Abstract
1. Antidromically identified supraoptic (SO) units were recorded in lactating rats anaesthetized with urethane (1·1 g/kg), and their activity was studied during milk ejection evoked by the suckling of the young. Fifty-eight SO units were recorded through 174 milk ejections. Each milk ejection was the result of a neurohypophysial release of an oxytocin pulse of 0·5-1·5 m-u.
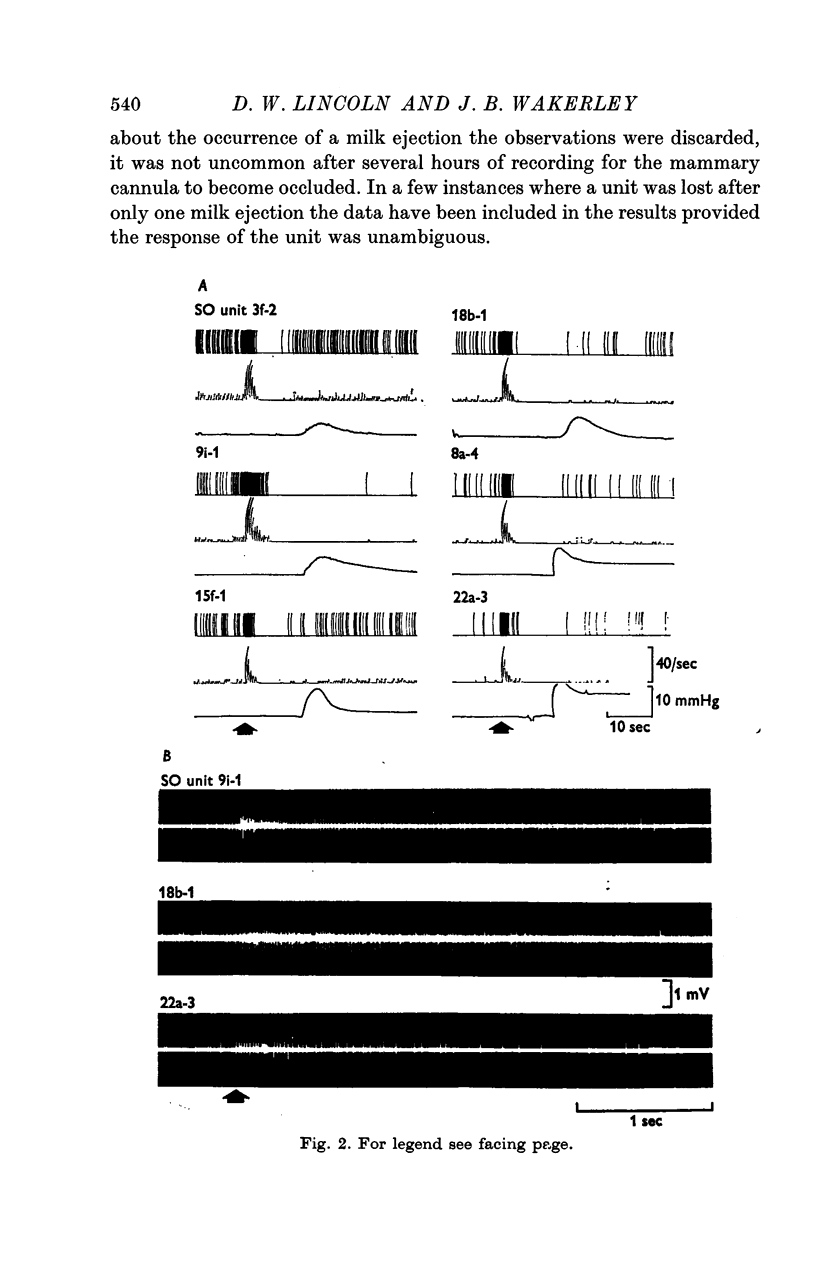
2. Fifty-five of the SO units displayed background activity and three were silent. The firing rates ranged from 0 to 15·4 spikes/sec, the distribution was exponential with 26% of the units firing at < 1 spike/sec. Sixteen (28%) of the SO units displayed a phasic pattern of activity characterized by periods of activity (6-132 sec) and silence (4-71 sec).
