Abstract
When used as an immunogen, Treponema pallidum repeat protein K (TprK) has been shown to attenuate syphilitic lesions upon homologous intradermal challenge in the rabbit model. To further explore this protein as a potential vaccine component, we sought to identify the immunogenic regions of TprK. The abilities of three recombinant peptides encompassing TprK to elicit T- and B-cell responses and to protect against challenge were examined. All three fragments elicited proliferative responses from splenocytes taken from infected rabbits. However, enzyme-linked immunosorbent assays indicated that only fragments 1 and 3 were consistently recognized by antisera from infected rabbits. Each fragment was also used to immunize rabbits that were subsequently challenged intradermally with infectious T. pallidum. All lesions on unimmunized control rabbits ulcerated and contained treponemes, while the lesions on rabbits immunized with fragment 1 were the least likely to have detectable treponemes (25%) and the least likely to ulcerate (37.5%). The lesions on rabbits immunized with fragment 3 showed intermediate results, and rabbits immunized with fragment 2 were the most likely of all those on immunized rabbits to have detectable treponemes (91.7%) and to ulcerate (66.7%). These results demonstrate that epitopes in fragment 1 are recognized by T cells and antibodies during infection and that immunization with this portion of TprK most effectively attenuates syphilitic lesion development.
Syphilis, caused by Treponema pallidum subsp. pallidum, is a significant public health burden, with approximately 30 million infected persons worldwide (18), largely focused in areas of low socioeconomic status and poor health care infrastructure (1, 2, 9, 39). Syphilis is also a problem in the United States and other developed countries, where there are periodic outbreaks (6, 12, 13, 21, 37). An additional problem associated with syphilitic lesions is the estimated three- to fivefold increase in the risk of acquiring human immunodeficiency virus (HIV) (45). Furthermore, HIV has been isolated from genital ulcers, suggesting an increased risk of HIV transmissibility from patients with syphilis (25, 38). T. pallidum is also capable of crossing the placenta to cause congenital syphilis. In some areas of Africa, congenital syphilis has been estimated to account for 25 to 30% of perinatal morbidity and mortality (22).
A vaccine against syphilis would be the ideal way to combat this public health burden. Protection has been demonstrated in the rabbit model by using complicated immunization protocols with gamma-irradiated, antiformin-treated, or 4°C “aged” treponemes (34, 35, 41). Although these regimens are impractical and not suitable for humans, they do demonstrate that protective immunity by vaccination is attainable. It has also been shown that when various recombinant and native proteins, including endoflagella, glycerophosphodiester phosphodiesterase, 4D, and Tp92, are used as immunogens, lesion development is altered upon intradermal challenge in the rabbit model (8, 10, 11, 16).
The relative roles of humoral and cellular immunity in protection against syphilis are not definitively known. Passively transferred antibody leads to delayed and altered lesion development upon intradermal challenge in the rabbit model but does not afford complete protection (7). Adoptive T-cell transfers are not possible in the most common animal model, the outbred rabbit. In the inbred guinea pig model, in which clinical disease is less apparent and the 50% infectious dose is several log units more than in rabbits or humans, adoptive T-cell transfers have prevented lesion formation but not infection (46). Similar results were obtained in the hamster model of T. pallidum subsp. pertenue and endemicum (27, 40). It is known that, in rabbits and humans, T cells infiltrate primary and secondary lesions and activate macrophages to phagocytose antibody-opsonized treponemes (5, 17, 28, 44). It is believed that a protective response against syphilis takes time to develop. Infected rabbits develop immunity against homologous isolates after 3 to 6 months of infection (5, 43). In humans, only patients who are infected for long periods of time before being treated develop altered lesions upon reinfection (32). Identifying what treponemal factors interact with the immune system throughout infection may serve to identify putative protective epitopes.
In our laboratories, when rabbits were immunized with a large portion (amino acids 37 to 478) of recombinant T. pallidum repeat protein K (TprK) and challenged intradermally, they developed lesions that were smaller, were less likely to ulcerate or have detectable treponemes, and healed more rapidly than lesions in control rabbits (14). To further explore which portions of TprK interact with the immune system and to examine the role of the previously unexplored C-terminal end, we have examined the abilities of three portions of TprK to interact with T and B cells during infection and to confer protection.
MATERIALS AND METHODS
Rabbits.
Outbred adult male New Zealand White rabbits were obtained from R & R Rabbitry (Stanwood, Wash.). Only rabbits seronegative for Treponema paraluiscuniculi (rabbit syphilis) were included in these studies. Rabbits were housed individually at 15 to 18°C and given antibiotic-free food and water. Experiments were approved by the University of Washington Animal Care Committee and conducted in accordance with institutional guidelines.
