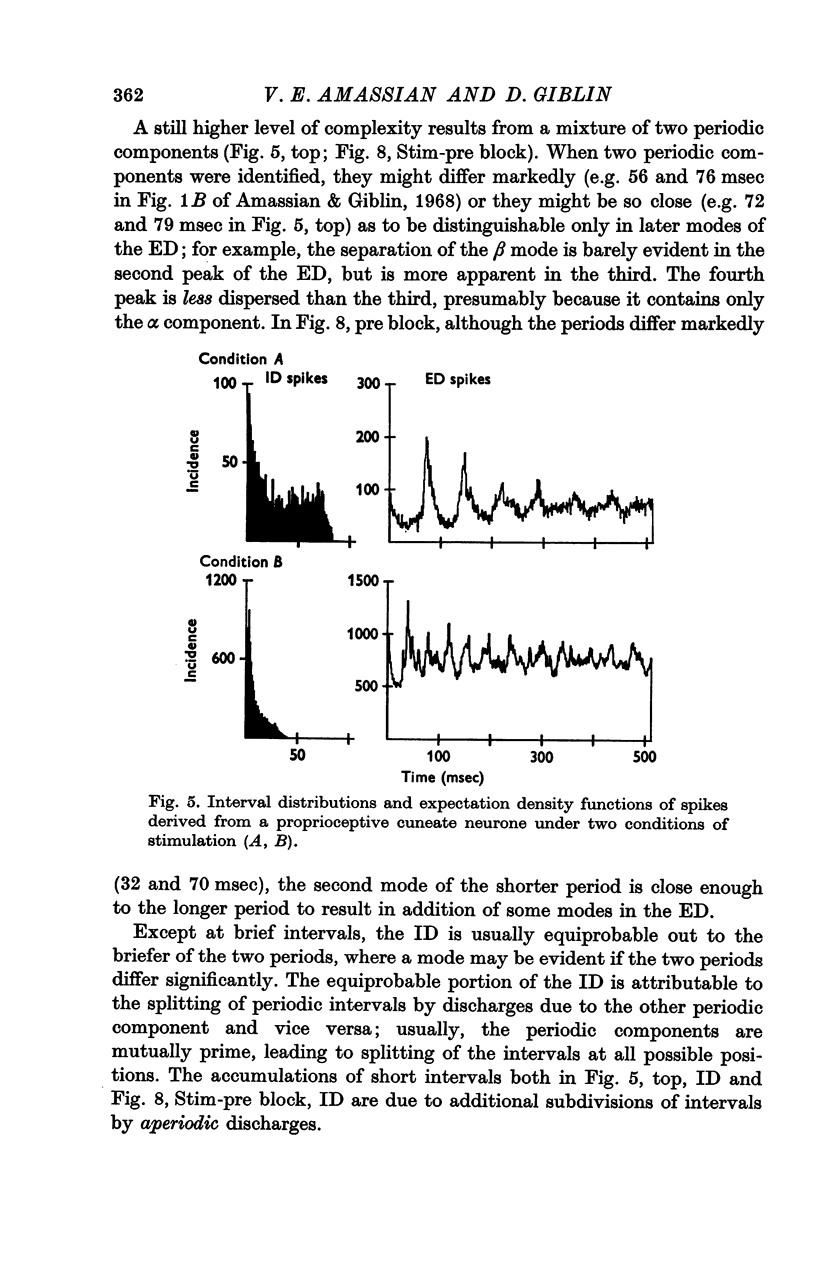
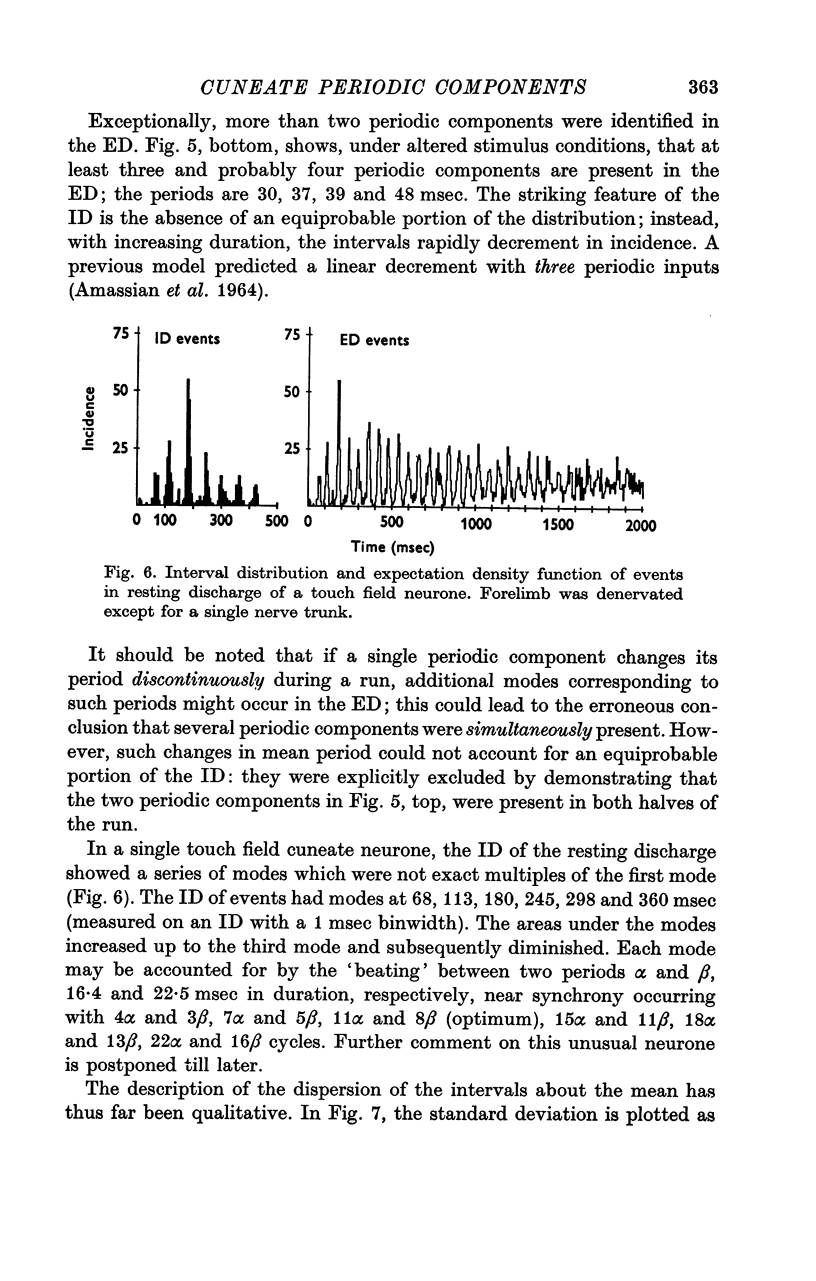
Abstract
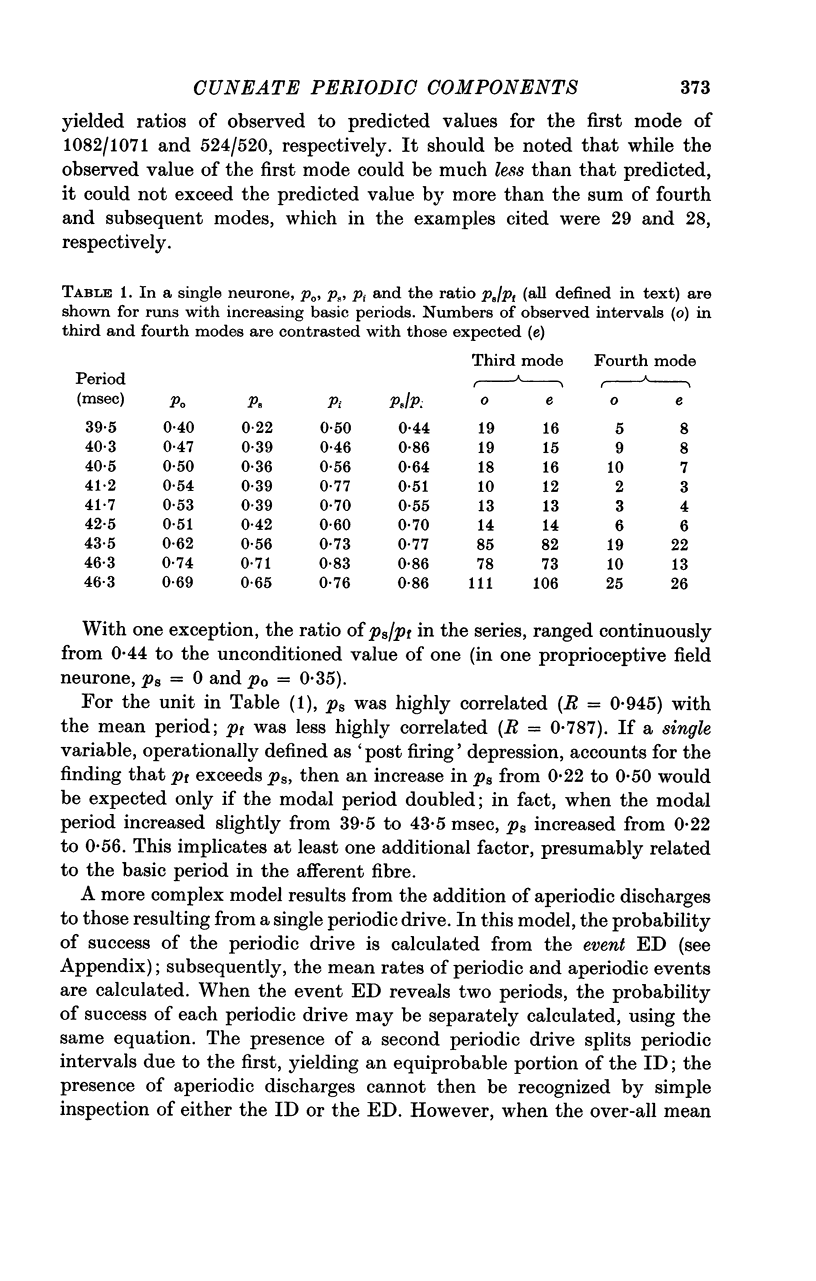
1. Individual cuneate touch, touch-hair and proprioceptive neurones often display periodic components in their steady-state resting or evoked discharges. The periods may be visible in the interspike interval distribution or may be revealed only by the expectation density function. Several levels of complexity were identified; from one to four mutually prime, periodic components may be present, with or without an aperiodic component.