3. Twenty-five of the non-phasic units displayed a large and stereotyped acceleration in spike activity some 10·5-17·4 sec before the rise in intramammary pressure at milk ejection. Units accelerated to rates between 9-66 spikes/sec, an increase of about thirtyfold (median) on background activity. The response was brief (0·9-4·7 sec) and was followed by a period of after-inhibition.
4. It was concluded from studies of double recordings and observations of multi-unit activity that all the responsive units were synchronously activated. The mean latency of 13·3 sec between the onset of the neurosecretory response and milk ejection was similar to that observed following the experimental release of endogenous oxytocin by electrical stimulation of the neurohypophysis (50 pulses/sec for 2-4 sec).
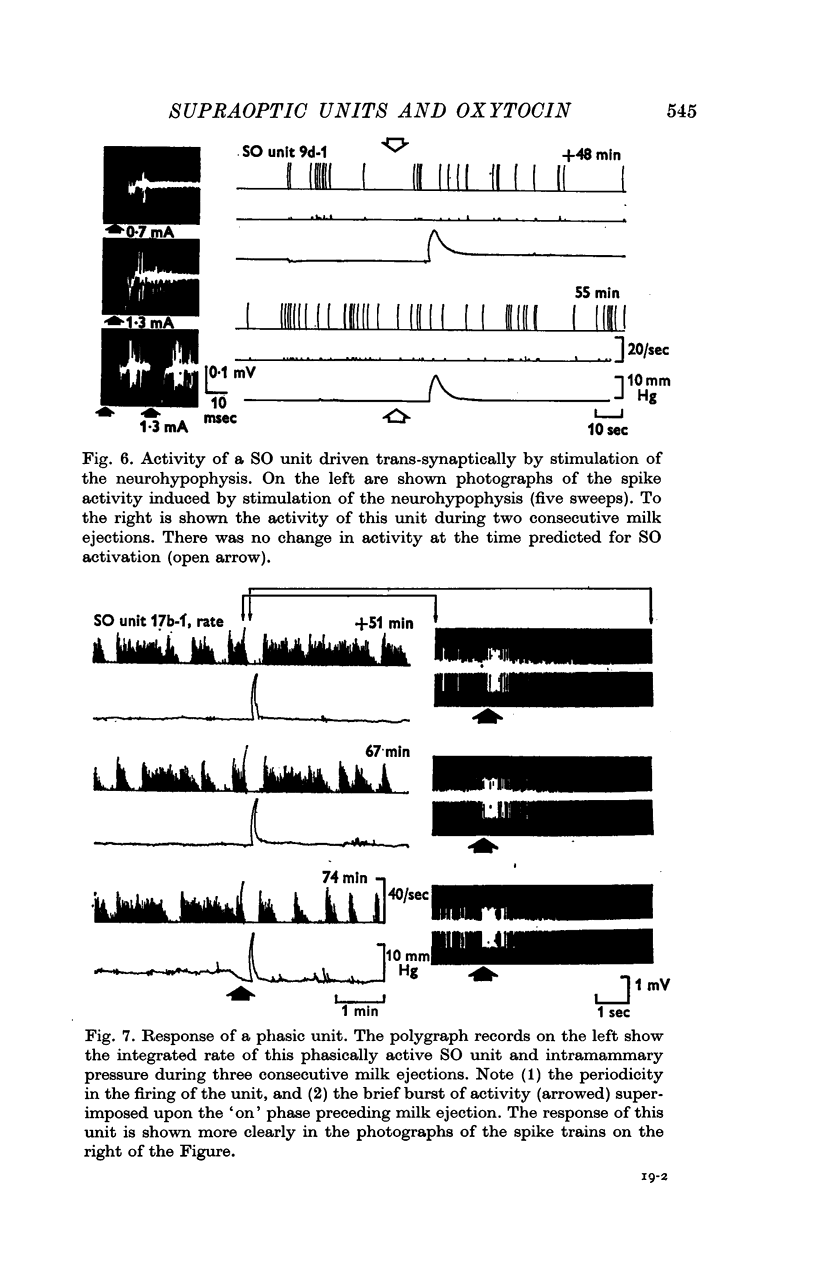
5. Four of the phasically active units were correlated with the oxytocin release for milk ejection. Three of these units displayed a burst of activity superimposed on the terminal portion of an active phase, some 10·2-14·7 sec before milk ejection. The fourth unit, recorded in conjunction with a responsive non-phasic unit, consistently switched from silence to activity coincident with the onset of the SO activation.
