Abstract
1. The purpose of this study was to study in rats and rabbits the ontogenetic development of the blood—brain barrier to macromolecules and the ontogenetic development of concentration differences between plasma and cerebrospinal fluid for ions which are known to be transported actively across the choroid plexus and the blood—brain barrier.
2. By comparing the development of concentration differences for ions with the development of the blood—brain barrier to macromolecules we wanted to evaluate an eventual relationship between the development of these two functions of the blood—brain barrier.
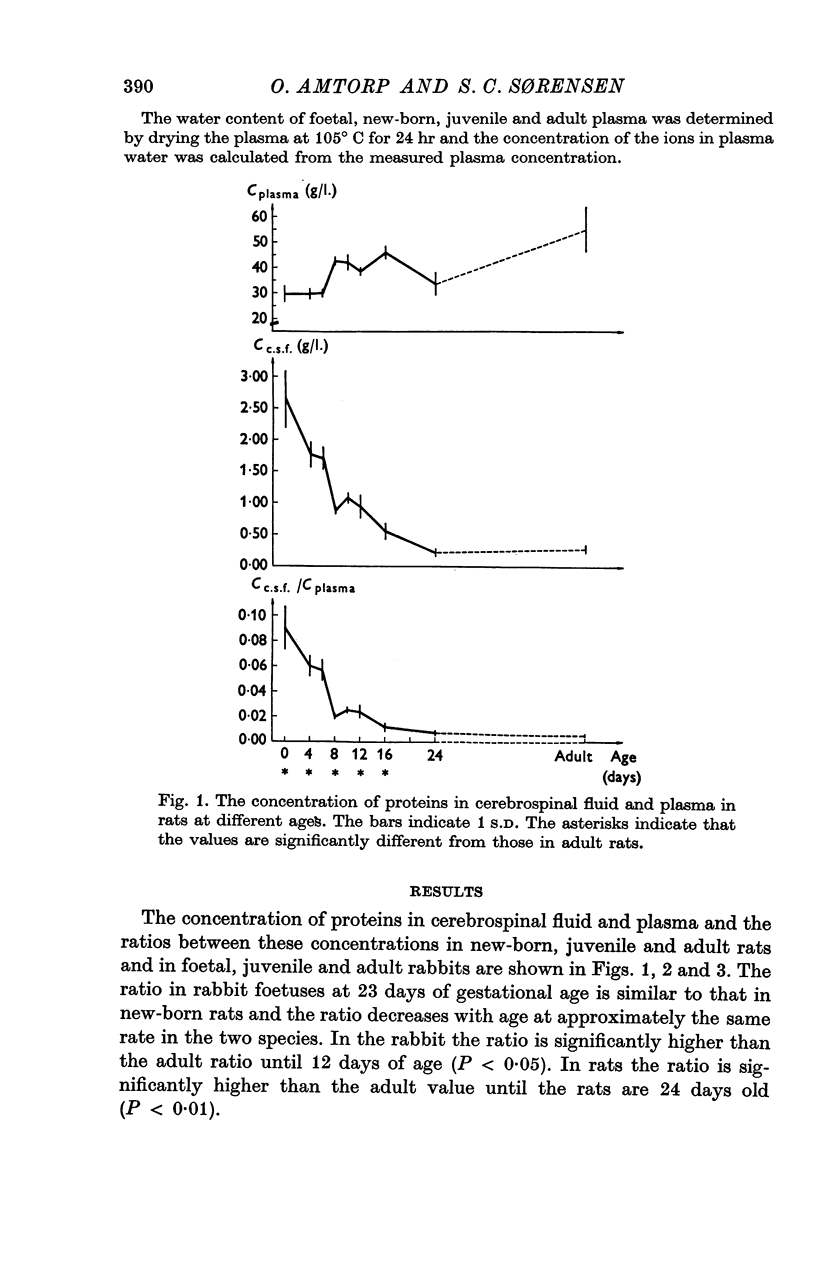
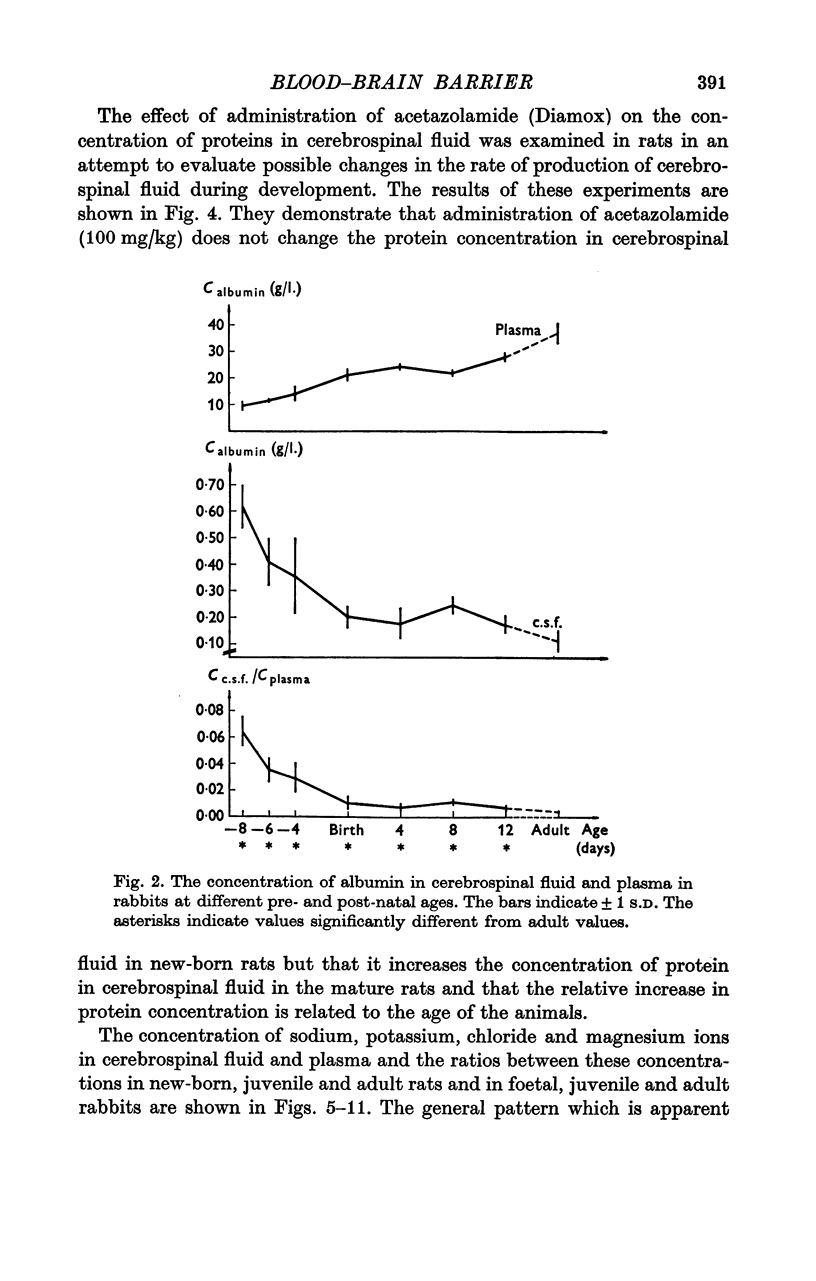
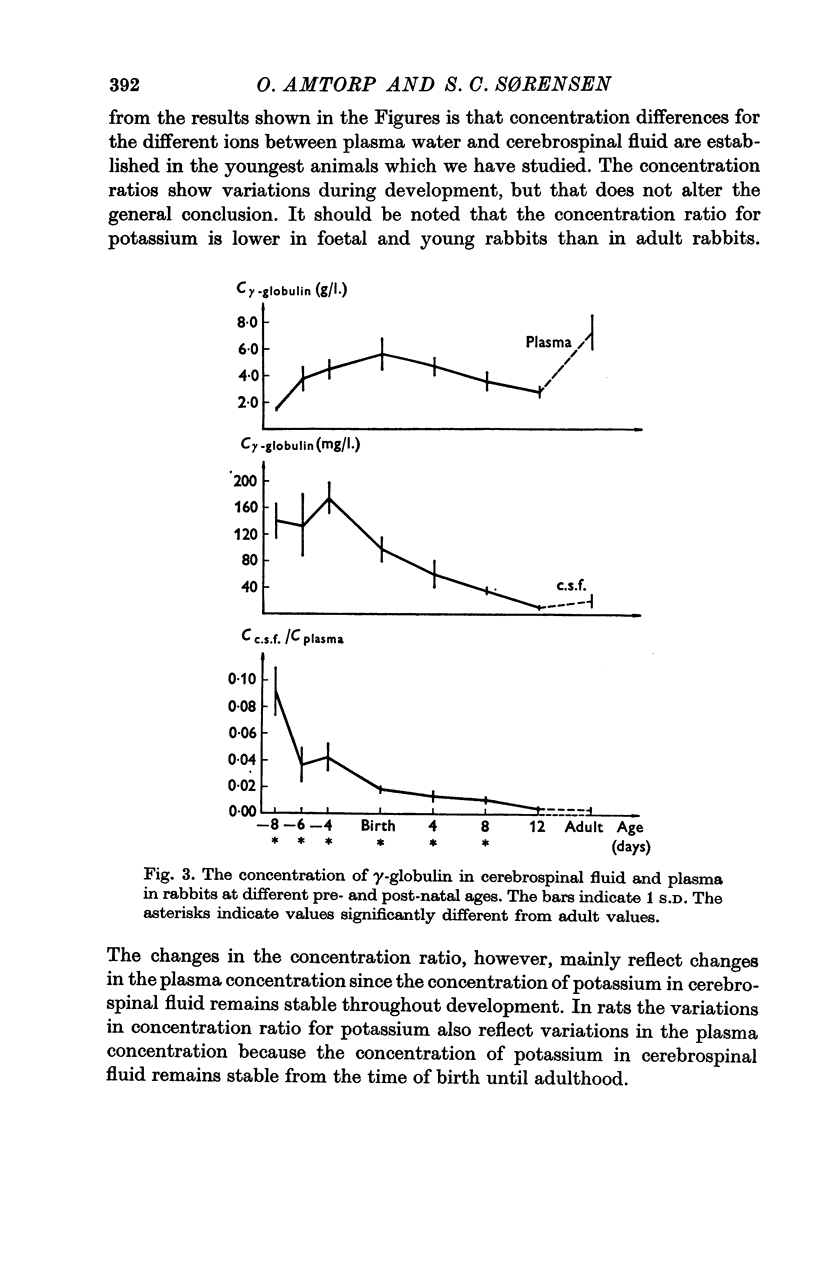
3. The concentration of protein in cerebrospinal fluid and plasma was measured in foetal, juvenile and adult rabbits and in new-born, juvenile and adult rats. The concentration of protein was similar in rabbit foetuses at 23 days of gestational age (term at 31 days) and in new-born rats, and the ratio decreases at approximately the same rate in the two species.
4. The high concentration of proteins in cerebrospinal fluid might reflect either a high rate of entry of protein into the brain or a low production rate of cerebrospinal fluid. Injection of Diamox® (100 mg/kg) 2 hr before sampling of cerebrospinal fluid did not change the concentration of protein in cerebrospinal fluid in new-born rats whereas it increased the concentration in older rats. This finding suggests that new-born rat produces little (if any) cerebrospinal fluid suggesting that the high concentration of protein in cerebrospinal fluid in new-born rats reflect a low rate of turnover of cerebrospinal fluid.
