Abstract
1. Molluscan neurones have been found to show six different types of response (three excitatory and three inhibitory) to the iontophoretic application of 5-hydroxytryptamine (5-HT). The pharmacological properties of the receptors and the ionic mechanisms associated with these responses have been analysed.
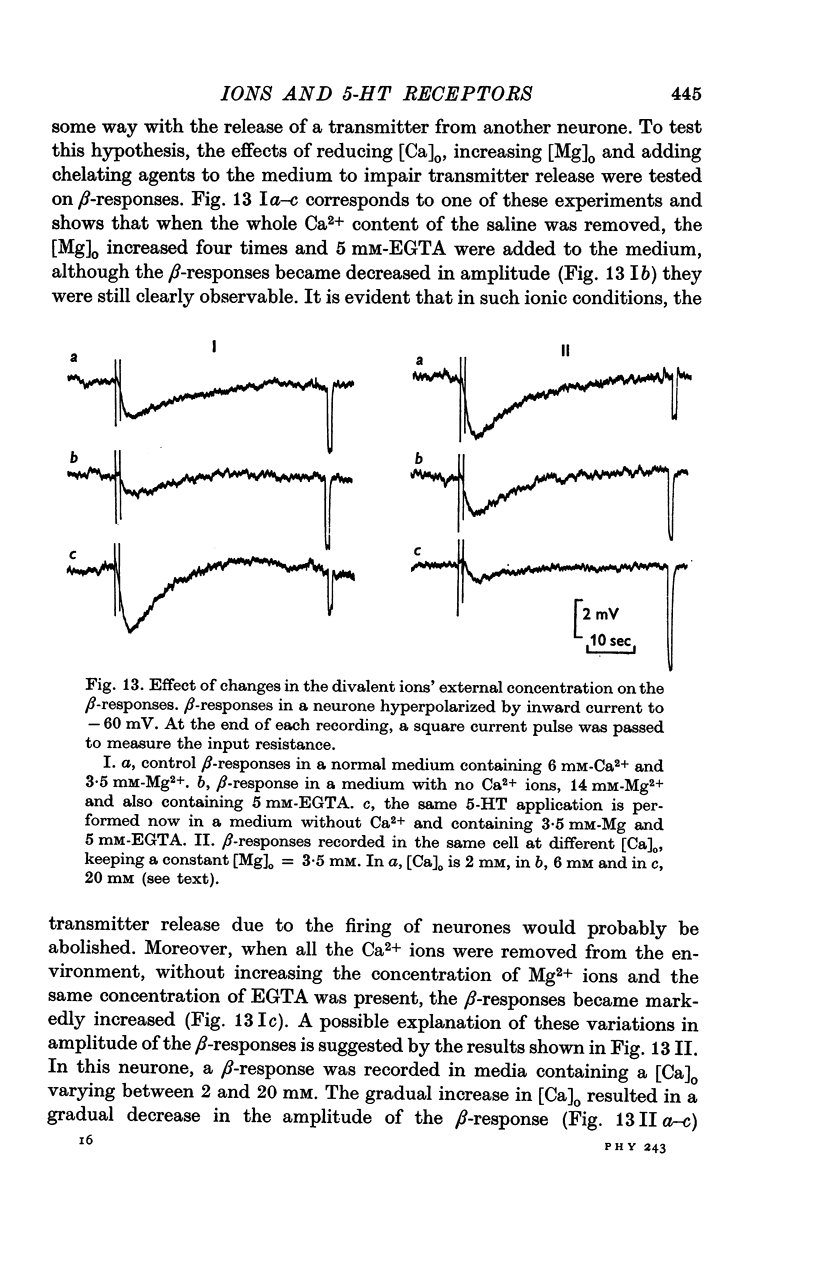
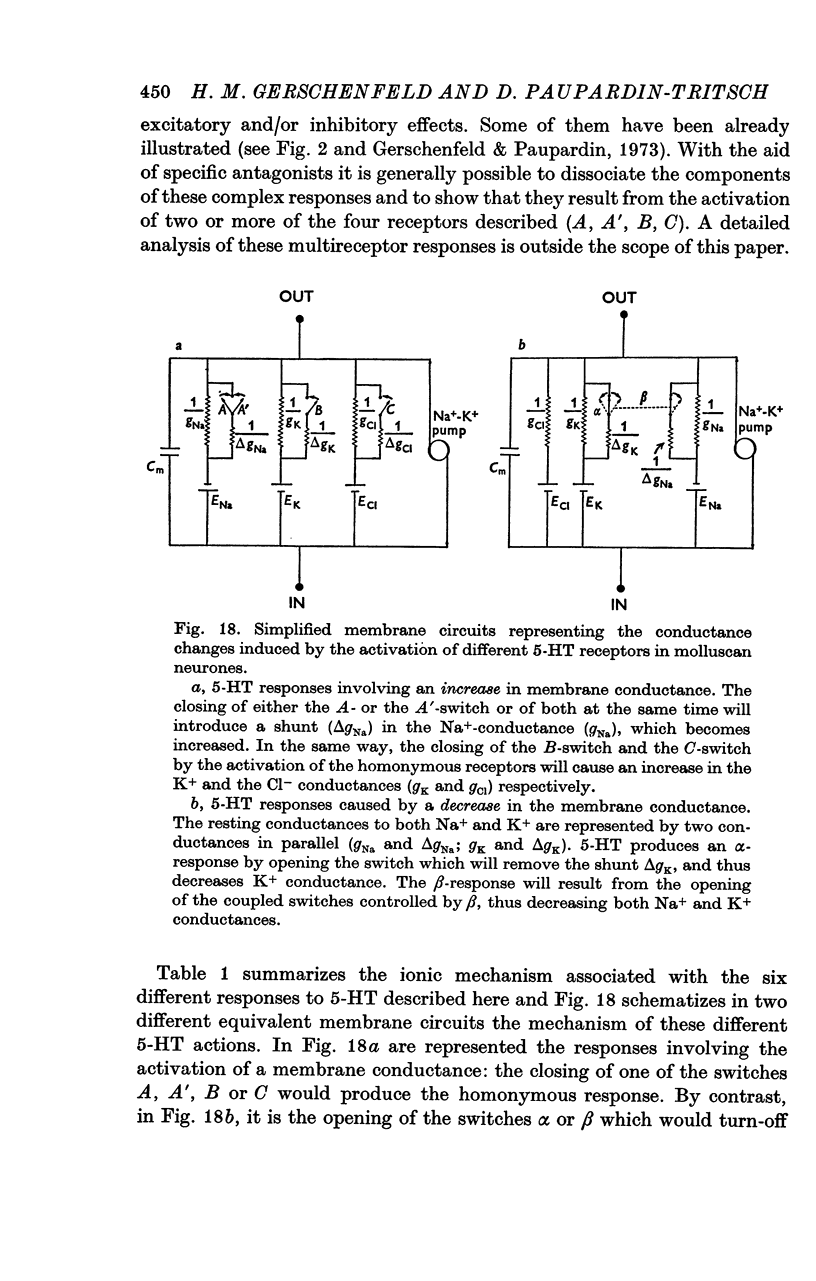
2. Four of the responses to 5-HT (named A, A′, B and C) are consequent upon an increase in membrane conductance whereas the other two (named α and β) are caused by a decrease in membrane conductance.