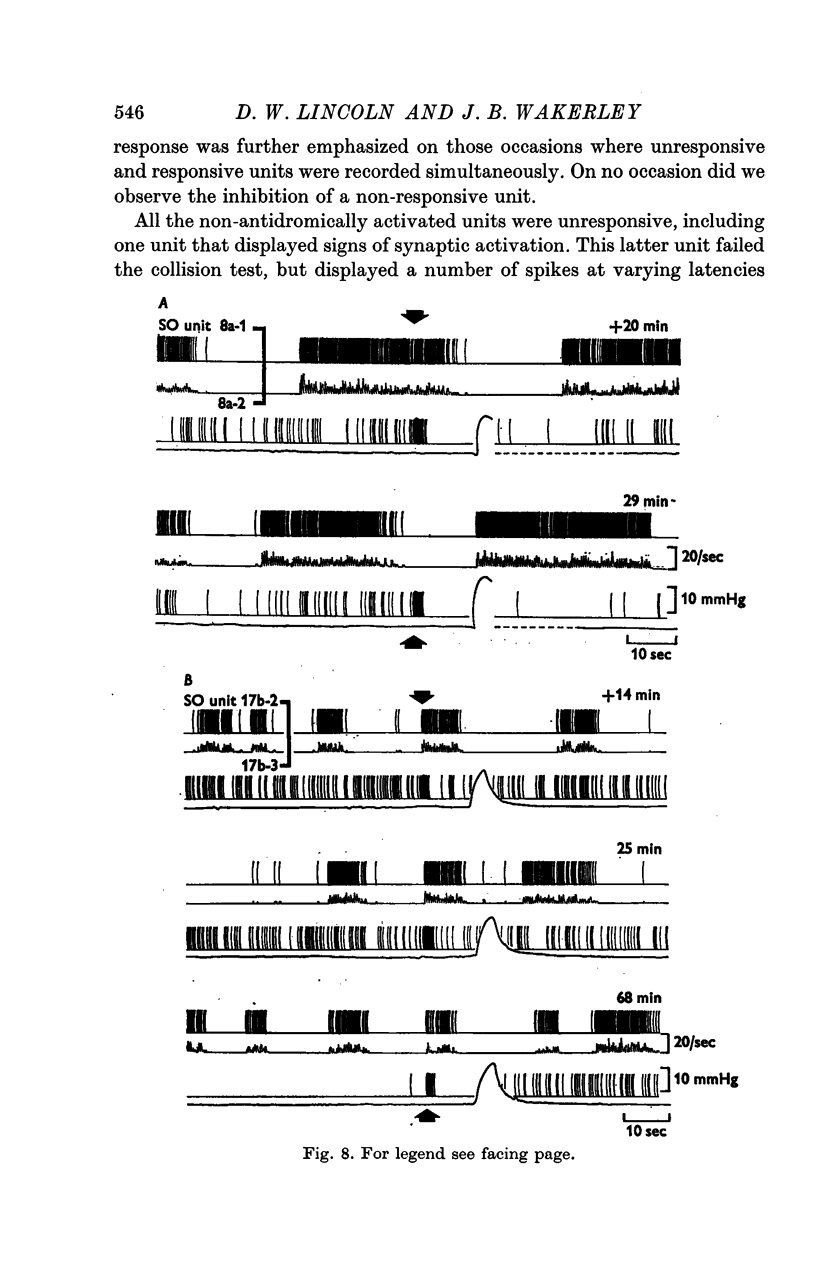
6. The remaining SO units and a further ten units that were not antidromically activated by neurohypophysial stimulation displayed no change in activity during either the period of neurosecretory activation or the period of after-inhibition.