Experimental infection with T. pallidum.
The T. pallidum subsp. pallidum Seattle Nichols strain was propagated by serial passage in rabbits as previously described (30). Six groups of four rabbits each were infected intratesticularly with 108 motile T. pallidum spirochetes.
Cloning, expression, and purification of recombinant proteins.
The open reading frame of tprK (GenBank accession number AF194369) was divided into three sections, and PCR primers were designed to amplify them as follows: fragment 1, encoding amino acids 37 to 273; fragment 2, encoding amino acids 274 to 348; and fragment 3, encoding amino acids 349 to 478 (Fig. 1). The fragments were amplified from T. pallidum Seattle Nichols strain DNA. The amplicons were cloned into the pCR3.1 T/A cloning vector (Invitrogen, Carlsbad, Calif.), sequenced, subcloned into the pRSET expression vector (Invitrogen), expressed, and purified by nickel chromatography as previously described (14, 26). All peptides were resuspended in phosphate-buffered saline (PBS) and evaluated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) for size and purity; concentrations were determined with a bicinchoninic acid protein assay (Pierce, Rockford, Ill.).
FIG. 1.
TprK fragments were designed. Hydrophilic N-terminal fragment 1 is italicized, hydrophobic middle fragment 2 is underlined, and hydrophilic C-terminal fragment 3 is in bold print. The dashes indicate the potential signal sequence cleavage site.
Immunizations and challenges.
Rabbits were immunized with 125 μg of the recombinant fragments in the MPL + TDM + CWS Adjuvant System (Sigma, St. Louis, Mo.). Each immunization was administered subcutaneously (12.5 μg), intradermally (37.5 μg), intramuscularly (50 μg), and intraperitoneally (25 μg) in accordance with the manufacturer's suggestions. Immunizations were administered every 3 weeks for six immunizations. Four rabbits were used for each condition, except for fragment 2 (n = 3), in which one rabbit died of unrelated causes. Within 11 to 13 days of the last boost, all immunized rabbits and four unimmunized control rabbits were challenged intradermally at eight sites on their shaved backs with 105 homologous Seattle Nichols T. pallidum spirochetes per site. Diameters and appearances of the lesions were recorded. Aspirates were taken from the lesions 16 days postchallenge and examined by dark-field (DF) microscopy for the presence of treponemes. Because treponemes were not found in all rabbits by DF microscopy, the Venereal Disease Research Laboratory (Atlanta, Ga.) and fluorescent treponemal antibody-absorbed tests were used to determine if the animals had seroconverted and thus were likely to be infected.
Lymphocyte proliferation assays.
Spleen cells were obtained from uninfected rabbits and rabbits infected for various amounts of time as previously described (3, 30). Briefly, 5 × 105 cells in 200 μl of culture medium were added to each well of 96-well flat-bottom culture plates and incubated in quadruplicate with the TprK fragments (12.5 μg/ml) as test antigens, 107 sonicated T. pallidum spirochetes as a treponeme-specific positive control, 20 μg of concanavalin A (Sigma) per ml as a positive T-cell control, or 10 μl of PBS alone as a background control. One-half microcurie of [methyl-3H]thymidine with a specific activity of 6.7 Ci (248 GBq)/mmol (DuPont NEN, Boston, Mass.) was added to each well after 3 days of incubation with sonicated T. pallidum or concanavalin A and after 4 days of incubation with test antigens. All cultures were harvested 24 h after addition of [3H]thymidine. The amount of tritium uptake was measured as an indication of proliferation. The mean of the experimental wells minus the mean of the wells with no antigen was calculated for each condition and each animal. The data represent the means ± standard errors for each condition from four rabbits per time point.
Enzyme-linked immunosorbent assays (ELISAs).
Ninety-six-well Maxisorb Immunoplates (Nunc, Naperville, Ill.) were coated with 50 μl of a 10-μg/ml concentration of recombinant protein in PBS (pH 7.2)-0.1% SDS and incubated at 4°C overnight. All wash steps used PBS-0.05% Tween 20 for 2 × 1 min and then 2 × 5 min. Plates were washed and then blocked with 200 μl of 3% nonfat dry milk (NFM) per well in PBS. Equal amounts of sera from four infected animals were pooled prior to testing. Sera from immunized rabbits were collected 10 days after the last boost. Sera were preadsorbed with crude lysate from an unrelated recombinant preparation to remove antibodies directed against Escherichia coli and the vector. One hundred microliters of sera diluted 1:20 in PBS with 1% NFM was added to each well, and the plates were incubated at 37°C for 1 h. The plates were washed, 100 μl of goat anti-rabbit immunoglobulin G heavy- and light-chain alkaline phosphatase conjugate (Sigma) diluted 1:2,000 in PBS-1% NFM was added to each well, and the plates were incubated for 1 h at room temperature. The plates were washed, developed for 15 min with 50 μl of para-nitrophenyl phosphate substrate (Sigma) at 1 mg/ml, and absorbance was measured by determining the optical density at 405 nm. The data reported are the means ± the standard errors of triplicate test wells minus the mean values of triplicate wells with no antigen.
