Full text
PDF























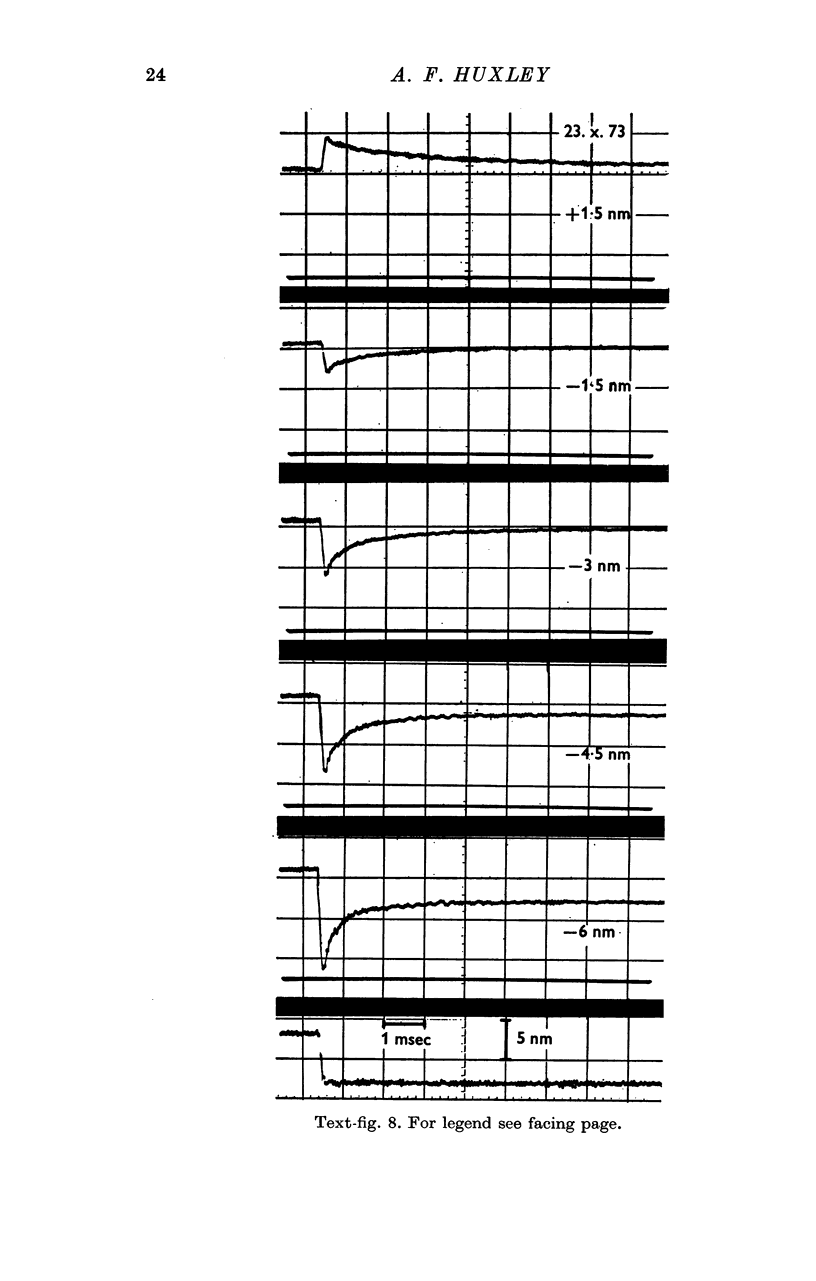


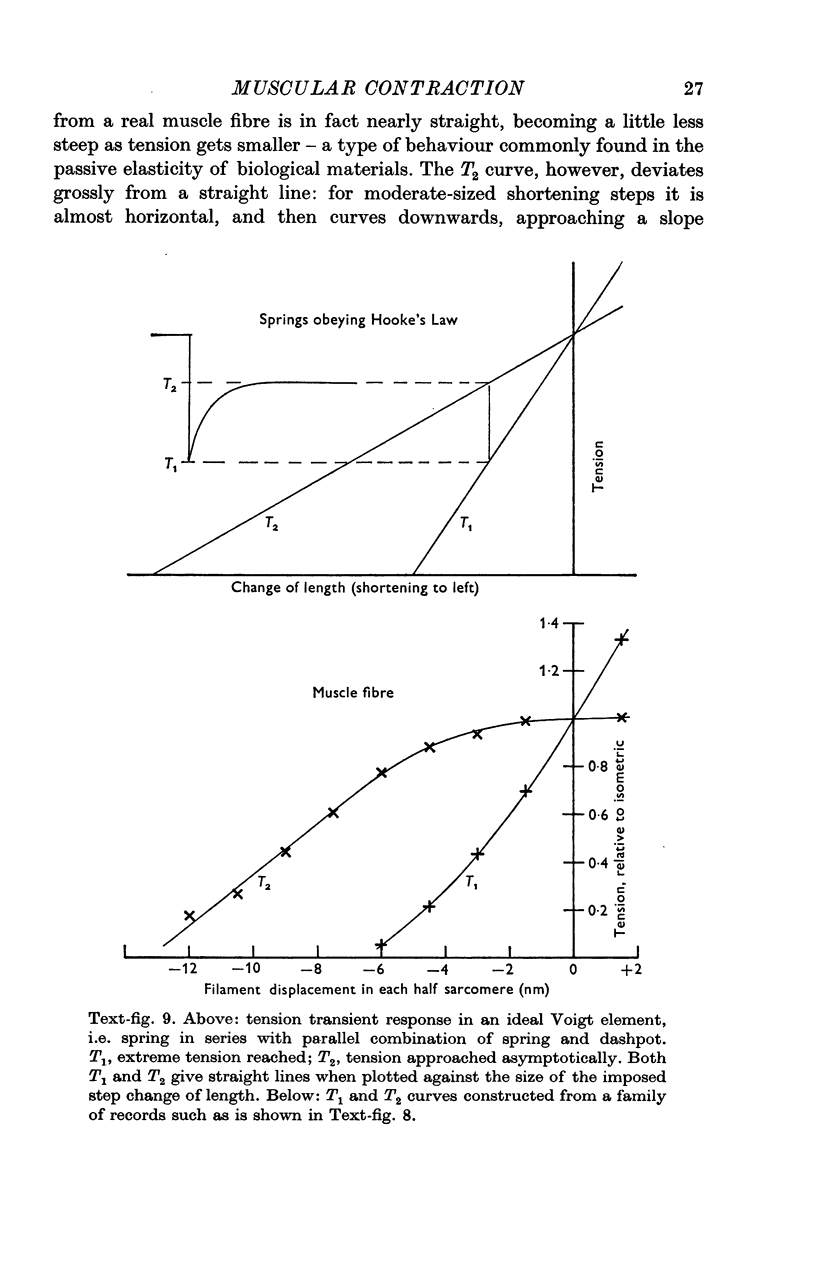

















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABBOTT B. C., AUBERT X. M. Changes of energy in a muscle during very slow stretches. Proc R Soc Lond B Biol Sci. 1951 Dec 31;139(894):104–117. doi: 10.1098/rspb.1951.0049. [DOI] [PubMed] [Google Scholar]
- ABBOTT B. C., AUBERT X. M., HILL A. V. The absorption of work by a muscle stretched during a single twitch or a short tetanus. Proc R Soc Lond B Biol Sci. 1951 Dec 31;139(894):86–104. doi: 10.1098/rspb.1951.0048. [DOI] [PubMed] [Google Scholar]
- AUBERT X. Réversibilité partielle de la contraction musculaire au cours de l'absorption du travail en cycle. Arch Int Physiol. 1948 Jun;55(4):348–361. doi: 10.3109/13813454809144858. [DOI] [PubMed] [Google Scholar]
- Adrian R. H., Peachey L. D. Reconstruction of the action potential of frog sartorius muscle. J Physiol. 1973 Nov;235(1):103–131. doi: 10.1113/jphysiol.1973.sp010380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley C. C., Ridgway E. B. On the relationships between membrane potential, calcium transient and tension in single barnacle muscle fibres. J Physiol. 1970 Jul;209(1):105–130. doi: 10.1113/jphysiol.1970.sp009158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood H. L., Hoyle G., Smyth T., Jr Mechanical and electrical responses of single innervated crab-muscle fibres. J Physiol. 1965 Oct;180(3):449–482. doi: 10.1113/jphysiol.1965.sp007712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert X., Lebacq J. The heat of shortening during the plateau of tetanic contraction and at the end of relaxation. J Physiol. 1971 Jul;216(1):181–200. doi: 10.1113/jphysiol.1971.sp009517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARANY E. H., EDMAN K. A. P., PALIS A. The influence of electrolytes on the rate of viscosity drop in ATP-actomyosin mixtures. Acta Physiol Scand. 1952 Feb 12;24(4):361–367. doi: 10.1111/j.1748-1716.1952.tb00851.x. [DOI] [PubMed] [Google Scholar]
- Bailey K. Tropomyosin: a new asymmetric protein component of the muscle fibril. Biochem J. 1948;43(2):271–279. doi: 10.1042/bj0430271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blangé T., Karemaker J. M., Kramer A. E. Elasticity as an expression of cross-bridge activity in rat muscle. Pflugers Arch. 1972;336(4):277–288. doi: 10.1007/BF00586953. [DOI] [PubMed] [Google Scholar]
- Brown L. M., Gonzalez-Serratos H., Huxley A. F. Electron microscopy of frog muscle fibres in extreme passive shortening. J Physiol. 1970 Jun;208(2):86P–88P. [PubMed] [Google Scholar]