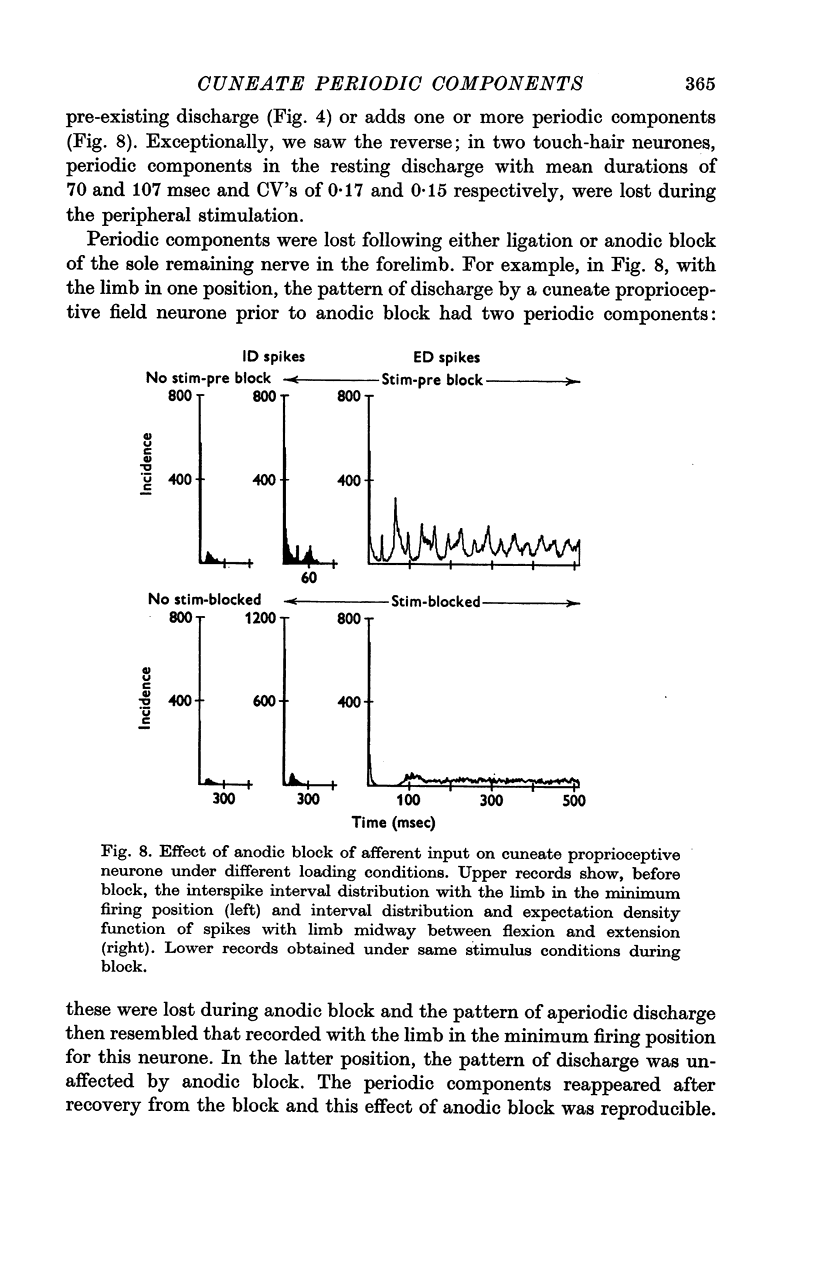
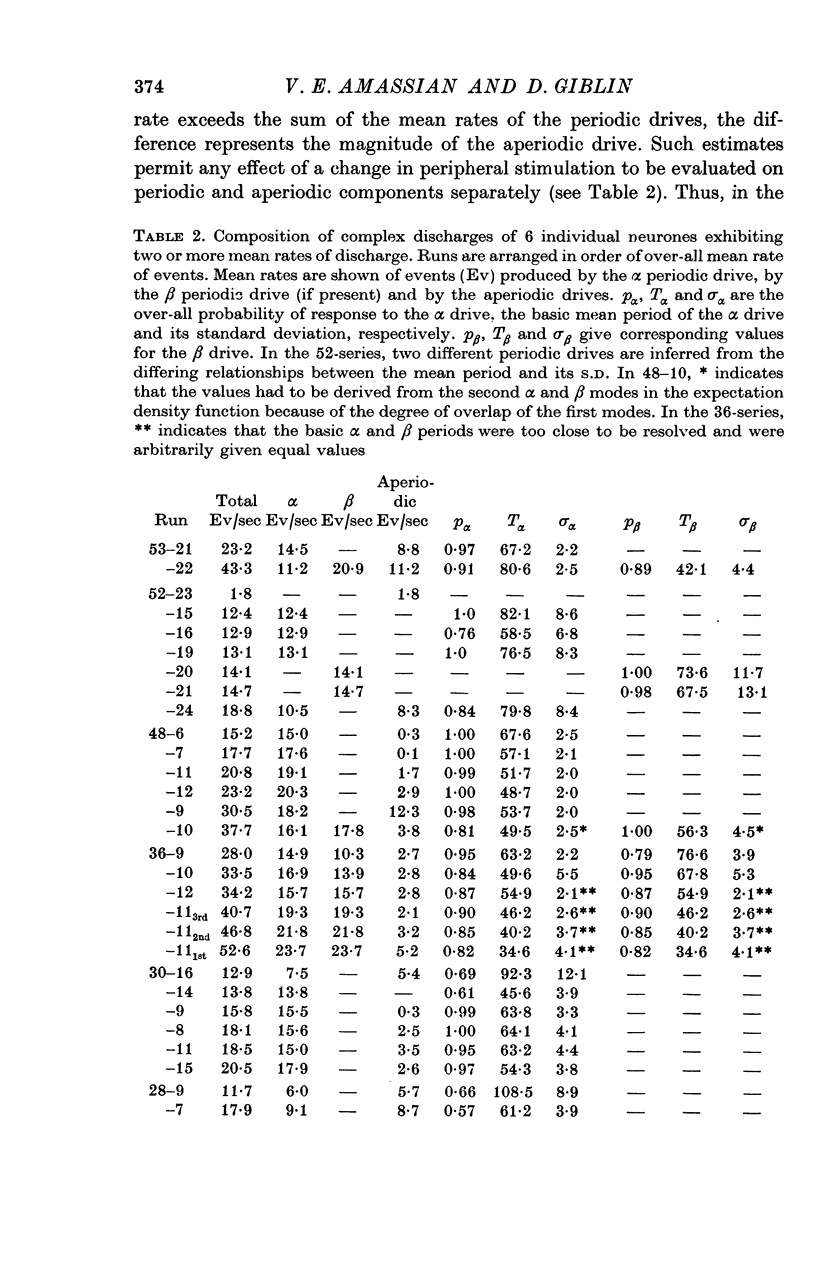
2. The periodic components usually depend on the peripheral input, as shown by their introduction or modification by peripheral stimulation and by their disappearance following deafferentation.