RESULTS
Protein expression.
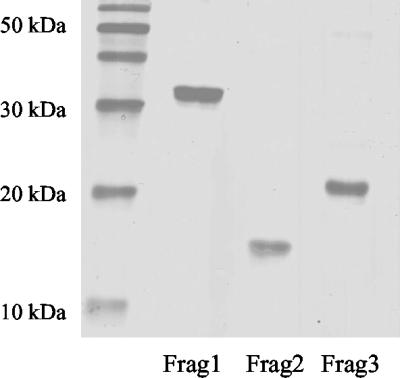
In previous studies, immunization with amino acids 37 to 348 of TprK resulted in promising attenuation of infection after challenge with T. pallidum Nichols, including reduced frequency of T. pallidum in lesions and reduced ulceration of lesions (14). To identify the immunogenic regions of TprK, the following three TprK fragments were studied: fragment 1, a hydrophilic N-terminal portion following the proposed signal sequence cleavage site, amino acids 37 to 273; fragment 2, a hydrophobic middle region, amino acids 274 to 348; and fragment 3, a hydrophilic C-terminal region, amino acids 349 to 478 (Fig. 1). Fragments 1 and 2 together comprise the region that was previously shown to alter lesion development (14), and fragment 3 was not previously studied. These fragments were expressed in E. coli, and clear bands representing purified proteins of the expected sizes were confirmed by SDS-PAGE (Fig. 2).
FIG. 2.
Confirmation of the purity and size of recombinant proteins by SDS-PAGE. The expected size of fragment 1 (Frag1) was approximately 30 kDa, that of fragment 2 (Frag2) was 12 kDa, and that of fragment 3 (Frag3) was 18 kDa.
T-cell responses during infection.
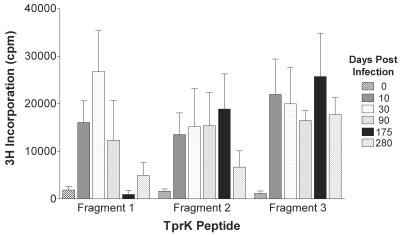
Because T cells are thought to play an important role in activating macrophages to clear treponemes from syphilitic lesions (28, 44), the ability of each of the fragments to elicit responses from T cells was examined. The optimal concentrations of the antigens were determined by titration (data not shown). All three TprK fragments were able to elicit proliferative responses in spleen cells from rabbits infected for 10 to 280 days with the homologous Seattle Nichols strain (Fig. 3). Sonicated T. pallidum elicited responses of greater than 35,000 cpm in all infected rabbits, while concanavalin A, a T-cell mitogen, elicited responses of greater than 35,000 cpm in infected and noninfected rabbits (data not shown). Cells that were not incubated with antigen incorporated <1,000 cpm (data not shown).
FIG. 3.
The ability of each of the TprK fragments to elicit splenocyte proliferation from groups of four rabbits infected with T. pallidum for 0 to 280 days was evaluated. Each bar represents the mean ± the standard error of tritiated thymidine incorporation of proliferating cells from all of the animals.
Antibody responses during infection.
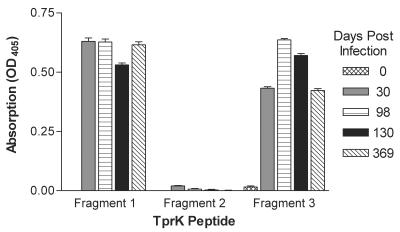
To determine the development of the humoral response during the course of infection, antibody responses to the fragments were evaluated at different times postinfection. Fragments 1 and 3 were recognized by antibodies from infected rabbits by 30 days postinfection and for at least 369 days postinfection (Fig. 4). In these four rabbits, fragment 2 was not recognized by antibodies at any point during infection (Fig. 4).
FIG. 4.