- CAIN D. F., INFANTE A. A., DAVIES R. E. Chemistry of muscle contraction. Adenosine triphosphate and phosphorylcreatine as energy supplies for single contractions of working muscle. Nature. 1962 Oct 20;196:214–217. doi: 10.1038/196214a0. [DOI] [PubMed] [Google Scholar]
- CARLSEN F., KNAPPEIS G. G., BUCHTHAL F. Ultrastructure of the resting and contracted striated muscle fiber at different degrees of stretch. J Biophys Biochem Cytol. 1961 Oct;11:95–117. doi: 10.1083/jcb.11.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASELLA C. Tensile force in total striated muscle, isolated fibre and sarcolemma. Acta Physiol Scand. 1950 Dec;21(4):380–401. doi: 10.1111/j.1748-1716.1950.tb00744.x. [DOI] [PubMed] [Google Scholar]
- Civan M. M., Podolsky R. J. Contraction kinetics of striated muscle fibres following quick changes in load. J Physiol. 1966 Jun;184(3):511–534. doi: 10.1113/jphysiol.1966.sp007929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantin L. L., Taylor S. R. Active and passive shortening in voltage-clamped frog muscle fibres. J Physiol. 1971 Oct;218 (Suppl):13P–15P. [PubMed] [Google Scholar]
- Costantin L. L. The role of sodium current in the radial spread of contraction in frog muscle fibers. J Gen Physiol. 1970 Jun;55(6):703–715. doi: 10.1085/jgp.55.6.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIES R. E. A MOLECULAR THEORY OF MUSCLE CONTRACTION: CALCIUM-DEPENDENT CONTRACTIONS WITH HYDROGEN BOND FORMATION PLUS ATP-DEPENDENT EXTENSIONS OF PART OF THE MYOSIN-ACTIN CROSS-BRIDGES. Nature. 1963 Sep 14;199:1068–1074. doi: 10.1038/1991068a0. [DOI] [PubMed] [Google Scholar]
- Dainty M., Kleinzeller A., Lawrence A. S., Miall M., Needham J., Needham D. M., Shen S. C. STUDIES ON THE ANOMALOUS VISCOSITY AND FLOW-BIREFRINGENCE OF PROTEIN SOLUTIONS : III. CHANGES IN THESE PROPERTIES OF MYOSIN SOLUTIONS IN RELATION TO ADENOSINETRIPHOSPHATE AND MUSCULAR CONTRACTION. J Gen Physiol. 1944 Mar 20;27(4):355–399. doi: 10.1085/jgp.27.4.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson V. A., Woledge R. C. The thermal effects of shortening in tetanic contractions of frog muscle. J Physiol. 1973 Sep;233(3):659–671. doi: 10.1113/jphysiol.1973.sp010328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EBASHI S. THIRD COMPONENT PARTICIPATING IN THE SUPERPRECIPITATION OF 'NATURAL ACTOMYOSIN'. Nature. 1963 Dec 7;200:1010–1010. doi: 10.1038/2001010a0. [DOI] [PubMed] [Google Scholar]
- ENDO M. ENTRY OF A DYE INTO THE SARCOTUBULAR SYSTEM OF MUSCLE. Nature. 1964 Jun 13;202:1115–1116. doi: 10.1038/2021115b0. [DOI] [PubMed] [Google Scholar]
- Ebashi S., Endo M. Calcium ion and muscle contraction. Prog Biophys Mol Biol. 1968;18:123–183. doi: 10.1016/0079-6107(68)90023-0. [DOI] [PubMed] [Google Scholar]
- Ebashi S., Endo M., Otsuki I. Control of muscle contraction. Q Rev Biophys. 1969 Nov;2(4):351–384. doi: 10.1017/s0033583500001190. [DOI] [PubMed] [Google Scholar]