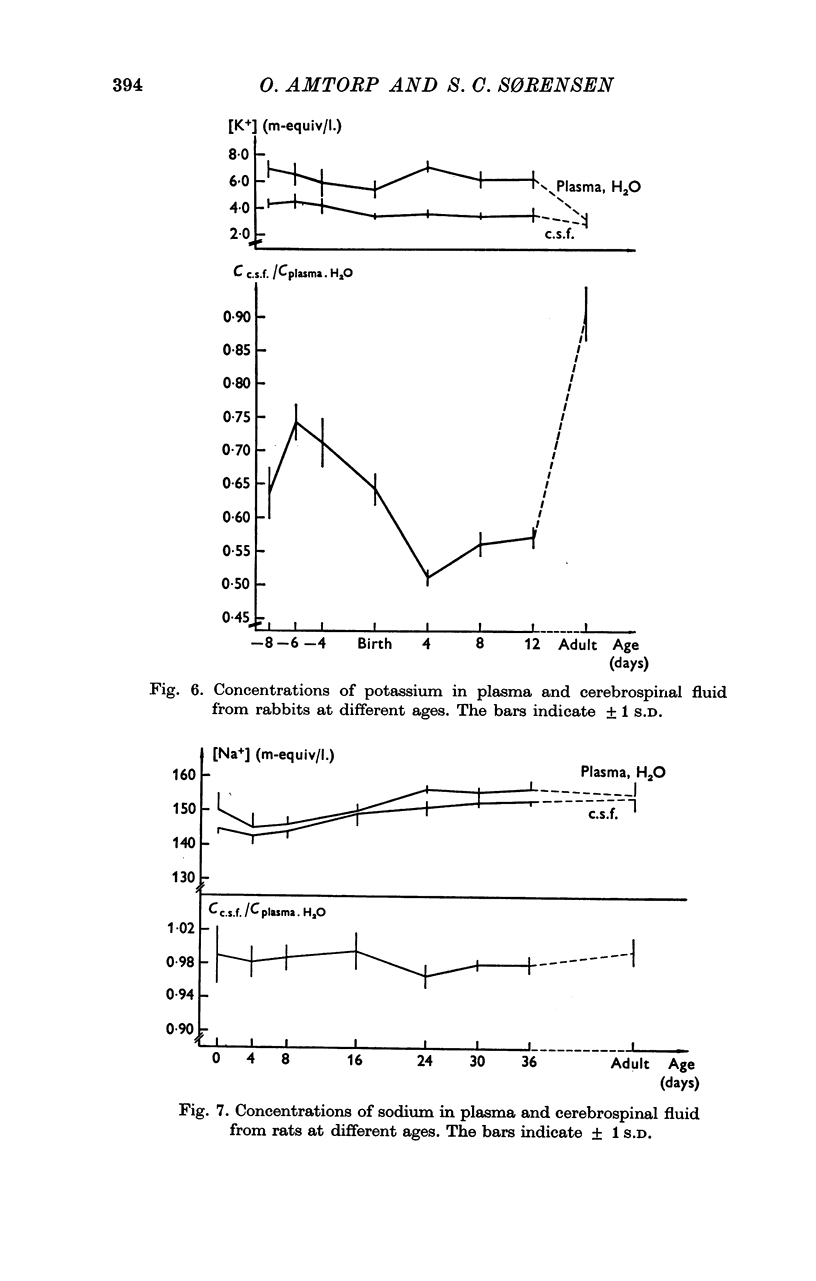
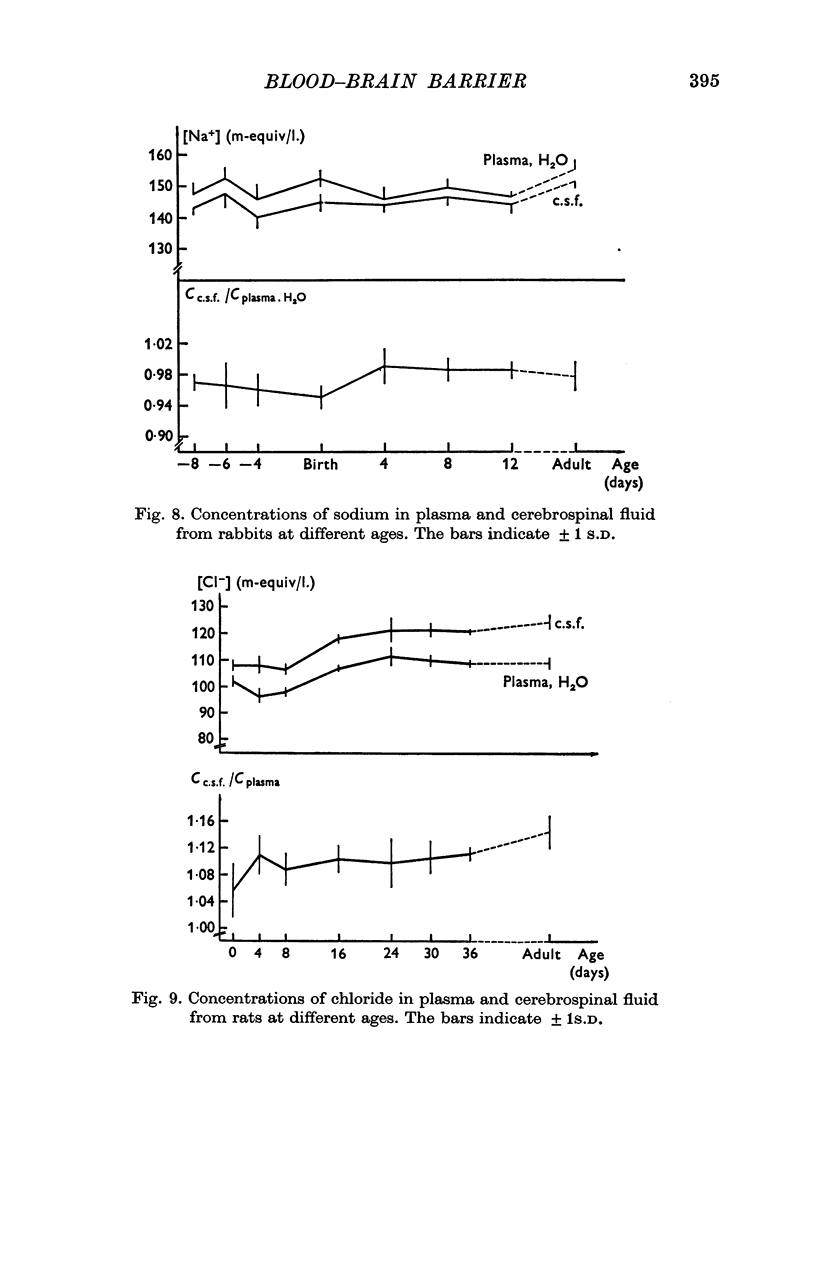
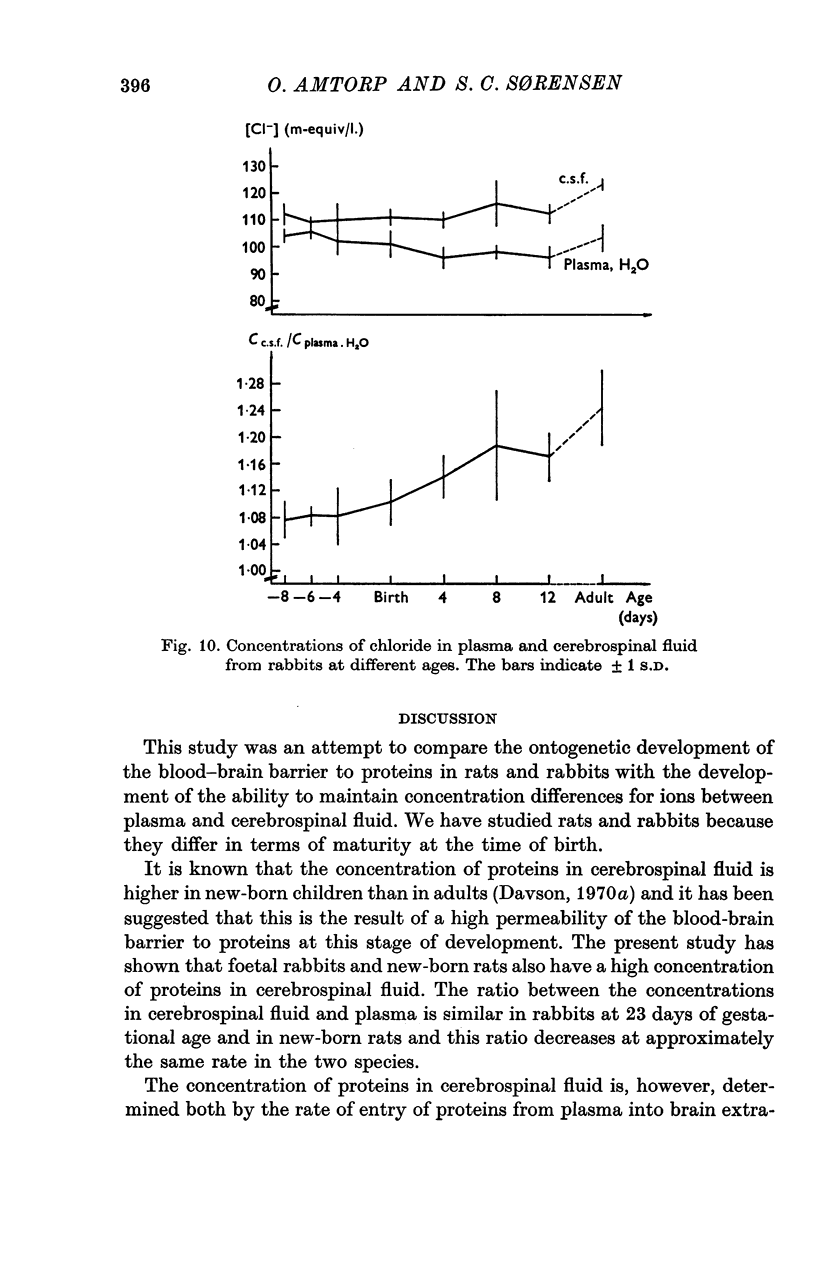