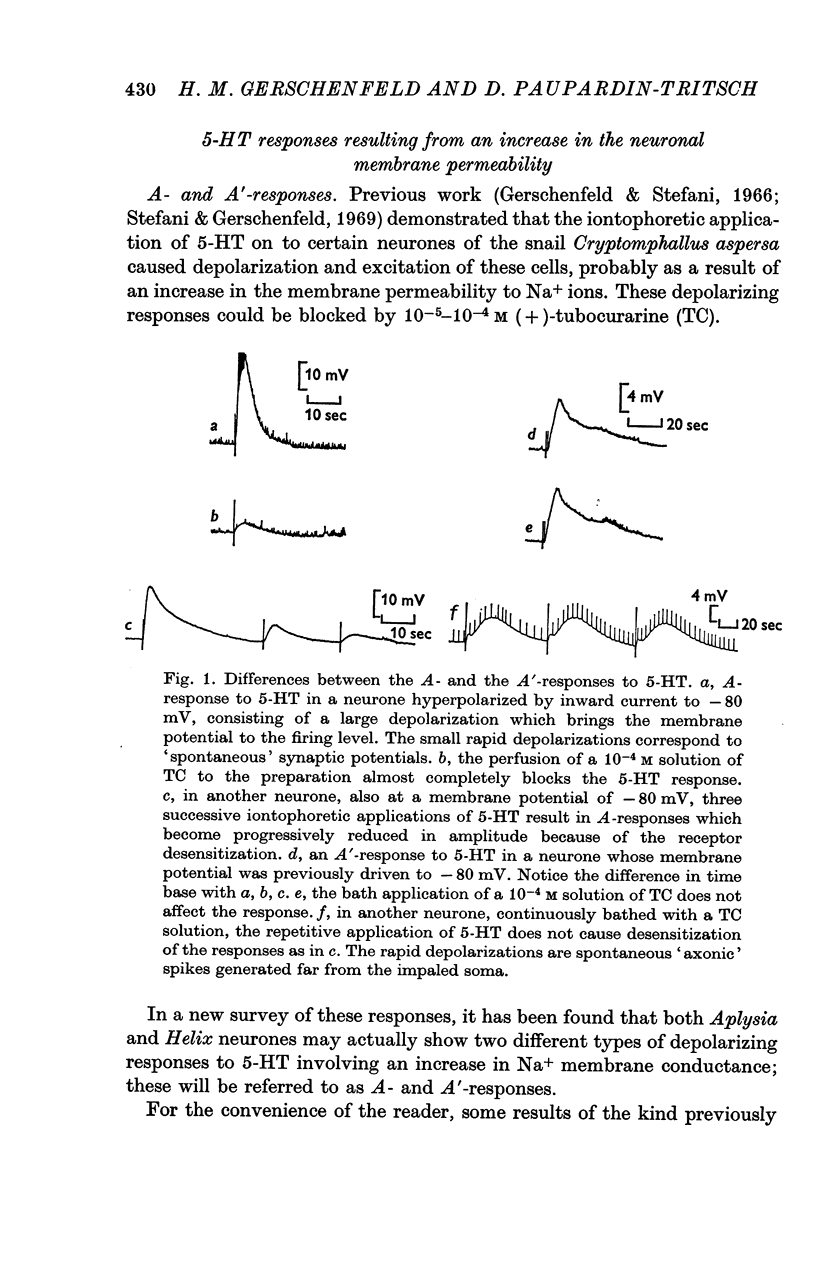
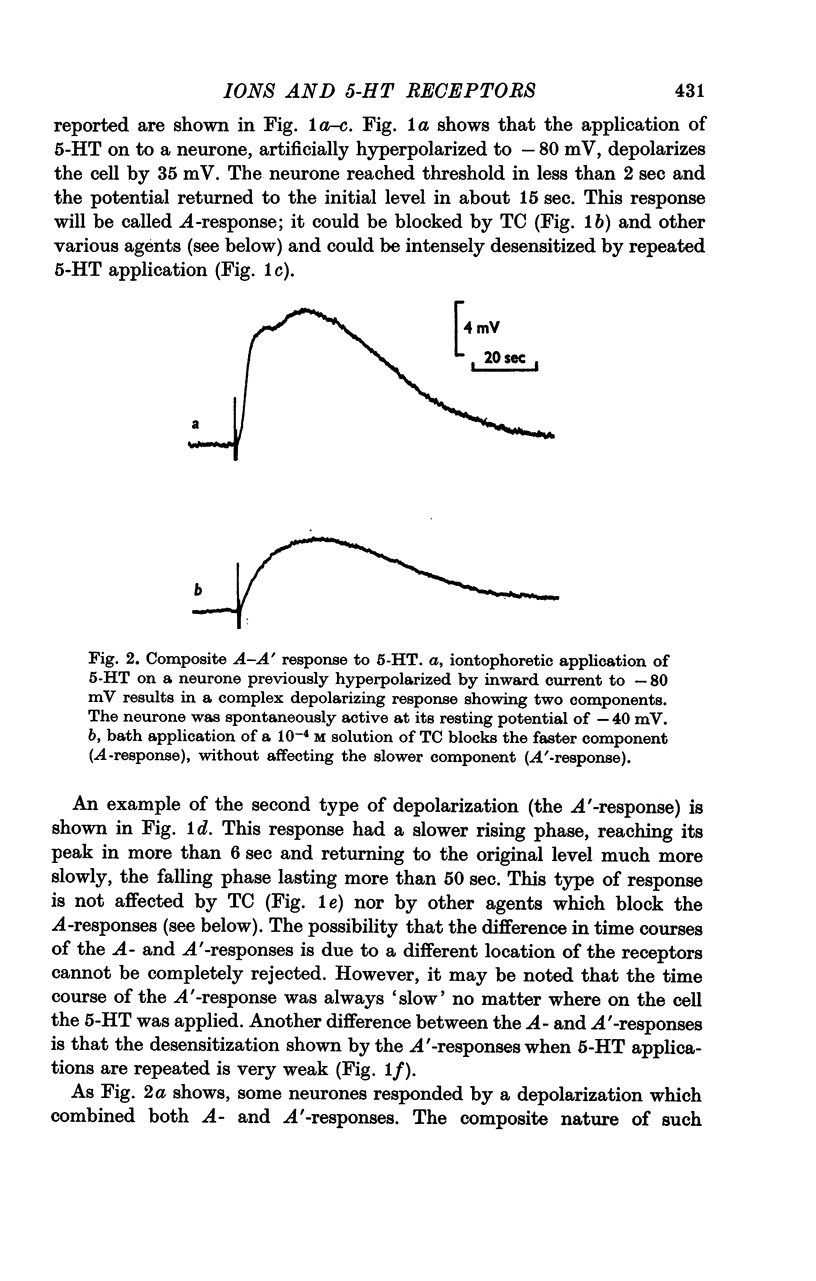
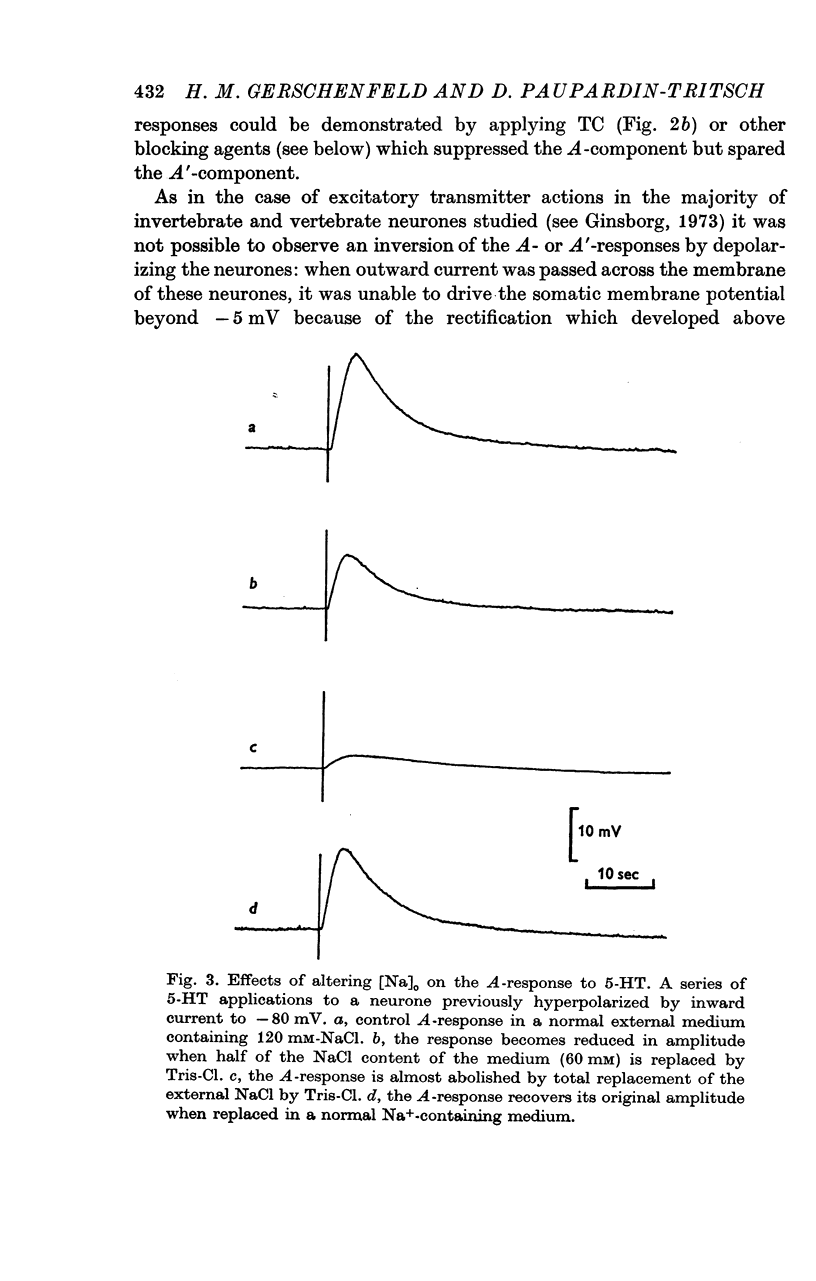
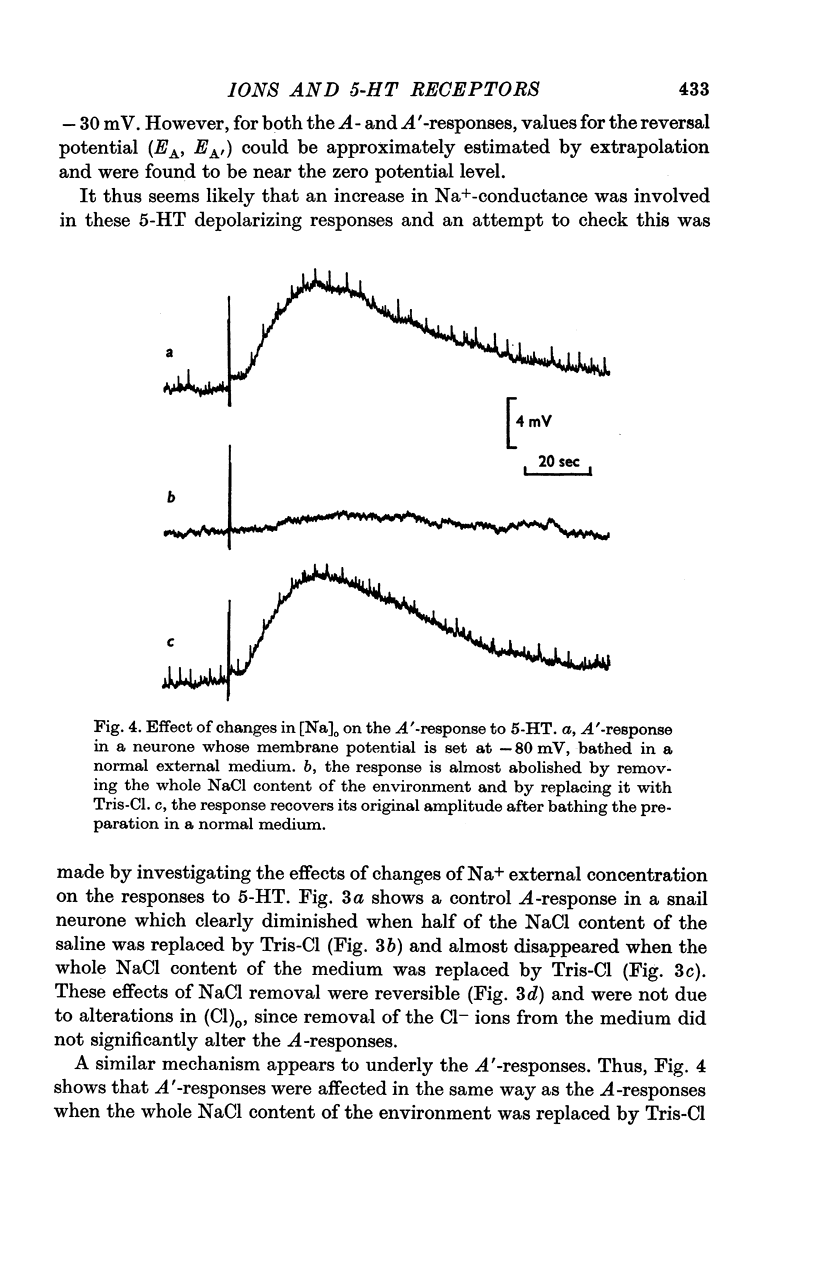
3. The A-response to 5-HT consists of a `fast' depolarization due to an increase mainly in Na+-conductance; the A′-response is a `slow' depolarization also associated with a Na+-conductance increase. Receptors mediating the A- and A′-depolarizations have different pharmacological properties and may exist side by side on the same neurone.