7. This activation of the SO neurones, in the formulation of oxytocin release and milk ejection, is the same as that we have previously observed in recordings from the paraventricular (PV) region, and the proportion of neurones displaying the response is similar: 48% in the SO nuclei, 58% in the PV nuclei. We conclude, since the SO nuclei contain 80% of the neurosecretory cells that project to the neurohypophysis, that the SO nuclei are as important, if not more so, than the PV nuclei in the control of oxytocin release.
Full text
PDF


















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BODIAN D., MAREN T. H. The effect of neuro- and adenohypophysectomy on retrograde degeneration in hypothalamic nuclei of the rat. J Comp Neurol. 1951 Jun;94(3):485–511. doi: 10.1002/cne.900940310. [DOI] [PubMed] [Google Scholar]
- BROOKS C. M., USHIYAMA J., LANGE G. Reactions of neurons in or near the supraoptic nuclei. Am J Physiol. 1962 Mar;202:487–490. doi: 10.1152/ajplegacy.1962.202.3.487. [DOI] [PubMed] [Google Scholar]
- BROWN L. M., GINSBURG M. Effect of anaesthetics and haemorrhage on the release of neurohypophysial antidiuretic hormone. Br J Pharmacol Chemother. 1956 Sep;11(3):236–244. doi: 10.1111/j.1476-5381.1956.tb01060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandaranayake R. C. Morphology of the accessory neurosecretory nuclei and of the retrochiasmatic part of the supraoptic nucleus of the rat. Acta Anat (Basel) 1971;80(1):14–22. doi: 10.1159/000143670. [DOI] [PubMed] [Google Scholar]
- Barker J. L., Crayton J. W., Nicoll R. A. Antidromic and orthodromic responses of paraventricular and supraoptic neurosecretory cells. Brain Res. 1971 Oct 29;33(2):353–366. doi: 10.1016/0006-8993(71)90108-9. [DOI] [PubMed] [Google Scholar]
- Bisset G. W., Clark B. J., Errington M. L. The hypothalamic neurosecretory pathways for the release of oxytocin and vasopressin in the cat. J Physiol. 1971 Aug;217(1):111–131. doi: 10.1113/jphysiol.1971.sp009562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisset G. W., Clark B. J., Haldar J. Blood levels of oxytocin and vasopressin during suckling in the rabbit and the problem of their independent release. J Physiol. 1970 Mar;206(3):711–722. doi: 10.1113/jphysiol.1970.sp009039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burford G. D., Jones C. W., Pickering B. T. Tentative identification of a vasopressin-neurophysin and an oxytocin-neurophysin in the rat. Biochem J. 1971 Oct;124(4):809–813. doi: 10.1042/bj1240809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROSS B. A., GREEN J. D. Activity of single neurones in the hypothalamus: effect of osmotic and other stimuli. J Physiol. 1959 Oct;148:554–569. doi: 10.1113/jphysiol.1959.sp006306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross B. A., Dyer R. G. Unit activity in rat diencephalic islands--the effect of anaesthetics. J Physiol. 1971 Jan;212(2):467–481. doi: 10.1113/jphysiol.1971.sp009336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross B. A., Novin D., Sundsten J. W. Antidromic activation of neurones in the paraventricular nucleus by stimulation in the neural lobe of the pituitary. J Physiol. 1969 Jul;203(1):68P–70P. [PubMed] [Google Scholar]
