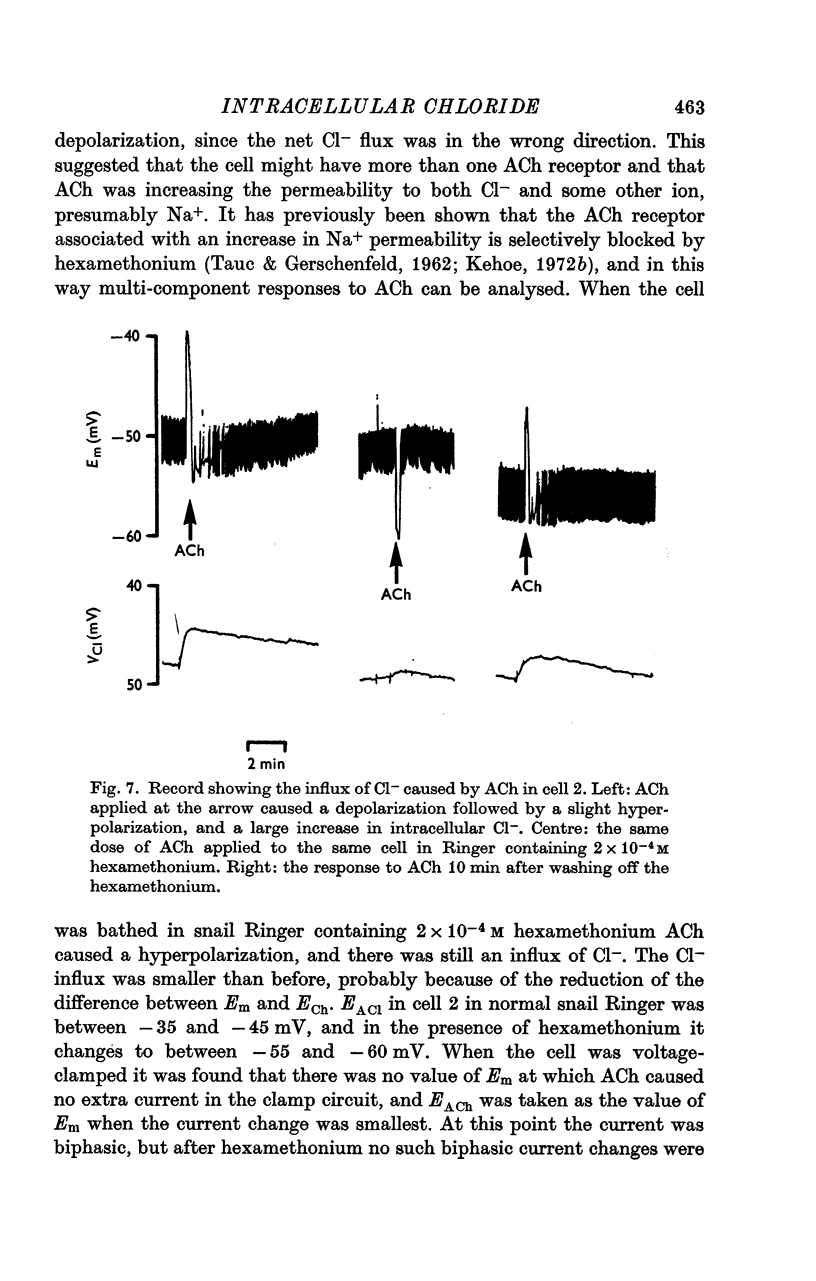
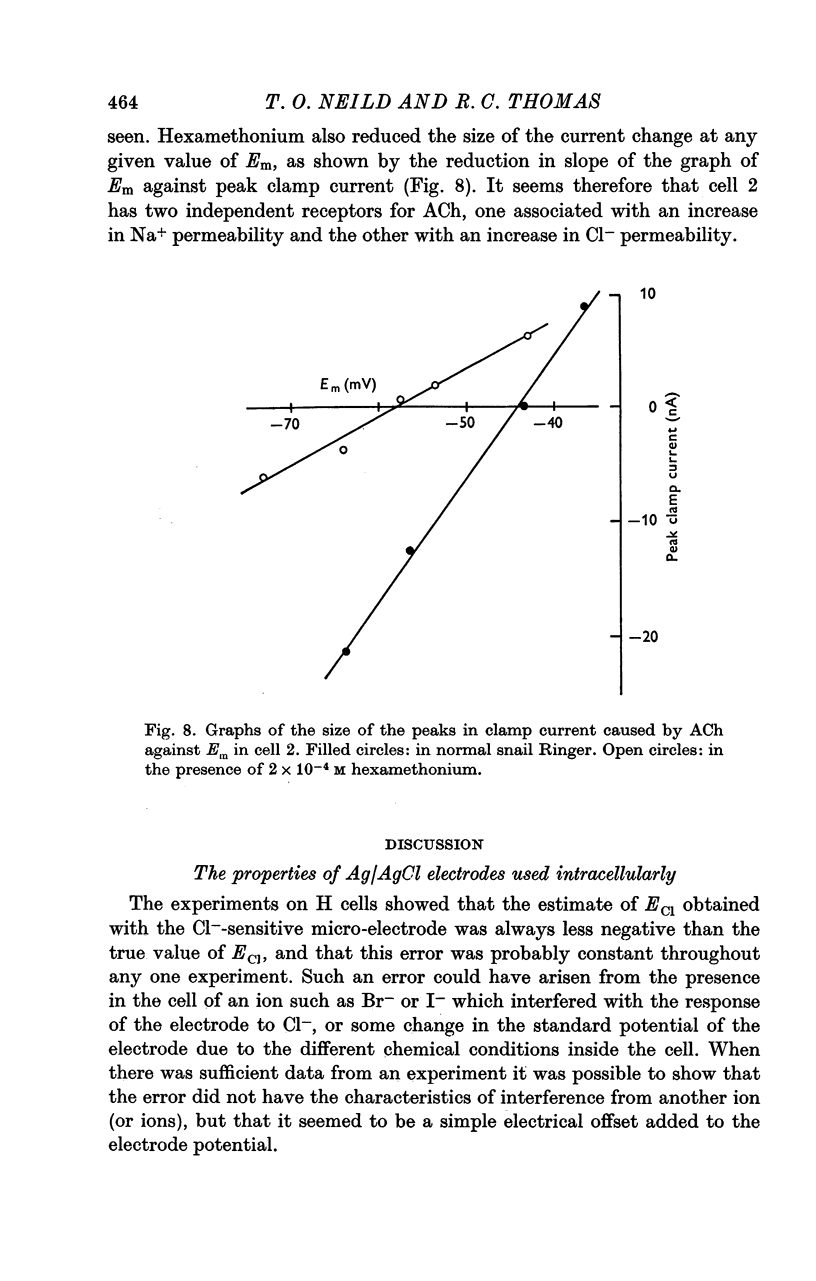
6. In certain D cells ACh also caused an increase in internal Cl- although it decreased the membrane potential. In the presence of hexamethonium, ACh caused a hyperpolarization and a smaller increase in internal chloride.