Evaluation by ELISA of the ability of antibodies from four rabbits infected for 0 to 369 days to recognize the three TprK fragments. Each bar represents the mean ± the standard error of absorbance measurements taken from triplicate wells. OD405, optical density at 405 nm.
Immunization and challenge studies.
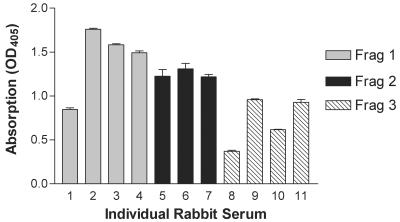
To determine the role of each of the peptides of TprK in protection, separate groups of rabbits were immunized with one of the fragments and subsequently challenged intradermally with the homologous Seattle Nichols strain. Sera from rabbits taken prior to immunization failed to recognize the TprK fragments (data not shown). However, immunized rabbits developed specific antibody responses to each of the immunizing peptides (Fig. 5).
FIG. 5.
Antisera from rabbits immunized with fragment (Frag) 1, 2, or 3 were tested in ELISAs for the ability to recognize their immunogens. Each bar represents the mean ± the standard error of absorbance measurements taken from triplicate wells. OD405, optical density at 405 nm.
As shown in Table 1, rabbits immunized with fragment 1 and challenged intradermally were the least likely to have detectable treponemes by DF microscopy (P < 0.001) and had the lowest proportion of ulcerative lesions (P < 0.001). Rabbits immunized with fragment 3 also had statistically significantly fewer lesions with detectable treponemes (P < 0.025) and lesions that ulcerated less frequently compared to the unimmunized controls (P < 0.001) but more than the rabbits immunized with fragment 1 (P < 0.001). The fragment 2-immunized rabbits had fewer ulcerative lesions compared to unimmunized controls (P < 0.001), but the proportion of DF+ lesions was not significantly different. All rabbits seroconverted, as indicated by Venereal Disease Research Laboratory and fluorescent treponemal antibody-absorbed serological tests (data not shown), and thus, even those fragment 1-immunized animals with no demonstrable spirochetes (n = 2) were considered to be infected.
TABLE 1.
Challenge results
| Immunogen | No. of DF+ lesions/total (%)a | P valueb | No. of ulcerative lesions/total (%) | P valueb |
|---|---|---|---|---|
| Fragment 1 | 8/32 (25) | <0.001 | 12/32 (37.5) | <0.001 |
| Fragment 2 | 22/24 (91.7) | >0.05 | 16/24 (66.7) | <0.001 |
| Fragment 3 | 27/32 (84.4) | <0.025 | 18/32 (56.3) | <0.001 |
| None | 32/32 (100) | 32/32 (100) |
Aspirates were taken from lesions and examined under a dark-field microscope for the presence of treponemes.
Chi-square test comparing test values to unimmunized-control values.
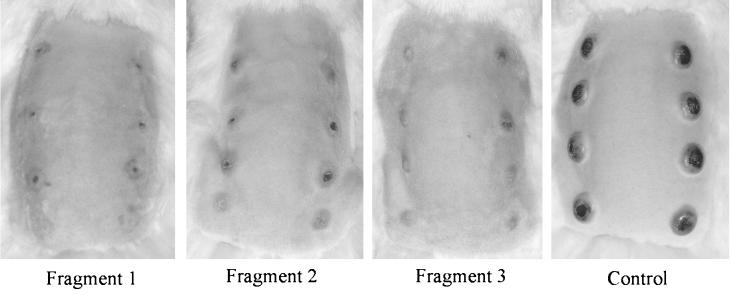
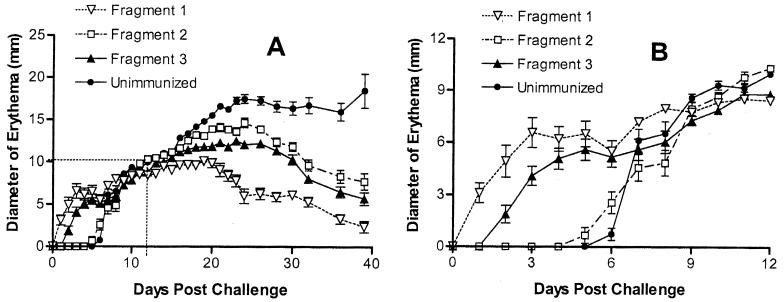
The lesions in rabbits immunized with fragment 1 were also the smallest, least indurated, and quickest to heal; intermediate results were seen with the lesions on rabbits immunized with fragment 2 or 3; and the unimmunized control rabbits had the largest, most ulcerative and indurated lesions that did not heal during the 40-day observation period (Fig. 6 and 7A). Erythema at the challenge sites was apparent on rabbits immunized with fragment 1 or 3 within 2 days postchallenge, followed by induration (thought to be a delayed-type hypersensitivity [DTH] response), whereas those immunized with fragment 2 and the unimmunized control rabbits did not develop any signs at the challenge sites until approximately a week after the challenge (Fig. 7B).