- Dreifuss J. J., Kalnins I., Kelly J. S., Ruf K. B. Action potentials and release of neurohypophysial hormones in vitro. J Physiol. 1971 Jul;215(3):805–817. doi: 10.1113/jphysiol.1971.sp009499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreifuss J. J., Kelly J. S. Recurrent inhibition of antidromically identified rat supraoptic neurones. J Physiol. 1972 Jan;220(1):87–103. doi: 10.1113/jphysiol.1972.sp009696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreifuss J. J., Kelly J. S. The activity of identified supraoptic neurones and their response to acetylcholine applied by iontophoresis. J Physiol. 1972 Jan;220(1):105–118. doi: 10.1113/jphysiol.1972.sp009697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyball R. E., Koizumi K. Electrical activity in the supraoptic and paraventricular nuclei associated with neurohypophysial hormone release. J Physiol. 1969 May;201(3):711–722. doi: 10.1113/jphysiol.1969.sp008783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyball R. E. Oxytocin and ADH secretion in relation to electrical activity in antidromically identified supraoptic and paraventricular units. J Physiol. 1971 Apr;214(2):245–256. doi: 10.1113/jphysiol.1971.sp009430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer R. G., Dyball R. E., Morris J. F. The effect of hypothalamic deafferentation upon the ultrastructure and hormone content of the paraventricular nucleus. J Endocrinol. 1973 Jun;57(3):509–516. doi: 10.1677/joe.0.0570509. [DOI] [PubMed] [Google Scholar]
- Folley S. J., Knaggs G. S. Milk-ejection activity (oxytocin) in the external jugular vein blood of the cow, goat and sow, in relation to the stimulus of milking or suckling. J Endocrinol. 1966 Feb;34(2):197–214. doi: 10.1677/joe.0.0340197. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):497–506. doi: 10.1113/jphysiol.1952.sp004719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldar J. Independent release of oxytocin and vasopressin during parturition in the rabbit. J Physiol. 1970 Mar;206(3):723–730. doi: 10.1113/jphysiol.1970.sp009040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris G. W., Manabe Y., Ruf K. B. A study of the parameters of electrical stimulation of unmyelinated fibres in the pituitary stalk. J Physiol. 1969 Jul;203(1):67–81. doi: 10.1113/jphysiol.1969.sp008850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward J. N., Jennings D. P. Activity of magnocellular neuroendocrine cells in the hypothalamus of unanaesthetized monkeys. II. Osmosensitivity of functional cell types in the supraoptic nucleus and the internuclear zone. J Physiol. 1973 Aug;232(3):545–572. doi: 10.1113/jphysiol.1973.sp010285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida A. The oxytocin release and the compound action potential evoked by electrical stimulation on the isolated neurohypophysis of the rat. Jpn J Physiol. 1970 Feb 15;20(1):84–96. doi: 10.2170/jjphysiol.20.84. [DOI] [PubMed] [Google Scholar]
- Jones C. W., Pickering B. T. Comparison of the effects of water deprivation and sodium chloride imbibition on the hormone content of the neurohypophysis of the rat. J Physiol. 1969 Aug;203(2):449–458. doi: 10.1113/jphysiol.1969.sp008874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi K., Yamashita H. Studies of antidromically identified neurosecretory cells of the hypothalamus by intracellular and extracellular recordings. J Physiol. 1972 Mar;221(3):683–705. doi: 10.1113/jphysiol.1972.sp009776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEDERIS K. Vasopressin and oxytocin in the mammalian hypothalamus. Gen Comp Endocrinol. 1961 Apr;1:80–89. doi: 10.1016/0016-6480(61)90027-2. [DOI] [PubMed] [Google Scholar]
- Lincoln D. W., Boer K., Swaab D. F. Measurement of intramammary pressure in rats by pressure sensitive radio-pill. Physiol Behav. 1974 Feb;12(2):289–292. doi: 10.1016/0031-9384(74)90185-1. [DOI] [PubMed] [Google Scholar]