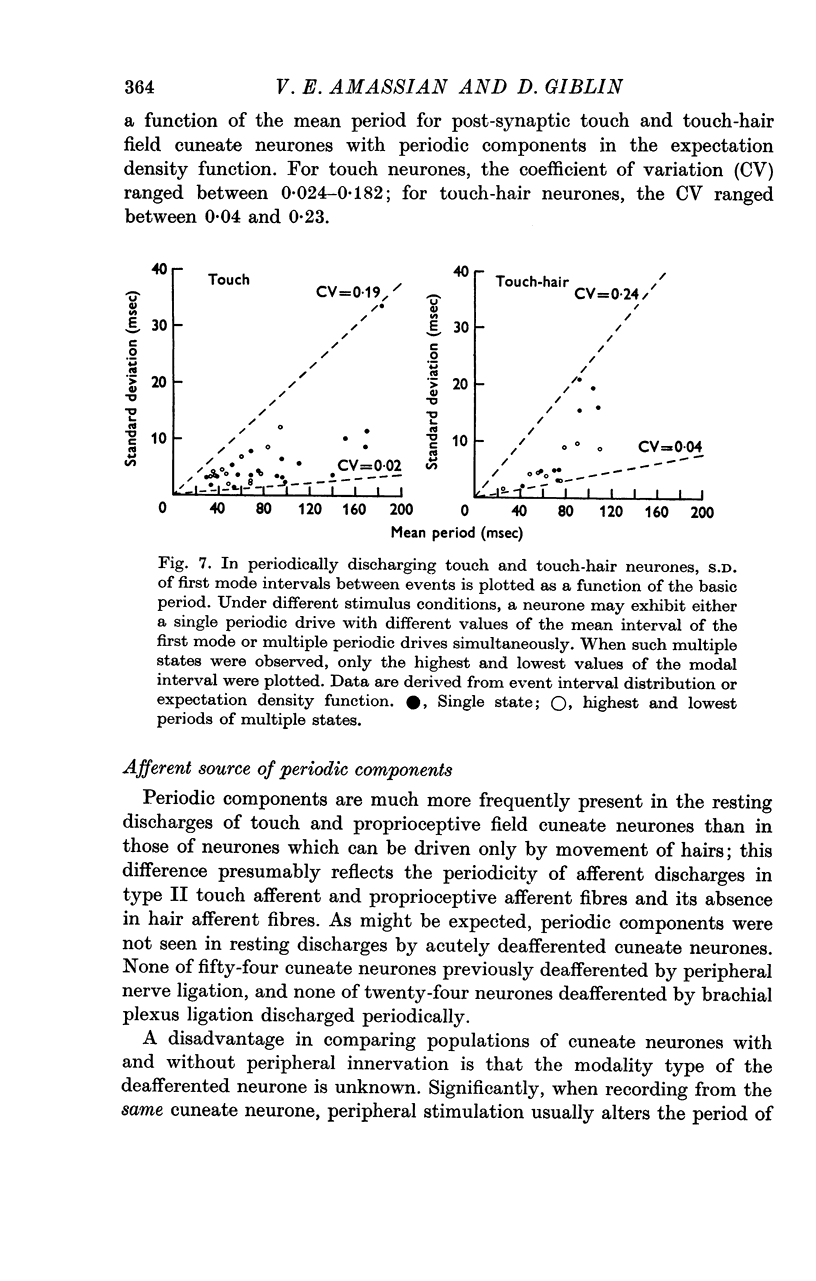
3. The coefficient of variation of periodic discharges by touch and touch-hair neurones was 0·024-0·23 (aperiodic, driven discharges usually had CV's of 0·64-0·77).
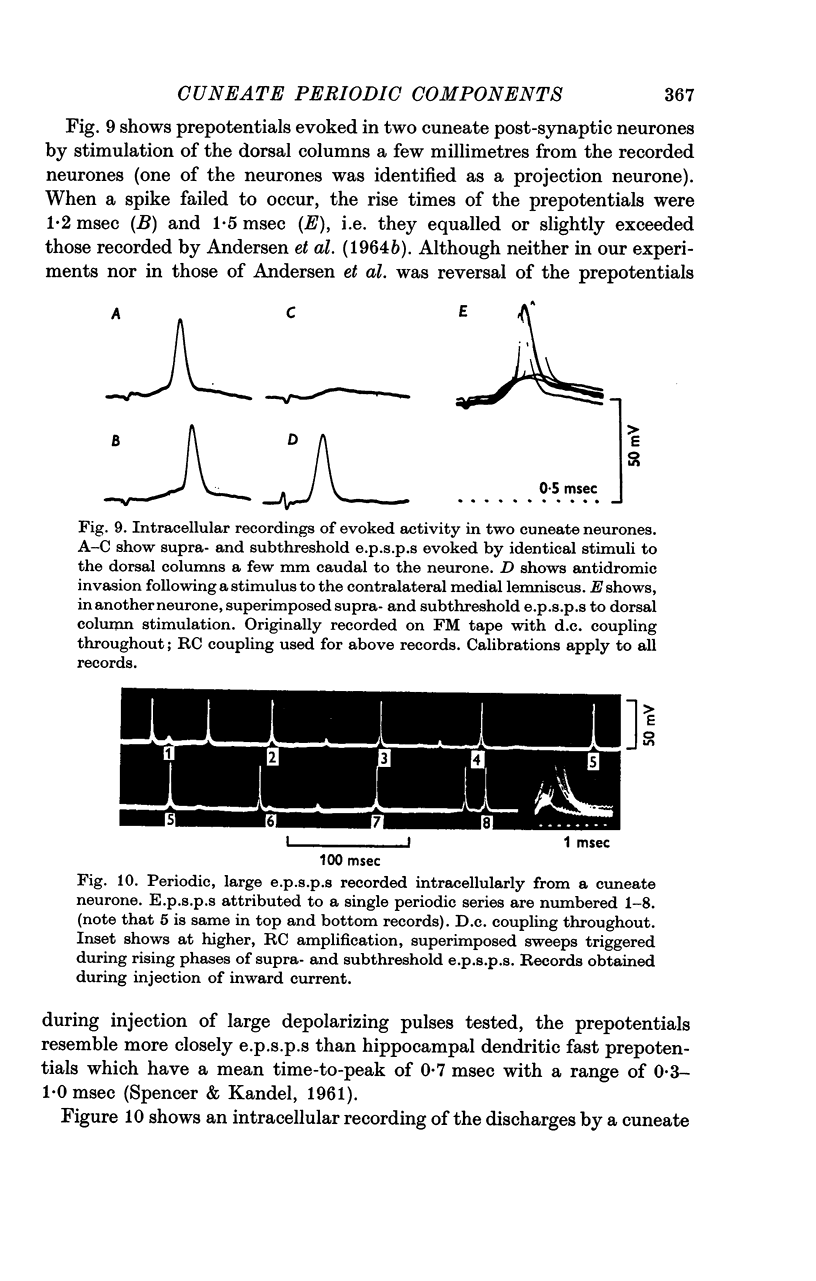
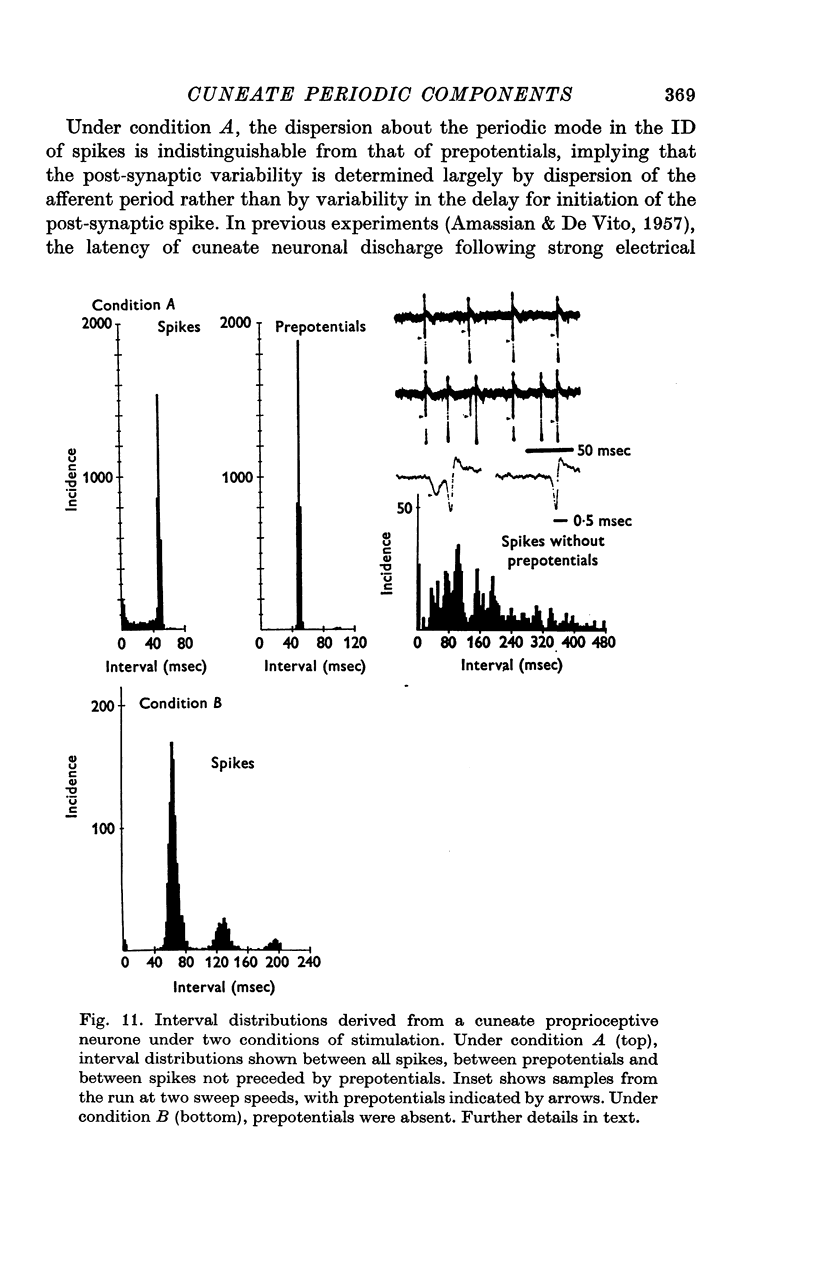
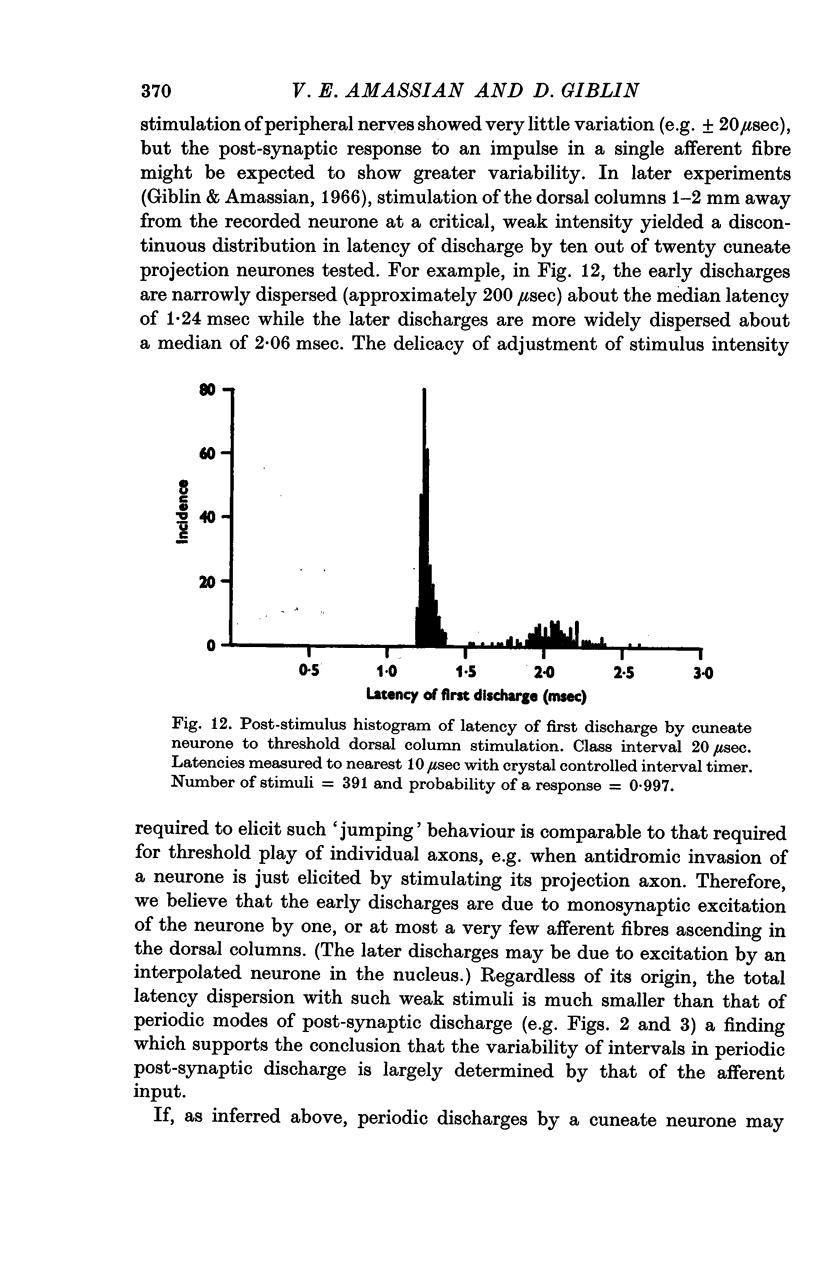
4. The occasional recording extracellularly of a periodically occurring, unitary prepotential preceding the spike and the intracellular recording of a periodic, `giant', unitary e.p.s.p. imply that an individual periodic impulse in an afferent fibre may elicit a post-synaptic discharge. Even with threshold stimulation, the timing of post-synaptic discharge was quite precise.