- Ebashi S., Kodama A. A new protein factor promoting aggregation of tropomyosin. J Biochem. 1965 Jul;58(1):107–108. doi: 10.1093/oxfordjournals.jbchem.a128157. [DOI] [PubMed] [Google Scholar]
- Elliott G. F., Rome E. M., Spencer M. A type of contraction hypothesis applicable to all muscles. Nature. 1970 May 2;226(5244):417–420. doi: 10.1038/226417a0. [DOI] [PubMed] [Google Scholar]
- Endo M. Entry of fluorescent dyes into the sarcotubular system of the frog muscle. J Physiol. 1966 Jul;185(1):224–238. doi: 10.1113/jphysiol.1966.sp007983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANZINI-ARMSTRONG C., PORTER K. R. SARCOLEMMAL INVAGINATIONS CONSTITUTING THE T SYSTEM IN FISH MUSCLE FIBERS. J Cell Biol. 1964 Sep;22:675–696. doi: 10.1083/jcb.22.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenn W. O. A quantitative comparison between the energy liberated and the work performed by the isolated sartorius muscle of the frog. J Physiol. 1923 Dec 28;58(2-3):175–203. doi: 10.1113/jphysiol.1923.sp002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenn W. O., Marsh B. S. Muscular force at different speeds of shortening. J Physiol. 1935 Nov 22;85(3):277–297. doi: 10.1113/jphysiol.1935.sp003318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenn W. O. The relation between the work performed and the energy liberated in muscular contraction. J Physiol. 1924 May 23;58(6):373–395. doi: 10.1113/jphysiol.1924.sp002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields R. W., Faber J. J. Biophysical analysis of the mechanical properties of the sarcolemma. Can J Physiol Pharmacol. 1970 Jun;48(6):394–404. doi: 10.1139/y70-062. [DOI] [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. Proceedings: Mechanism of early tension recovery after a quick release in tetanized muscle fibres. J Physiol. 1974 Jul;240(2):42P–43P. [PubMed] [Google Scholar]
- Gelfan S. The submaximal responses of the single muscle fibre. J Physiol. 1933 Dec 30;80(3):285–295. doi: 10.1113/jphysiol.1933.sp003089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert C., Kretzschmar K. M., Wilkie D. R., Woledge R. C. Chemical change and energy output during muscular contraction. J Physiol. 1971 Oct;218(1):163–193. doi: 10.1113/jphysiol.1971.sp009609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Serratos H. Inward spread of activation in vertebrate muscle fibres. J Physiol. 1971 Feb;212(3):777–799. doi: 10.1113/jphysiol.1971.sp009356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. M., Huxley A. F., Julian F. J. Tension development in highly stretched vertebrate muscle fibres. J Physiol. 1966 May;184(1):143–169. doi: 10.1113/jphysiol.1966.sp007908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. M., Huxley A. F., Julian F. J. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol. 1966 May;184(1):170–192. doi: 10.1113/jphysiol.1966.sp007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANSON J., HUXLEY H. E. Structural basis of the cross-striations in muscle. Nature. 1953 Sep 19;172(4377):530–532. doi: 10.1038/172530b0. [DOI] [PubMed] [Google Scholar]