FIG. 6.
Representative photos of rabbits taken 23 days post intradermal challenge at eight locations on their backs are shown.
FIG. 7.
(A) Measurements of diameters of erythematous lesions in all rabbits postchallenge. (B) Enlargement of the boxed area in graph A.
DISCUSSION
The nature of the protective immune response to syphilis is not fully defined. Because of earlier promising results with TprK (14), we sought to identify the region of TprK that is responsible for attenuating lesion development and to further characterize the immune response to TprK. Although Hazlett et al. failed to reproduce our previously reported immunization results (20), the data presented in this study confirm and expand our earlier studies. The hydrophilic N-terminal portion of TprK interacts with T and B cells during infection, and immunization with that peptide clearly attenuates lesion development following challenge.
It is known that rabbits infected for greater than 3 months are immune to homologous challenge (5, 43). Therefore, T- and B-cell epitopes identified during infection may be protective. All three recombinant fragments of TprK were able to elicit proliferative responses from splenocytes of infected rabbits as early as 10 days postinfection and for at least 280 days postinfection. These results indicate that there are multiple T-cell epitopes within TprK and that TprK is expressed during infection. The presence of specific antibody responses from 30 days postinfection until at least 369 days postinfection also suggests that TprK is expressed during infection. Fragments 1 and 3 were readily recognized by antibodies from infected rabbits, while fragment 2 was not. There are clearly B-cell epitopes in fragment 2 because immunization with recombinant fragment 2 led to specific antibody responses, but these epitopes are not dominant epitopes during infection. This lack of anti-fragment 2 antibodies in infection-immune animals suggests that these antibodies may not play a role in protection.
When each of the fragments was used to immunize rabbits that were subsequently intradermally challenged, the rabbits immunized with fragment 1 showed the most apparent effects of immunization, those immunized with fragment 3 showed intermediate results, and those immunized with fragment 2 were most similar to unimmunized controls. Rabbits immunized with fragment 1 or 3 developed early erythema and induration, indicative of a T-cell-mediated DTH response, while the fragment 2-immunized rabbits and unimmunized controls did not develop clinical signs until approximately a week after the challenge. These results are consistent with the hypothesis that a DTH response is important for clearance of treponemes and protection against syphilis. Sites of primary and secondary syphilitic lesions are infiltrated by T lymphocytes and macrophages, leading to the clearance of treponemes (17, 28). There are many other lines of evidence indicating that cellular immune responses are critical in controlling infection. It is known that HIV and posttransplant immunosuppressed patients can show relapses and exacerbated signs of disease (19, 23, 24). Rabbits given corticosteroids at different times postinfection have a decreased ability to clear treponemes and can suffer reemergence of disease (29, 33, 42). It is also known, however, that efficient phagocytosis and killing of treponemes by activated macrophages are dependent on opsonic antibodies (4, 31). Furthermore, it has been shown that passive antibody transfers lead to altered lesion development in the rabbit model but fail to prevent infection (7). Taken together, these results suggest that protection is not possible without a cellular immune response but that antibodies also play a role in protection. Therefore, an effective vaccine against syphilis would most likely need to elicit both T- and B-cell responses.
Between the two groups of rabbits that exhibited a DTH-like response, the rabbits immunized with fragment 1 exhibited more dramatic lesion attenuation than those immunized with fragment 3. To further examine the antibody responses, synthetic 20-amino-acid peptides of TprK were tested against sera from infected and immunized rabbits (36). ELISAs indicated that antisera from fragment 1-immunized rabbits reacted with a peptide recognized by antisera from infected rabbits. In contrast, antisera from rabbits immunized with fragment 3 failed to react with epitopes that are recognized by antisera from infected rabbits (36). N-terminal fragment 1 may have conferred the most lesion protection in the these studies because it was able to elicit B-cell responses that may be most relevant to the native protein.
TprK has been shown to have seven discrete variable regions that vary among and within isolates (15). Experiments have shown that the T-cell epitopes recognized during infection are in the highly conserved regions of TprK (36). If immunization with TprK results in a protective DTH response based on the conserved TprK T-cell epitopes, incorporation of these epitopes may be useful in a cross-protective multicomponent vaccine. However, B-cell epitope mapping experiments have shown that infection-induced antibodies are directed to variable regions of TprK (36). If these B-cell epitopes are protective, then heterologous protection conferred by these epitopes would be limited. More studies addressing the nature of a protective immune response and the role that TprK variation may play in protection are warranted.