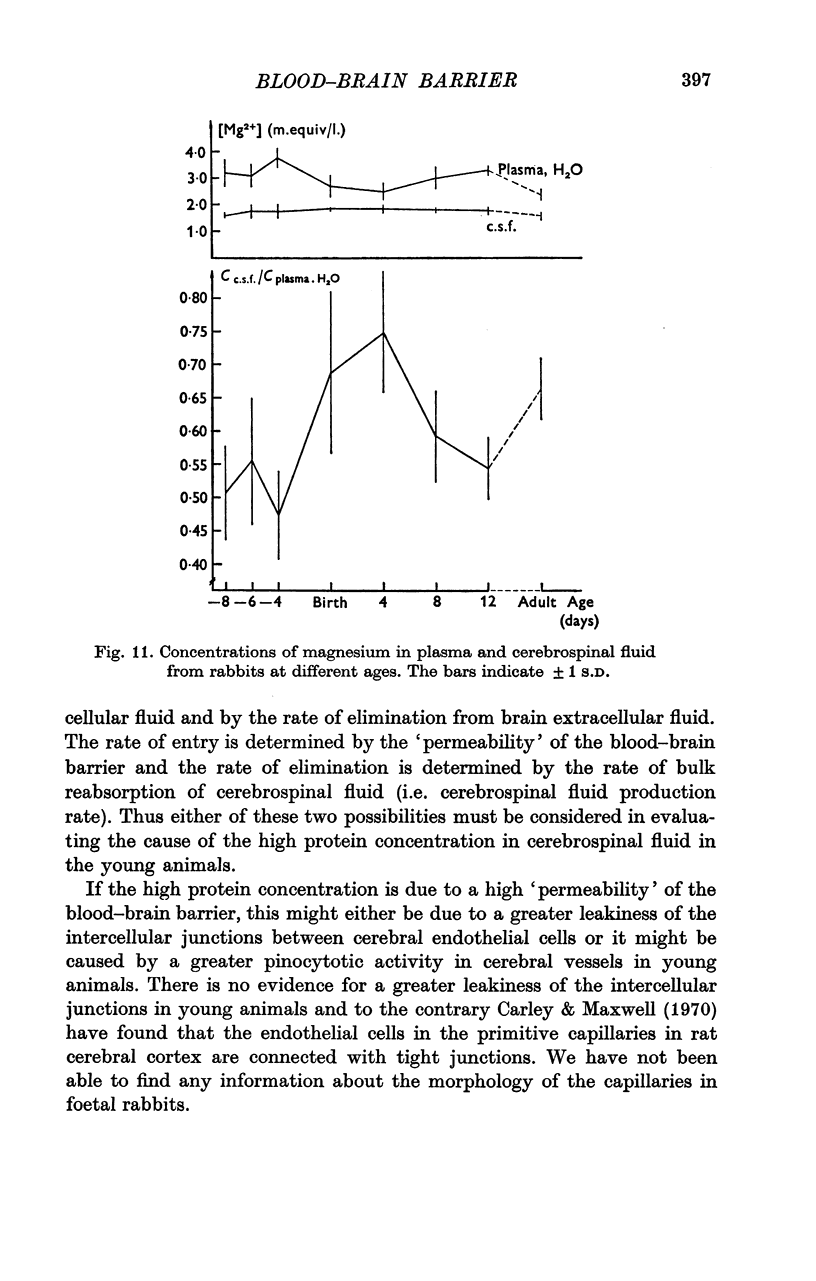
5. The concentration of sodium, potassium, chloride and magnesium in plasma and cisternal cerebrospinal fluid was measured in rabbits of different age, from 23 days of gestation until adulthood, and in rats of different ages from birth until adulthood.