- HILL A. V. THE EFFECT OF LOAD ON THE HEAT OF SHORTENING OF MUSCLE. Proc R Soc Lond B Biol Sci. 1964 Jan 14;159:297–318. doi: 10.1098/rspb.1964.0004. [DOI] [PubMed] [Google Scholar]
- HILL A. V. The heat of activation and the heat of shortening in a muscle twitch. Proc R Soc Lond B Biol Sci. 1949 Jun 23;136(883):195–211. doi: 10.1098/rspb.1949.0019. [DOI] [PubMed] [Google Scholar]
- HILL A. V. Work and heat in a muscle twitch. Proc R Soc Lond B Biol Sci. 1949 Jun 23;136(883):220–228. doi: 10.1098/rspb.1949.0021. [DOI] [PubMed] [Google Scholar]
- HILL D. K. THE SPACE ACCESSIBLE TO ALBUMIN WITHIN THE STRIATED MUSCLE FIBRE OF THE TOAD. J Physiol. 1964 Dec;175:275–294. doi: 10.1113/jphysiol.1964.sp007517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F. A high-power interference microscope. J Physiol. 1954 Jul 28;125(1):11–3P. [PubMed] [Google Scholar]
- HUXLEY A. F. Applications of an interference microscope. J Physiol. 1952 Aug;117(4):52P–53P. [PubMed] [Google Scholar]
- HUXLEY A. F., GORDON A. M. Striation patterns in active and passive shortening of muscle. Nature. 1962 Jan 20;193:280–281. doi: 10.1038/193280b0. [DOI] [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- HUXLEY A. F., NIEDERGERKE R. Measurement of the striations of isolated muscle fibres with the interference microscope. J Physiol. 1958 Dec 30;144(3):403–425. doi: 10.1113/jphysiol.1958.sp006110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F., NIEDERGERKE R. Structural changes in muscle during contraction; interference microscopy of living muscle fibres. Nature. 1954 May 22;173(4412):971–973. doi: 10.1038/173971a0. [DOI] [PubMed] [Google Scholar]
- HUXLEY A. F., PEACHEY L. D. The maximum length for contraction in vertebrate straiated muscle. J Physiol. 1961 Apr;156:150–165. doi: 10.1113/jphysiol.1961.sp006665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F., TAYLOR R. E. Function of Krause's membrane. Nature. 1955 Dec 3;176(4492):1068–1068. doi: 10.1038/1761068a0. [DOI] [PubMed] [Google Scholar]
- HUXLEY A. F., TAYLOR R. E. Local activation of striated muscle fibres. J Physiol. 1958 Dec 30;144(3):426–441. doi: 10.1113/jphysiol.1958.sp006111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY H. E. EVIDENCE FOR CONTINUITY BETWEEN THE CENTRAL ELEMENTS OF THE TRIADS AND EXTRACELLULAR SPACE IN FROG SARTORIUS MUSCLE. Nature. 1964 Jun 13;202:1067–1071. doi: 10.1038/2021067b0. [DOI] [PubMed] [Google Scholar]