Even though the fragments did not confer complete protection and the rabbits were infected, as indicated by positive serological tests, the lesions of immunized rabbits ulcerated less frequently and healed more rapidly. If such a vaccine were to be used in humans, it would presumably lower the transmissibility of syphilis infection to contacts although not protect the immunized person against infection. The effect of such immunization in reducing syphilis progression in an infected individual cannot be predicted. An even greater effect of immunization may be achievable if protein folding, the delivery system, and the immunization doses and schedules are optimized. Even though complete protection may not be attained with TprK alone, components of TprK may be useful in combination with other antigens in a vaccine.
Acknowledgments
We thank Barbara Molini, Lynn Barrett, Caroline Cameron, Stacy Smith, and Karin Haverlah for excellent technical assistance and advice.
This work was supported by U.S. Public Health Service grants AI-34616, AI-42143 (S. A. Lukehart), and AI-43456 (W. C. Van Voorhis) from the National Institutes of Health. C. A. Morgan was supported by institutional training grant AI-07509 from the National Institutes of Health.
Editor: D. L. Burns
REFERENCES
- 1.Adler, M. W. 1996. Sexually transmitted diseases: control in developing countries. Genitourin. Med. 72:83-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agacfidan, A., and P. Kohl. 1999. Sexually transmitted diseases (STDs) in the world. FEMS Immunol. Med. Microbiol. 24:431-435. [DOI] [PubMed] [Google Scholar]
- 3.Arroll, T. W., A. Centurion-Lara, S. A. Lukehart, and W. C. Van Voorhis. 1999. T-cell responses to Treponema pallidum subsp. pallidum antigens during the course of experimental syphilis infection. Infect. Immun. 67:4757-4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker-Zander, S. A., and S. A. Lukehart. 1992. Macrophage-mediated killing of opsonized Treponema pallidum. J. Infect. Dis. 165:69-74. [DOI] [PubMed] [Google Scholar]
- 5.Baker-Zander, S., and S. Sell. 1980. A histopathologic and immunologic study of the course of syphilis in the experimentally infected rabbit. Demonstration of long-lasting cellular immunity. Am. J. Pathol. 101:387-413. [PMC free article] [PubMed] [Google Scholar]
- 6.Battu, V. R., P. J. Homer, P. K. Taylor, A. E. Jephcott, and S. I. Egglestone. 1997. Locally acquired heterosexual outbreak of syphilis in Bristol. Lancet 350:1100-1101. [DOI] [PubMed] [Google Scholar]
- 7.Bishop, N. H., and J. N. Miller. 1976. Humoral immunity in experimental syphilis. I. The demonstration of resistance conferred by passive immunization. J. Immunol. 117:191-207. [PubMed] [Google Scholar]
- 8.Borenstein, L. A., J. D. Radolf, T. E. Fehniger, D. R. Blanco, J. N. Miller, and M. A. Lovett. 1988. Immunization of rabbits with recombinant Treponema pallidum surface antigen 4D alters the course of experimental syphilis. J. Immunol. 140:2415-2421. [PubMed] [Google Scholar]
- 9.Borisenko, K. K., L. I. Tichonova, and A. M. Renton. 1999. Syphilis and other sexually transmitted infections in the Russian Federation. Int. J. Sex. Transm. Dis. AIDS 10:665-668. [DOI] [PubMed] [Google Scholar]
- 10.Cameron, C. E., C. Castro, S. A. Lukehart, and W. C. Van Voorhis. 1998. Function and protective capacity of Treponema pallidum subsp. pallidum glycerophosphodiester phosphodiesterase. Infect. Immun. 66:5763-5770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cameron, C. E., S. A. Lukehart, C. Castro, B. Molini, C. Godornes, and W. C. Van Voorhis. 2000. Opsonic potential, protective capacity, and sequence conservation of the Treponema pallidum subspecies pallidum Tp92. J. Infect. Dis. 181:1401-1413. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. 1998. Congenital syphilis—United States. Morb. Mortal. Wkly. Rep. 48:757-761. [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. 1999. Primary and secondary syphilis—United States. Morb. Mortal. Wkly. Rep. 48:873-878. [PubMed] [Google Scholar]