6. Concentration differences between plasma and cerebrospinal fluid for these were established in the youngest animals examined, indicating that the active transport mechanisms for these ions were functioning at an age where the concentration of protein in cerebrospinal fluid was very high.
7. The maintenance of concentration differences for ions at a time where the concentration of proteins in cerebrospinal fluid is high, is difficult to explain if the high concentration of proteins in cerebrospinal fluid is due to a leakiness of the intercellular junctions between cerebral endothelial cells. However, the findings might be explained either by a low rate of production of cerebrospinal fluid in the youngest animals and/or by a pinocytotic transfer of proteins across the blood—brain barrier in these animals.
8. In rats concentration gradients for ions are established at an age (new-borns) with a low or absent bulk formation of cerebrospinal fluid and at an age where the capillaries are still not enveloped by astrocytic foot processes. These facts suggest that the active transport mechanisms for the ions must be located in the cerebral endothelial cells.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bass N. H., Lundborg P. Postnatal development of bulk flow in the cerebrospinal fluid system of the albino rat: clearance of carboxyl-( 14 C)inulin after intrathecal infusion. Brain Res. 1973 Mar 30;52:323–332. doi: 10.1016/0006-8993(73)90668-9. [DOI] [PubMed] [Google Scholar]
- Bito L. Z., Myers R. E. The ontogenesis of haematoencephalic cation transport processes in the rhesus monkey. J Physiol. 1970 May;208(1):153–170. doi: 10.1113/jphysiol.1970.sp009111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury M. W., Crowder J., Desai S., Reynolds J. M., Reynolds M., Saunders N. R. Electrolytes and water in the brain and cerebrospinal fluid of the foetal sheep and guinea-pig. J Physiol. 1972 Dec;227(2):591–610. doi: 10.1113/jphysiol.1972.sp010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B. M., Falchuk K. H., Keimowitz R. I., Berliner R. W. The relationship between peritubular capillary protein concentration and fluid reabsorption by the renal proximal tubule. J Clin Invest. 1969 Aug;48(8):1519–1531. doi: 10.1172/JCI106118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brightman M. W., Reese T. S. Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol. 1969 Mar;40(3):648–677. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caley D. W., Maxwell D. S. Development of the blood vessels and extracellular spaces during postnatal maturation of rat cerebral cortex. J Comp Neurol. 1970 Jan;138(1):31–47. doi: 10.1002/cne.901380104. [DOI] [PubMed] [Google Scholar]
- DONAHUE S., PAPPAS G. D. The fine structure of capillaries in the cerebral cortex of the rat at various stages of development. Am J Anat. 1961 May;108:331–347. doi: 10.1002/aja.1001080307. [DOI] [PubMed] [Google Scholar]
- Davson H., Hollingsworth J. R. Active transport of 131-I across the blood-brain barrier. J Physiol. 1973 Sep;233(2):327–347. doi: 10.1113/jphysiol.1973.sp010310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson R. K., Woodbury D. M. Penetration of 14C-inulin and 14C-sucrose into brain, cerebrospinal fluid, and skeletal muscle of developing rats. Exp Brain Res. 1969;7(3):181–194. doi: 10.1007/BF00239028. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Olsson Y., Klatzo I., Sourander P., Steinwall O. Blood-brain barrier to albumin in embryonic new born and adult rats. Acta Neuropathol. 1968 Mar 4;10(2):117–122. doi: 10.1007/BF00691305. [DOI] [PubMed] [Google Scholar]
- Reese T. S., Karnovsky M. J. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J Cell Biol. 1967 Jul;34(1):207–217. doi: 10.1083/jcb.34.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siggaard-Andersen O., Bull B. S. Semiautomatic pipetting of ultramicro volumes of sample and reagent. Am J Clin Pathol. 1967 Nov;48(5):502–509. doi: 10.1093/ajcp/48.5.502. [DOI] [PubMed] [Google Scholar]
- Simionescu M., Simionescu N., Palade G. E. Morphometric data on the endothelium of blood capillaries. J Cell Biol. 1974 Jan;60(1):128–152. doi: 10.1083/jcb.60.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westergaard E., Brightman M. W. Transport of proteins across normal cerebral arterioles. J Comp Neurol. 1973 Nov 1;152(1):17–44. doi: 10.1002/cne.901520103. [DOI] [PubMed] [Google Scholar]