- HUXLEY H. E. Electron microscope studies of the organisation of the filaments in striated muscle. Biochim Biophys Acta. 1953 Nov;12(3):387–394. doi: 10.1016/0006-3002(53)90156-5. [DOI] [PubMed] [Google Scholar]
- HUXLEY H. E., HANSON J. Quantitative studies on the structure of cross-striated myofibrils. I. Investigations by interference microscopy. Biochim Biophys Acta. 1957 Feb;23(2):229–249. doi: 10.1016/0006-3002(57)90325-6. [DOI] [PubMed] [Google Scholar]
- HUXLEY H. E. STRUCTURAL ARRANGEMENTS AND THE CONTRACTION MECHANISM IN STRIATED MUSCLE. Proc R Soc Lond B Biol Sci. 1964 Oct 27;160:442–448. doi: 10.1098/rspb.1964.0054. [DOI] [PubMed] [Google Scholar]
- HUXLEY H. E. The double array of filaments in cross-striated muscle. J Biophys Biochem Cytol. 1957 Sep 25;3(5):631–648. doi: 10.1083/jcb.3.5.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY H. E. X-ray analysis and the problem of muscle. Proc R Soc Lond B Biol Sci. 1953 Mar 11;141(902):59–62. doi: 10.1098/rspb.1953.0017. [DOI] [PubMed] [Google Scholar]
- HUXLEY H., HANSON J. Changes in the cross-striations of muscle during contraction and stretch and their structural interpretation. Nature. 1954 May 22;173(4412):973–976. doi: 10.1038/173973a0. [DOI] [PubMed] [Google Scholar]
- Hanson J. Recent x-ray diffraction studies of muscle. Q Rev Biophys. 1968 Jun;1(2):177–216. doi: 10.1017/s0033583500000536. [DOI] [PubMed] [Google Scholar]
- Huxley A. F. A note suggesting that the cross-bridge attachment during muscle contraction may take place in two stages. Proc R Soc Lond B Biol Sci. 1973 Feb 27;183(1070):83–86. doi: 10.1098/rspb.1973.0006. [DOI] [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. A quick phase in the series-elastic component of striated muscle, demonstrated in isolated fibres from the frog. J Physiol. 1970 Jun;208(2):52P–53P. [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Mechanical properties of the cross-bridges of frog striated muscle. J Physiol. 1971 Oct;218 (Suppl):59P–60P. [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971 Oct 22;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Brown W. The low-angle x-ray diagram of vertebrate striated muscle and its behaviour during contraction and rigor. J Mol Biol. 1967 Dec 14;30(2):383–434. doi: 10.1016/s0022-2836(67)80046-9. [DOI] [PubMed] [Google Scholar]
- Huxley H. E. The structural basis of muscular contraction. Proc R Soc Lond B Biol Sci. 1971 Jun 29;178(1051):131–149. doi: 10.1098/rspb.1971.0057. [DOI] [PubMed] [Google Scholar]
- Jöbsis F. F., O'Connor M. J. Calcium release and reabsorption in the sartorius muscle of the toad. Biochem Biophys Res Commun. 1966 Oct 20;25(2):246–252. doi: 10.1016/0006-291x(66)90588-2. [DOI] [PubMed] [Google Scholar]