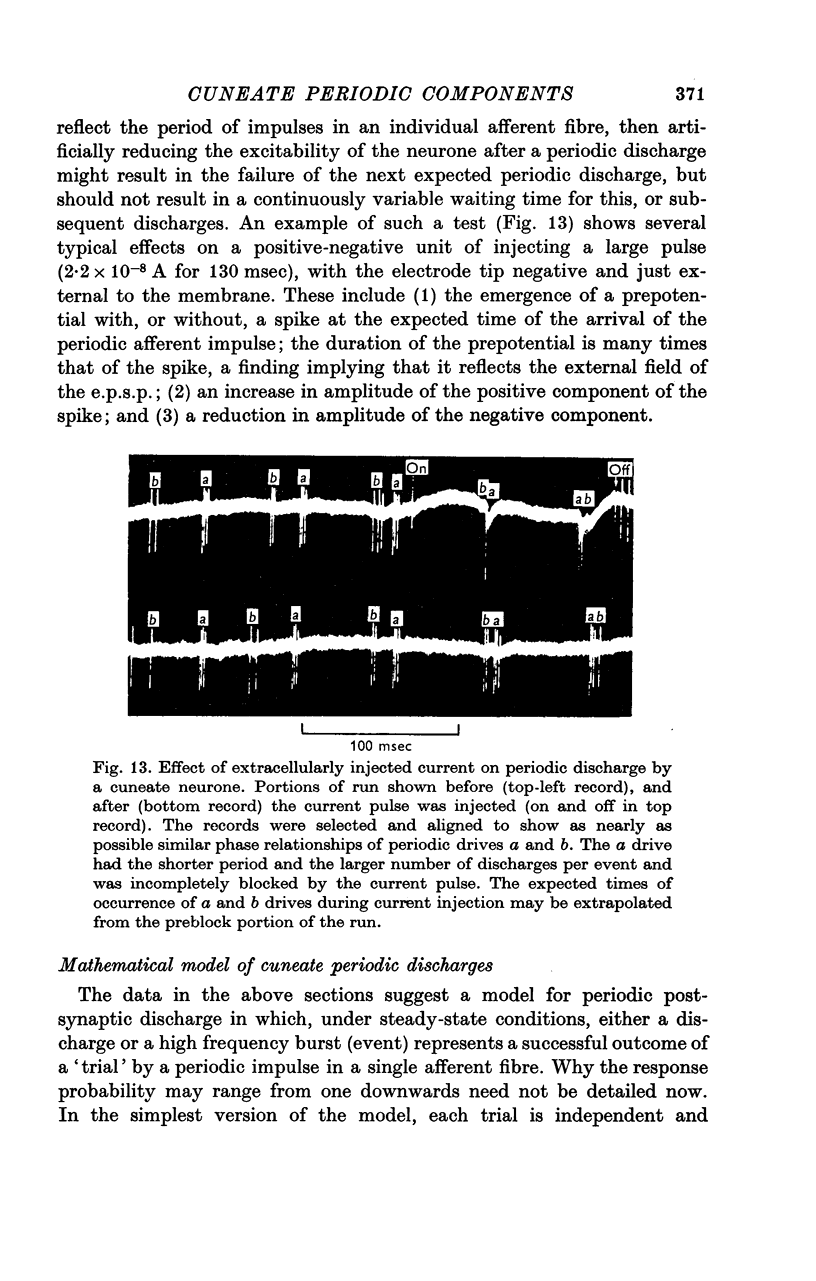
5. The periodicity did not appear to reflect a cycle of subnormality intrinsic to the post-synaptic neurone, because a current pulse injected through a micro-electrode lying just external to the membrane could block the next expected periodic discharge, but did not continuously vary its waiting time.
6. A mathematical model that fits some discharge patterns predicts the interval distribution from an over-all probability of response to the periodic input: each periodic afferent impulse is treated as an independent trial resulting in success (post-synaptic discharge) or failure. Some other interval distributions are fitted by a `conditioned' model in which a success reduces the probability of response at the next periodic trial.