7. It is concluded that the intracellular Cl- in both H and D cells is about 8·3 mM, giving an ECl of about -58 mV.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chiarandini D. J., Gerschenfeld H. M. Ionic mechanism of cholinergic inhibition in molluscan neurons. Science. 1967 Jun 23;156(3782):1595–1596. doi: 10.1126/science.156.3782.1595. [DOI] [PubMed] [Google Scholar]
- Chiarandini D. J., Stefani E., Gerschenfeld H. M. Ionic mechanisms of cholinergic excitation in molluscan neurons. Science. 1967 Jun 23;156(3782):1597–1599. doi: 10.1126/science.156.3782.1597. [DOI] [PubMed] [Google Scholar]
- Gayton D. C., Hinke J. A. The location of chloride in single striated muscle fibers of the giant barnacle. Can J Physiol Pharmacol. 1968 Mar;46(2):213–219. doi: 10.1139/y68-035. [DOI] [PubMed] [Google Scholar]
- Gerschenfeld H. M. Chemical transmission in invertebrate central nervous systems and neuromuscular junctions. Physiol Rev. 1973 Jan;53(1):1–119. doi: 10.1152/physrev.1973.53.1.1. [DOI] [PubMed] [Google Scholar]
- HINKE J. A. The measurement of sodium and potassium activities in the squid axon by means of cation-selective glass micro-electrodes. J Physiol. 1961 Apr;156:314–335. doi: 10.1113/jphysiol.1961.sp006678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinke J. A., Gayton D. C. Transmembrane K + and Cl - activity gradients for the muscle fiber of the giant barnacle. Can J Physiol Pharmacol. 1971 Apr;49(4):312–322. doi: 10.1139/y71-034. [DOI] [PubMed] [Google Scholar]
- KERKUT G. A., THOMAS R. C. THE EFFECT OF ANION INJECTION AND CHANGES IN THE EXTERNAL POTASSIUM AND CHLORIDE CONCENTRATION ON THE REVERSAL POTENTIALS OF THE IPSP AND ACETYLCHOLINE. Comp Biochem Physiol. 1964 Feb;11:199–213. doi: 10.1016/0010-406x(64)90163-x. [DOI] [PubMed] [Google Scholar]
- KEYNES R. D. CHLORIDE IN THE SQUID GIANT AXON. J Physiol. 1963 Dec;169:690–705. doi: 10.1113/jphysiol.1963.sp007289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe J. Ionic mechanisms of a two-component cholinergic inhibition in Aplysia neurones. J Physiol. 1972 Aug;225(1):85–114. doi: 10.1113/jphysiol.1972.sp009930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe J. Three acetylcholine receptors in Aplysia neurones. J Physiol. 1972 Aug;225(1):115–146. doi: 10.1113/jphysiol.1972.sp009931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkut G. A., French M. C., Walker R. J. The location of axonal pathways of identifiable neurones of Helix aspersa using the dye Procion yellow M-4R. Comp Biochem Physiol. 1970 Feb 15;32(4):681–690. doi: 10.1016/0010-406x(70)90820-0. [DOI] [PubMed] [Google Scholar]
- Kerkut G. A., Pitman R. M., Walker R. J. Iontophoretic application of acetylcholine and GABA onto insect central neurones. Comp Biochem Physiol. 1969 Nov 15;31(4):611–633. doi: 10.1016/0010-406x(69)90063-2. [DOI] [PubMed] [Google Scholar]
- Sato M., Austin G., Yai H., Maruhashi J. The ionic permeability changes during acetylcholine-induced responses of Aplysia ganglion cells. J Gen Physiol. 1968 Mar;51(3):321–345. doi: 10.1085/jgp.51.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickholm A., Wallin B. G. Intracellular chloride activity of crayfish giant axons. Nature. 1965 Nov 20;208(5012):790–791. doi: 10.1038/208790a0. [DOI] [PubMed] [Google Scholar]
- TAUC L., GERSCHENFELD H. M. A cholinergic mechanism of inhibitory synaptic transmission in a molluscan nervous system. J Neurophysiol. 1962 Mar;25:236–262. doi: 10.1152/jn.1962.25.2.236. [DOI] [PubMed] [Google Scholar]
- Tasaki I., Singer I. Some problems involved in electric measurements of biological systems. Ann N Y Acad Sci. 1968 Feb 1;148(1):36–53. doi: 10.1111/j.1749-6632.1968.tb20339.x. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. Intracellular sodium activity and the sodium pump in snail neurones. J Physiol. 1972 Jan;220(1):55–71. doi: 10.1113/jphysiol.1972.sp009694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. Membrane current and intracellular sodium changes in a snail neurone during extrusion of injected sodium. J Physiol. 1969 Apr;201(2):495–514. doi: 10.1113/jphysiol.1969.sp008769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin B. G. Intracellular ion concentrations in single crayfish axons. Acta Physiol Scand. 1967 Jul-Aug;70(3):419–430. doi: 10.1111/j.1748-1716.1967.tb03640.x. [DOI] [PubMed] [Google Scholar]