- Katz B. The relation between force and speed in muscular contraction. J Physiol. 1939 Jun 14;96(1):45–64. doi: 10.1113/jphysiol.1939.sp003756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushmerick M. J., Larson R. E., Davies R. E. The chemical energetics of muscle contraction. I. Activation heat, heat of shortening and ATP utilization for activation-relaxation processes. Proc R Soc Lond B Biol Sci. 1969 Dec 23;174(1036):293–313. doi: 10.1098/rspb.1969.0095. [DOI] [PubMed] [Google Scholar]
- Lymn R. W., Taylor E. W. Mechanism of adenosine triphosphate hydrolysis by actomyosin. Biochemistry. 1971 Dec 7;10(25):4617–4624. doi: 10.1021/bi00801a004. [DOI] [PubMed] [Google Scholar]
- MOMMAERTS W. F. H. M. The scattering of light in myosin solutions. I. The angular dissymmetry and the molecular length. J Biol Chem. 1951 Feb;188(2):553–557. [PubMed] [Google Scholar]
- MOMMAERTS W. F. The effect of adenosinetriphosphate upon actomyosin solutions, studied with a recording dual beam light-scattering photometer. J Gen Physiol. 1956 Jul 20;39(6):821–830. doi: 10.1085/jgp.39.6.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORALES M., BOTTS J. A model for the elementary process in muscle action. Arch Biochem Biophys. 1952 Jun;37(2):283–300. doi: 10.1016/0003-9861(52)90193-8. [DOI] [PubMed] [Google Scholar]
- Matsubara I., Elliott G. F. X-ray diffraction studies on skinned single fibres of frog skeletal muscle. J Mol Biol. 1972 Dec 30;72(3):657–669. doi: 10.1016/0022-2836(72)90183-0. [DOI] [PubMed] [Google Scholar]
- McClare C. W. A "molecular energy" muscle model. J Theor Biol. 1972 Jun;35(3):569–595. doi: 10.1016/0022-5193(72)90151-8. [DOI] [PubMed] [Google Scholar]
- McClare C. W. Chemical machines, Maxwell's demon and living organisms. J Theor Biol. 1971 Jan;30(1):1–34. doi: 10.1016/0022-5193(71)90033-6. [DOI] [PubMed] [Google Scholar]
- NEEDHAM D. M. Myosin and adenosinetriphosphate in relation to muscle contraction. Biochim Biophys Acta. 1950 Jan;4(1-3):42–49. doi: 10.1016/0006-3002(50)90007-2. [DOI] [PubMed] [Google Scholar]
- Oplatka A. On the mechanochemistry of muscular contraction. J Theor Biol. 1972 Feb;34(2):379–403. doi: 10.1016/0022-5193(72)90169-5. [DOI] [PubMed] [Google Scholar]
- PAGE S. G., HUXLEY H. E. FILAMENT LENGTHS IN STRIATED MUSCLE. J Cell Biol. 1963 Nov;19:369–390. doi: 10.1083/jcb.19.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PODOLSKY R. J. Kinetics of muscular contraction: the approach to the steady state. Nature. 1960 Nov 19;188:666–668. doi: 10.1038/188666a0. [DOI] [PubMed] [Google Scholar]