- 14.Centurion-Lara, A., C. Castro, L. Barrett, C. Cameron, M. Mostowfi, W. C. Van Voorhis, and S. A. Lukehart. 1999. Treponema pallidum major sheath protein homologue TprK is a target of opsonic antibody and the protective immune response. J. Exp. Med. 189:647-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centurion-Lara, A., C. Godornes, C. Castro, W. C. Van Voorhis, and S. A. Lukehart. 2000. The tprK gene is heterogeneous among Treponema pallidum strains and has multiple alleles. Infect. Immun. 68:824-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Champion, C. I., J. N. Miller, L. A. Borenstein, M. A. Lovett, and D. R. Blanco. 1990. Immunization with Treponema pallidum endoflagella alters the course of experimental rabbit syphilis. Infect. Immun. 58:3158-3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engelkens, H. J. H., F. J. W. ten Kate, J. Judanarso, V. D. Vuzevski, J. B. H. J. van Lier, J. C. J. Godschalk, J. J. van der Sluis, and E. Stolz. 1993. The localization of treponemes and characterization of the inflammatory infiltrate in skin biopsies from patients with primary or secondary syphilis, or early infectious yaws. Genitourin. Med. 69:102-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerbase, A. C., J. T. Rowley, D. H. Heymann, S. F. Berkley, and P. Piot. 1998. Global prevalence and incidence estimates of selected curable STDs. Sex. Transm. Infect. 74(Suppl. 1):S12-S16. [PubMed] [Google Scholar]
- 19.Gregory, N., M. Sanchez, and M. R. Buchness. 1990. The spectrum of syphilis in patients with human immunodeficiency virus infection. J. Am. Acad. Dermatol. 22:1061-1067. [DOI] [PubMed] [Google Scholar]
- 20.Hazlett, K. R., T. J. Sellati, T. T. Nguyen, D. L. Cox, M. L. Clawson, M. J. Caimano, and J. D. Radolf. 2001. The TprK protein of Treponema pallidum is periplasmic and is not a target of opsonic antibody or protective immunity. J. Exp. Med. 193:1015-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins, S. P., A. Sukthankar, M. Mahto, R. R. Jarvis, and H. B. Lacey. 2000. Syphilis increases in Manchester, UK. Lancet 355:1466.. [DOI] [PubMed] [Google Scholar]
- 22.Hira, S. K., G. J. Bhat, A. V. Ratnam, S. L. Nyaywa, S. J. Phiri, L. M. Sandala, G. K. Kukelabai, R. C. Mulenga, I. Nyerenda, and N. Ng'andu. 1987. Maternal and congenital syphilis in Zambia—some epidemiologic aspects. Afr. J. Sex. Transm. Dis. 3:3-6. [Google Scholar]
- 23.Johns, D. R., M. Tiernewy, and D. Felsenstein. 1987. Alteration in the natural history of neurosyphilis by concurrent infection with the human immunodeficiency virus. N. Engl. J. Med. 315:1569-1572. [DOI] [PubMed] [Google Scholar]
- 24.Katz, D. A., and J. R. Berger. 1989. Neurosyphilis in acquired immunodeficiency syndrome. Arch. Neurol. 45:895-898. [DOI] [PubMed] [Google Scholar]
- 25.Kreiss, J. K., R. Coombs, F. A. Plummer, K. K. Holmes, B. Nikora, W. Cameron, E. Ngugi, J. O. Ndinya Achola, and L. Corey. 1989. Isolation of human immunodeficiency virus from genital ulcers in Nairobi prostitutes. J. Infect. Dis. 160:380-384. [DOI] [PubMed] [Google Scholar]
- 26.Kroll, D. J., H. Abdel-Malek Abdel-Hafiz, T. Marcell, S. Simpson, C. Y. Chen, A. Guiterrez-Hartmann, J. W. Lustbader, and J. P. Hoeffler. 1993. A multifunctional prokaryotic protein expression system: overproduction, affinity purification, and selective detection. DNA Cell Biol. 12:441-453. [DOI] [PubMed] [Google Scholar]
- 27.Liu, H., B. M. Steiner, J. D. Alder, D. K. Baertschy, and R. F. Schell. 1990. Immune T cells sorted by flow cytometry confer protection against infection with Treponema pallidum subsp. pertenue in hamsters. Infect. Immun. 58:1685-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lukehart, S. A., S. A. Baker-Zander, R. M. C. Lloyd, and S. Sell. 1980. Characterization of lymphocyte responsiveness in early experimental syphilis. II. Nature of cellular infiltration and Treponema pallidum distribution in testicular lesions. J. Immunol. 124:461-467. [PubMed] [Google Scholar]