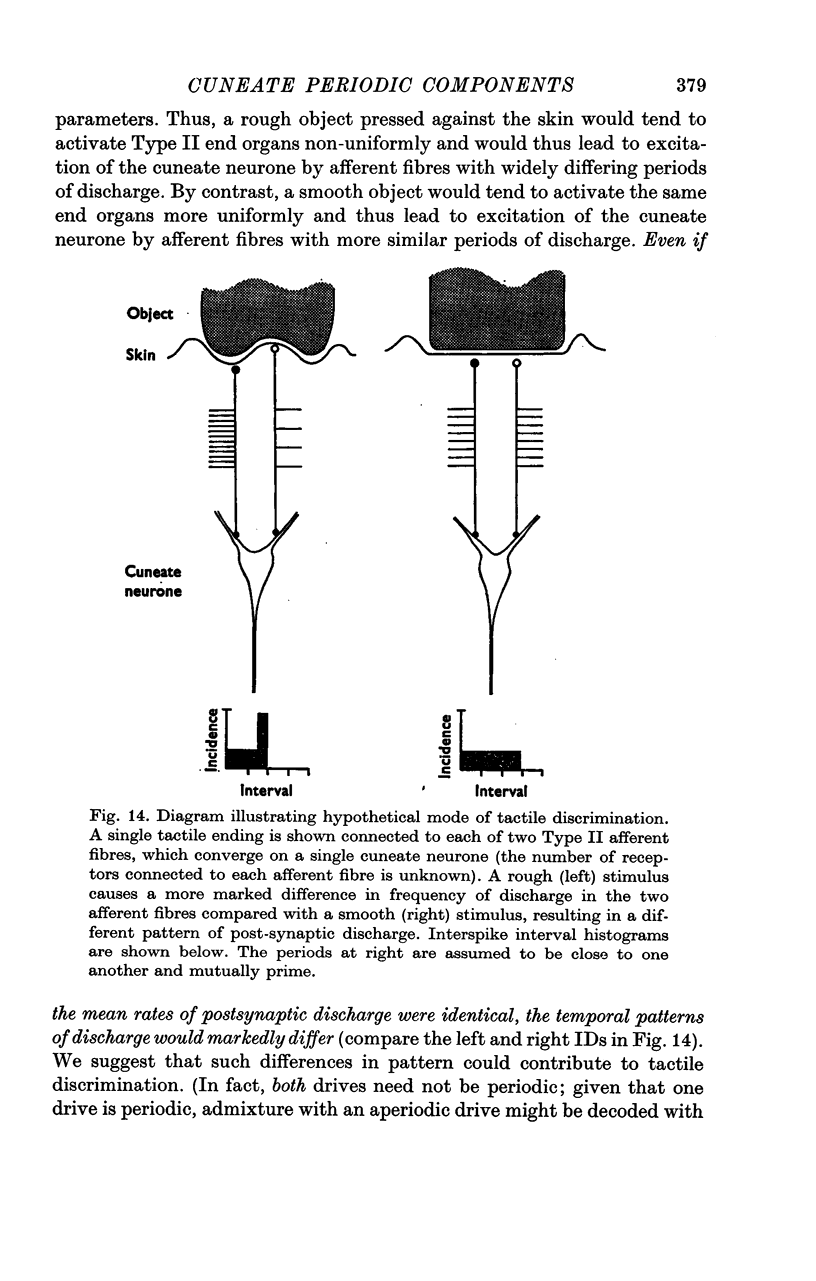
7. The discussion includes a hypothesis of tactile discrimination, in which the output of a sensory relay, e.g. cuneate neurone, signals both the number of afferent fibres converging upon it and the afferent period in each.
Full text
PDF

















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMASSIAN V. E. Evoked single cortical unit activity in the somatic sensory areas. Electroencephalogr Clin Neurophysiol. 1953 Aug;5(3):415–438. doi: 10.1016/0013-4694(53)90084-4. [DOI] [PubMed] [Google Scholar]
- ANDERSEN P., ECCLES J. C., OSHIMA T., SCHMIDT R. F. MECHANISMS OF SYNAPTIC TRANSMISSION IN THE CUNEATE NUCLEUS. J Neurophysiol. 1964 Nov;27:1096–1116. doi: 10.1152/jn.1964.27.6.1096. [DOI] [PubMed] [Google Scholar]
- ANDERSEN P., ECCLES J. C., SCHMIDT R. F., YOKOTA T. IDENTIFICATION OF RELAY CELLS AND INTERNEURONS IN THE CUNEATE NUCLEUS. J Neurophysiol. 1964 Nov;27:1080–1095. doi: 10.1152/jn.1964.27.6.1080. [DOI] [PubMed] [Google Scholar]
- BISHOP P. O., BURKE W., DAVIS R. The identification of single units in central visual pathways. J Physiol. 1962 Aug;162:409–431. doi: 10.1113/jphysiol.1962.sp006942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISHOP P. O., LEVICK W. R., WILLIAMS W. O. STATISTICAL ANALYSIS OF THE DARK DISCHARGE OF LATERAL GENICULATE NEURONES. J Physiol. 1964 Apr;170:598–612. doi: 10.1113/jphysiol.1964.sp007352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron D. H., Matthews B. H. The interpretation of potential changes in the spinal cord. J Physiol. 1938 Apr 14;92(3):276–321. doi: 10.1113/jphysiol.1938.sp003603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G. Cutaneous afferent fibre collaterals in the dorsal columns of the cat. Exp Brain Res. 1968;5(4):293–305. doi: 10.1007/BF00235904. [DOI] [PubMed] [Google Scholar]
- Chambers M. R., Andres K. H., von Duering M., Iggo A. The structure and function of the slowly adapting type II mechanoreceptor in hairy skin. Q J Exp Physiol Cogn Med Sci. 1972 Oct;57(4):417–445. doi: 10.1113/expphysiol.1972.sp002177. [DOI] [PubMed] [Google Scholar]
- Chambers M. R., Iggo A. Slowly-adapting cutaneous mechanoreceptors. J Physiol. 1967 Sep;192(2):26P–27P. [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide E., Fedina L., Jansen J., Lundberg A., Vyklický L. Properties of Clarke's column neurones. Acta Physiol Scand. 1969 Sep-Oct;77(1):125–144. doi: 10.1111/j.1748-1716.1969.tb04558.x. [DOI] [PubMed] [Google Scholar]
- Eide E., Fedina L., Jansen J., Lundberg A., Vyklický L. Unitary components in the activation of Clarke's column neurones. Acta Physiol Scand. 1969 Sep-Oct;77(1):145–158. doi: 10.1111/j.1748-1716.1969.tb04559.x. [DOI] [PubMed] [Google Scholar]
- GORDON G., JUKES M. G. DESCENDING INFLUENCES ON THE EXTEROCEPTIVE ORGANIZATIONS OF THE CAT'S GRACILE NUCLEUS. J Physiol. 1964 Sep;173:291–319. doi: 10.1113/jphysiol.1964.sp007457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORDON G., JUKES M. G. DUAL ORGANIZATION OF THE EXTEROCEPTIVE COMPONENTS OF THE CAT'S GRACILE NUCLEUS. J Physiol. 1964 Sep;173:263–290. doi: 10.1113/jphysiol.1964.sp007456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORDON G., PAINE C. H. Functional organization in nucleus gracilis of the cat. J Physiol. 1960 Sep;153:331–349. doi: 10.1113/jphysiol.1960.sp006537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo A., Krnjević K., Schwartz S. Patterns of firing in cuneate neurones and some effects of Flaxedil. Exp Brain Res. 1968;5(2):87–101. doi: 10.1007/BF00238699. [DOI] [PubMed] [Google Scholar]