- PODOLSKY R. J. THE MAXIMUM SARCOMERE LENGTH FOR CONTRACTION OF ISOLATED MYOFIBRILS. J Physiol. 1964 Jan;170:110–123. doi: 10.1113/jphysiol.1964.sp007317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PODOLSKY R. J. The chemical thermodynamics and molecular mechanism of muscular contraction. Ann N Y Acad Sci. 1959 Feb 6;72(12):522–537. doi: 10.1111/j.1749-6632.1959.tb44180.x. [DOI] [PubMed] [Google Scholar]
- POLISSAR M. J. Physical chemistry of contractile process in muscle. IV. Estimates of size of contractile unit. Am J Physiol. 1952 Mar;168(3):805–811. doi: 10.1152/ajplegacy.1952.168.3.805. [DOI] [PubMed] [Google Scholar]
- Page S. G. Fine structure of tortoise skeletal muscle. J Physiol. 1968 Aug;197(3):709–715. doi: 10.1113/jphysiol.1968.sp008583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page S. G. Structure and some contractile properties of fast and slow muscles of the chicken. J Physiol. 1969 Nov;205(1):131–145. doi: 10.1113/jphysiol.1969.sp008956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolsky R. J., Nolan A. C., Zaveler S. A. Cross-bridge properties derived from muscle isotonic velocity transients. Proc Natl Acad Sci U S A. 1969 Oct;64(2):504–511. doi: 10.1073/pnas.64.2.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle J. W. The contractile mechanism of insect fibrillar muscle. Prog Biophys Mol Biol. 1967;17:1–60. doi: 10.1016/0079-6107(67)90003-x. [DOI] [PubMed] [Google Scholar]
- RAMAMOORTHY B., CHATTERJEE B. G., DAKSHINAMURTI C., GULATI K. C. A rapid routine method for the estimation of nicotine in tobacco. Nature. 1952 Jan 19;169(4290):112–112. doi: 10.1038/169112a0. [DOI] [PubMed] [Google Scholar]
- RICE R. V. Conformation of individual macromolecular particles from myosin solution. Biochim Biophys Acta. 1961 Sep 30;52:602–604. doi: 10.1016/0006-3002(61)90427-9. [DOI] [PubMed] [Google Scholar]
- ROWE A. J. THE CONTRACTILE PROTEINS OF SKELETAL MUSCLE. Proc R Soc Lond B Biol Sci. 1964 Oct 27;160:437–441. doi: 10.1098/rspb.1964.0053. [DOI] [PubMed] [Google Scholar]
- Rapoport S. I. Mechanical properties of the sarcolemma and myoplasm in frog muscle as a function of sarcomere length. J Gen Physiol. 1972 May;59(5):559–585. doi: 10.1085/jgp.59.5.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgway E. B., Ashley C. C. Calcium transients in single muscle fibers. Biochem Biophys Res Commun. 1967 Oct 26;29(2):229–234. doi: 10.1016/0006-291x(67)90592-x. [DOI] [PubMed] [Google Scholar]
- Rüdel R., Taylor S. R. Striated muscle fibers: facilitation of contraction at short lengths by caffeine. Science. 1971 Apr 23;172(3981):387–389. doi: 10.1126/science.172.3981.387. [DOI] [PubMed] [Google Scholar]
- SHIMOMURA O., JOHNSON F. H., SAIGA Y. Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan, Aequorea. J Cell Comp Physiol. 1962 Jun;59:223–239. doi: 10.1002/jcp.1030590302. [DOI] [PubMed] [Google Scholar]