- Lincoln D. W., Hill A., Wakerley J. B. The milk-ejection reflex of the rat: an intermittent function not abolished by surgical levels of anaesthesia. J Endocrinol. 1973 Jun;57(3):459–476. doi: 10.1677/joe.0.0570459. [DOI] [PubMed] [Google Scholar]
- Lincoln D. W. Unit activity in the hypothalamus, septum and preoptic area of the rat: characteristics of spontaneous activity and the effect of oestrogen. J Endocrinol. 1967 Feb;37(2):177–189. doi: 10.1677/joe.0.0370177. [DOI] [PubMed] [Google Scholar]
- Livett B. G., Uttenthal L. O., Hope D. B. Localization of neurophysin-II in the hypothalamo-neurohypophysial system of the pig by immunofluorescence histochemistry. Philos Trans R Soc Lond B Biol Sci. 1971 Jun 17;261(839):371–378. doi: 10.1098/rstb.1971.0067. [DOI] [PubMed] [Google Scholar]
- NIBBELINK D. W. Paraventricular nuclei, neurohypophysis, and parturition. Am J Physiol. 1961 Jun;200:1229–1232. doi: 10.1152/ajplegacy.1961.200.6.1229. [DOI] [PubMed] [Google Scholar]
- Negoro H., Holland R. C. Inhibition of unit activity in the hypothalamic paraventricular nucleus following antidromic activation. Brain Res. 1972 Jul 20;42(2):385–402. doi: 10.1016/0006-8993(72)90538-0. [DOI] [PubMed] [Google Scholar]
- Negoro H., Visessuwan S., Holland R. C. Reflex activation of paraventricular nucleus units during the reproductive cycle and in ovariectomized rats treated with oestrogen or progesterone. J Endocrinol. 1973 Dec;59(3):559–567. doi: 10.1677/joe.0.0590559. [DOI] [PubMed] [Google Scholar]
- Roberts J. S., Share L. Oxytocin in plasma of pregnant, lactating and cycling ewes during vaginal stimulation. Endocrinology. 1968 Aug;83(2):272–278. doi: 10.1210/endo-83-2-272. [DOI] [PubMed] [Google Scholar]
- Voloschin L. M., Tramezzani J. H. The neural input of the milk ejection reflex in the hypothalamus. Endocrinology. 1973 Apr;92(4):973–983. doi: 10.1210/endo-92-4-973. [DOI] [PubMed] [Google Scholar]
- Wakerley J. B., Dyball R. E., Lincoln D. W. Milk ejection in the rat: the result of a selective release of oxytocin. J Endocrinol. 1973 Jun;57(3):557–558. doi: 10.1677/joe.0.0570557. [DOI] [PubMed] [Google Scholar]
- Wakerley J. B., Lincoln D. W. Intermittent release of oxytocin during suckling in the rat. Nat New Biol. 1971 Oct 6;233(40):180–181. doi: 10.1038/newbio233180a0. [DOI] [PubMed] [Google Scholar]
- Wakerley J. B., Lincoln D. W. The milk-ejection reflex of the rat: a 20- to 40-fold acceleration in the firing of paraventricular neurones during oxytocin release. J Endocrinol. 1973 Jun;57(3):477–493. doi: 10.1677/joe.0.0570477. [DOI] [PubMed] [Google Scholar]
- Wakerly J. B., Lincoln D. W. Phasic discharge of antidromically identified units in the paraventricular nucleus of the hypothalamus. Brain Res. 1971 Jan 8;25(1):192–194. doi: 10.1016/0006-8993(71)90580-4. [DOI] [PubMed] [Google Scholar]
- Watkins W. B., Evans J. J. Demonstration of neurophysin in the hypothalamo-neurohypophysial system of the normal and dehydrated rat by the use of cross-species reactive anti-neurophysins. Z Zellforsch Mikrosk Anat. 1972;131(2):149–170. doi: 10.1007/BF00306924. [DOI] [PubMed] [Google Scholar]
- Yagi K., Azuma T., Matsuda K. Neurosecretory cell: capable of conducting impulse in rats. Science. 1966 Nov 11;154(3750):778–779. doi: 10.1126/science.154.3750.778. [DOI] [PubMed] [Google Scholar]
- Zimmerman E. A., Hsu K. C., Robinson A. G., Carmel P. W., Frantz A. G., Tannenbaum M. Studies of neurophysin secreting neurons with immunoperoxidase techniques employing antibody to bovine neurophysin. I. Light microscopic findings in monkey and bovine tissues. Endocrinology. 1973 Mar;92(3):931–940. doi: 10.1210/endo-92-3-931. [DOI] [PubMed] [Google Scholar]