- 29.Lukehart, S. A., S. A. Baker-Zander, R. M. Lloyd, and S. Sell. 1981. Effect of cortisone administration on host-parasite relationships in early experimental syphilis. J. Immunol. 127:1361-1368. [PubMed] [Google Scholar]
- 30.Lukehart, S. A., S. A. Baker-Zander, and S. Sell. 1980. Characterization of lymphocyte responsiveness in early experimental syphilis. I. In vitro response to mitogens and Treponema pallidum antigens. J. Immunol. 124:454-460. [PubMed] [Google Scholar]
- 31.Lukehart, S. A., and J. N. Miller. 1978. Demonstration of the in vitro phagocytosis of Treponema pallidum by rabbit peritoneal macrophages. J. Immunol. 121:2014-2024. [PubMed] [Google Scholar]
- 32.Magnuson, H. J., E. W. Thomas, S. Olansky, B. I. Kaplan, L. De Mello, and J. C. Cutler. 1956. Inoculation syphilis in human volunteers. Medicine 35:33-82. [DOI] [PubMed] [Google Scholar]
- 33.McLeod, C. P., and H. J. Magnuson. 1956. Effect of cortisone on latent syphilis in rabbits and mice. J. Immunol. 76:373-376. [PubMed] [Google Scholar]
- 34.Metzger, M., E. Michalska, J. Podwinska, and W. Smogor. 1969. Immunogenic properties of the protein component of Treponema pallidum. Br. J. Vener. Dis. 45:299-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller, J. N. 1973. Immunity in experimental syphilis. VI. Successful vaccination of rabbits with Treponema pallidum, Nichols strain, attenuated by gamma-irradiation. J. Immunol. 110:1206-1215. [PubMed] [Google Scholar]
- 36.Morgan, C. A., B. J. Molini, S. A. Lukehart, and W. C. Van Voorhis. 2002. Segregation of B and T cell epitopes of Treponema pallidum TprK to variable and conserved regions during experimental syphilis infection. J. Immunol. 169:952-957. [DOI] [PubMed] [Google Scholar]
- 37.Panchaud, C. S. Singh, D. Feivelson, and J. E. Darroch. 2000. Sexually transmitted diseases among adolescents in developed countries. Fam. Plann. Perspect. 32:24-32, 45. [PubMed] [Google Scholar]
- 38.Plummer, F. A., M. A. Wainberg, P. Plourde, P. Jessamine, L. J. D'Costa, I. A. Wamola, and A. R. Ronald. 1990. Detection of human immunodeficiency virus type 1 (HIV-1) in genital ulcer exudates of HIV-1-infected men by culture and gene amplification. J. Infect. Dis. 161:810-811. [DOI] [PubMed] [Google Scholar]
- 39.Renton, A. M., K. K. Borisenko, A. Meheus, and A. Gromyko. 1998. Epidemics of syphilis in the newly independent states of the former Soviet Union. Sex. Transm. Infect. 74:165-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schell, R. F., J. K. Chan, and J. L. Le Frock. 1979. Endemic syphilis: passive transfer of resistance with serum and cells in hamsters. J. Infect. Dis. 140:378-383. [DOI] [PubMed] [Google Scholar]
- 41.Tani, T., R. Inoue, and O. Asano. 1951. Studies on the preventive inoculation against syphilis. Jpn. Med. J. 4:71-86. [DOI] [PubMed] [Google Scholar]
- 42.Turner, T. B., and D. H. Hollander. 1954. Studies on the mechanism of action of cortisone in experimental syphilis. Am. J. Syph. Gonor. Vener. Dis. 38:371-387. [PubMed] [Google Scholar]
- 43.Turner, T. B., and D. H. Hollander. 1957. Biology of the treponematoses. World Health Organization, Geneva, Switzerland.
- 44.Van Voorhis, W. C., L. K. Barrett, D. M. Koelle, J. M. Nasio, F. A. Plummer, and S. A. Lukehart. 1996. Primary and secondary syphilis lesions contain mRNA for Th1 cytokines. J. Infect. Dis. 173:491-495. [DOI] [PubMed] [Google Scholar]
- 45.Wasserheit, J. N. 1991. Epidemiological synergy: interrelationships between HIV infection and other STDs, p. 47-72. In L. C. Chen et al. (ed.), AIDS and women's reproductive health. Plenum Press, New York, N.Y.
- 46.Wicher, V., K. Wicher, A. Jakubowski, and S. M. Nakeeb. 1987. Adoptive transfer of immunity to Treponema pallidum Nichols infection in inbred strain 2 and C4D guinea pigs. Infect. Immun. 55:2502-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]