- SJOSTRAND F. S. The connections between A- and I-band filaments in striated frog muscle. J Ultrastruct Res. 1962 Oct;7:225–246. doi: 10.1016/s0022-5320(62)90020-5. [DOI] [PubMed] [Google Scholar]
- SPENCER M., WORTHINGTON C. R. A hypothesis of contraction in striated muscle. Nature. 1960 Jul 30;187:388–391. doi: 10.1038/187388a0. [DOI] [PubMed] [Google Scholar]
- STRICKHOLM A. Excitation currents and impedence of a small electrically isolated area of the muscle cell surface. J Cell Comp Physiol. 1962 Oct;60:149–167. doi: 10.1002/jcp.1030600205. [DOI] [PubMed] [Google Scholar]
- Schneider M. F., Chandler W. K. Voltage dependent charge movement of skeletal muscle: a possible step in excitation-contraction coupling. Nature. 1973 Mar 23;242(5395):244–246. doi: 10.1038/242244a0. [DOI] [PubMed] [Google Scholar]
- Strickholm A. Local sarcomere contraction in fast muscle fibres. Nature. 1966 Nov 19;212(5064):835–836. doi: 10.1038/212835a0. [DOI] [PubMed] [Google Scholar]
- Sugi H., Ochi R. The mode of transverse spread of contraction initiated by local activation in single frog muscle fibers. J Gen Physiol. 1967 Oct;50(9):2167–2176. doi: 10.1085/jgp.50.9.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor E. W. Chemistry of muscle contraction. Annu Rev Biochem. 1972;41(10):577–616. doi: 10.1146/annurev.bi.41.070172.003045. [DOI] [PubMed] [Google Scholar]
- Taylor S. R., Rüdel R. Striated muscle fibers: inactivation of contraction induced by shortening. Science. 1970 Feb 6;167(3919):882–884. doi: 10.1126/science.167.3919.882. [DOI] [PubMed] [Google Scholar]
- Ullrick W. C. A theory of contraction for striated muscle. J Theor Biol. 1967 Apr;15(1):53–69. doi: 10.1016/0022-5193(67)90043-4. [DOI] [PubMed] [Google Scholar]
- Walcott B., Ridgway E. B. The ultrastructure of myosin-extracted striated muscle fibers. Am Zool. 1967 Aug;7(3):499–504. doi: 10.1093/icb/7.3.499. [DOI] [PubMed] [Google Scholar]
- Wilkie D. R. Heat work and phosphorylcreatine break-down in muscle. J Physiol. 1968 Mar;195(1):157–183. doi: 10.1113/jphysiol.1968.sp008453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woledge R. C. The energetics of tortoise muscle. J Physiol. 1968 Aug;197(3):685–707. doi: 10.1113/jphysiol.1968.sp008582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L. C., Dowben R. M., Kornacker K. The molecular mechanism of force generation in striated muscle. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1199–1205. doi: 10.1073/pnas.66.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZOBEL C. R., CARLSON F. D. An electron microscopic investigation of myosin and some of its aggregates. J Mol Biol. 1963 Jul;7:78–89. doi: 10.1016/s0022-2836(63)80020-0. [DOI] [PubMed] [Google Scholar]