- Iggo A., Muir A. R. The structure and function of a slowly adapting touch corpuscle in hairy skin. J Physiol. 1969 Feb;200(3):763–796. doi: 10.1113/jphysiol.1969.sp008721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JABBUR S. J., TOWE A. L. Cortical excitation of neurons in dorsal column nuclei of cat, including an analysis of pathways. J Neurophysiol. 1961 Sep;24:499–509. doi: 10.1152/jn.1961.24.5.499. [DOI] [PubMed] [Google Scholar]
- Jansen J. K., Nicolaysen K., Rudjord T. Discharge pattern of neurons of the dorsal spinocerebellar tract activated by static extension of primary endings of muscle spindles. J Neurophysiol. 1966 Nov;29(6):1061–1086. doi: 10.1152/jn.1966.29.6.1061. [DOI] [PubMed] [Google Scholar]
- KRUGER L., SIMINOFF R., WITKOVSKY P. Single neuron analysis of dorsal column nuclei and spinal nucleus of trigeminal in cat. J Neurophysiol. 1961 Jul;24:333–349. doi: 10.1152/jn.1961.24.4.333. [DOI] [PubMed] [Google Scholar]
- KUNO M. MECHANSIM OF FACILITATION AND DEPRESSION OF THE EXCITATORY SYNAPTIC POTENTIAL IN SPINAL MOTONEURONES. J Physiol. 1964 Dec;175:100–112. doi: 10.1113/jphysiol.1964.sp007505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUNO M. QUANTAL COMPONENTS OF EXCITATORY SYNAPTIC POTENTIALS IN SPINAL MOTONEURONES. J Physiol. 1964 Dec;175:81–99. doi: 10.1113/jphysiol.1964.sp007504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M., Miyahara J. T. Factors responsible for multiple discharge of neurons in Clarke's column. J Neurophysiol. 1968 Jul;31(4):624–638. doi: 10.1152/jn.1968.31.4.624. [DOI] [PubMed] [Google Scholar]
- MOUNTCASTLE V. B. Modality and topographic properties of single neurons of cat's somatic sensory cortex. J Neurophysiol. 1957 Jul;20(4):408–434. doi: 10.1152/jn.1957.20.4.408. [DOI] [PubMed] [Google Scholar]
- PERL E. R., WHITLOCK D. G., GENTRY J. R. Cutaneous projection to second-order neurons of the dorsal column system. J Neurophysiol. 1962 May;25:337–358. doi: 10.1152/jn.1962.25.3.337. [DOI] [PubMed] [Google Scholar]
- POGGIO G. F., VIERNSTEIN L. J. TIME SERIES ANALYSIS OF IMPULSE SEQUENCES OF THALAMIC SOMATIC SENSORY NEURONS. J Neurophysiol. 1964 Jul;27:517–545. doi: 10.1152/jn.1964.27.4.517. [DOI] [PubMed] [Google Scholar]
- Petit D., Burgess P. R. Dorsal column projection of receptors in cat hairy skin supplied by myelinated fibers. J Neurophysiol. 1968 Nov;31(6):849–855. doi: 10.1152/jn.1968.31.6.849. [DOI] [PubMed] [Google Scholar]
- RALL W. Branching dendritic trees and motoneuron membrane resistivity. Exp Neurol. 1959 Nov;1:491–527. doi: 10.1016/0014-4886(59)90046-9. [DOI] [PubMed] [Google Scholar]
- Sanderson A. C., Kozak W. M., Calvert T. W. Distribution coding in the visual pathway. Biophys J. 1973 Mar;13(3):218–244. doi: 10.1016/S0006-3495(73)85982-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenga E., Verveen A. A. Membrane noise and ion transport in the node of Ranvier. Biomembranes. 1972;3:473–482. doi: 10.1007/978-1-4684-0961-1_32. [DOI] [PubMed] [Google Scholar]
- Uddenberg N. Functional organization of long, second-order afferents in the dorsal funiculus. Exp Brain Res. 1968;4(4):377–382. doi: 10.1007/BF00235702. [DOI] [PubMed] [Google Scholar]
- WINTER D. L. N. GRACILIS OF CAT. FUNCTIONAL ORGANIZATION AND CORTICOFUGAL EFFECTS. J Neurophysiol. 1965 Jan;28:48–70. doi: 10.1152/jn.1965.28.1.48. [DOI] [PubMed] [Google Scholar]
- Walberg F. The fine structure of the cuneate nucleus in normal cats and following interruption of afferent fibres. An electron microscopical study with particular reference to findings made in glees and nauta sections. Exp Brain Res. 1966;2(2):107–128. doi: 10.1007/BF00240401. [DOI] [PubMed] [Google Scholar]
- ten Hoopen M. Impulse sequences of thalamic neurons--an attempted theoretical interpretation. Brain Res. 1966 Dec;3(2):123–140. doi: 10.1016/0006-8993(66)90071-0. [DOI] [PubMed] [Google Scholar]