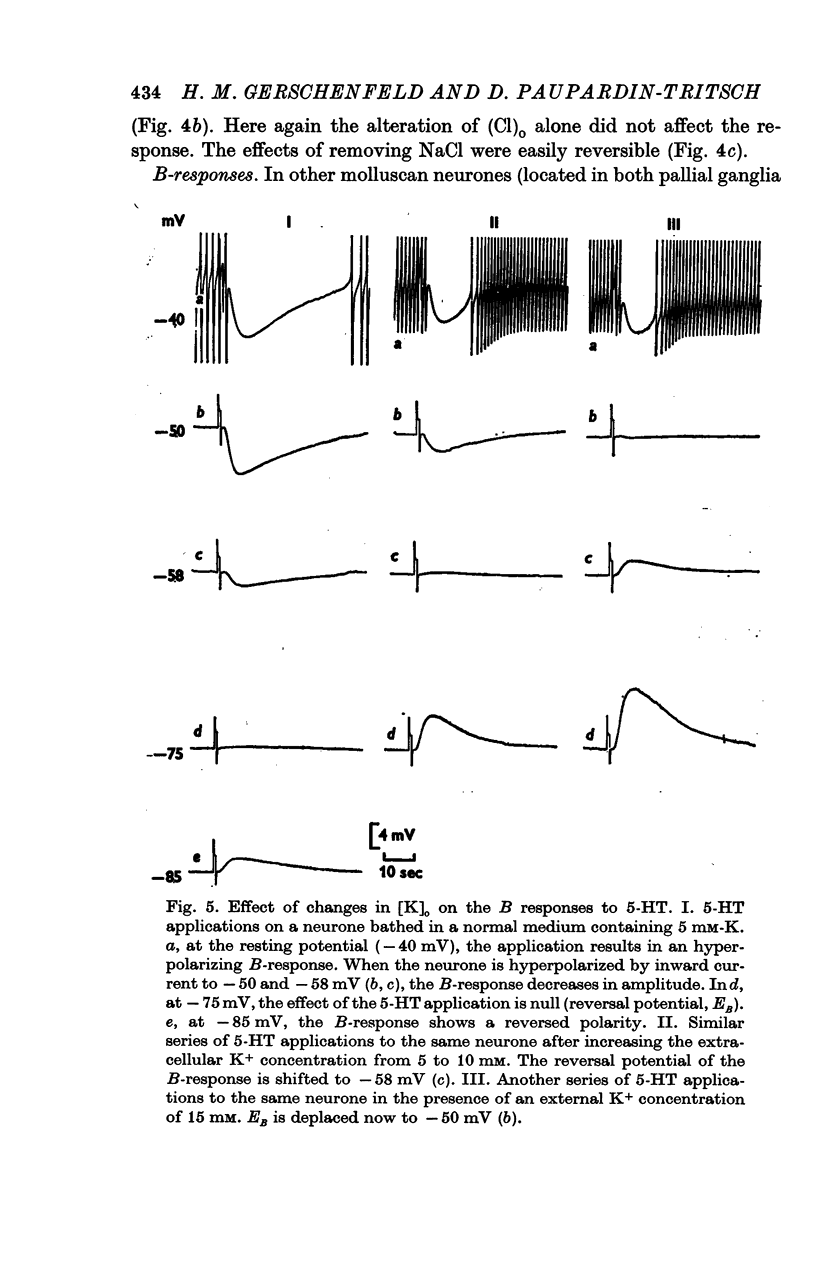
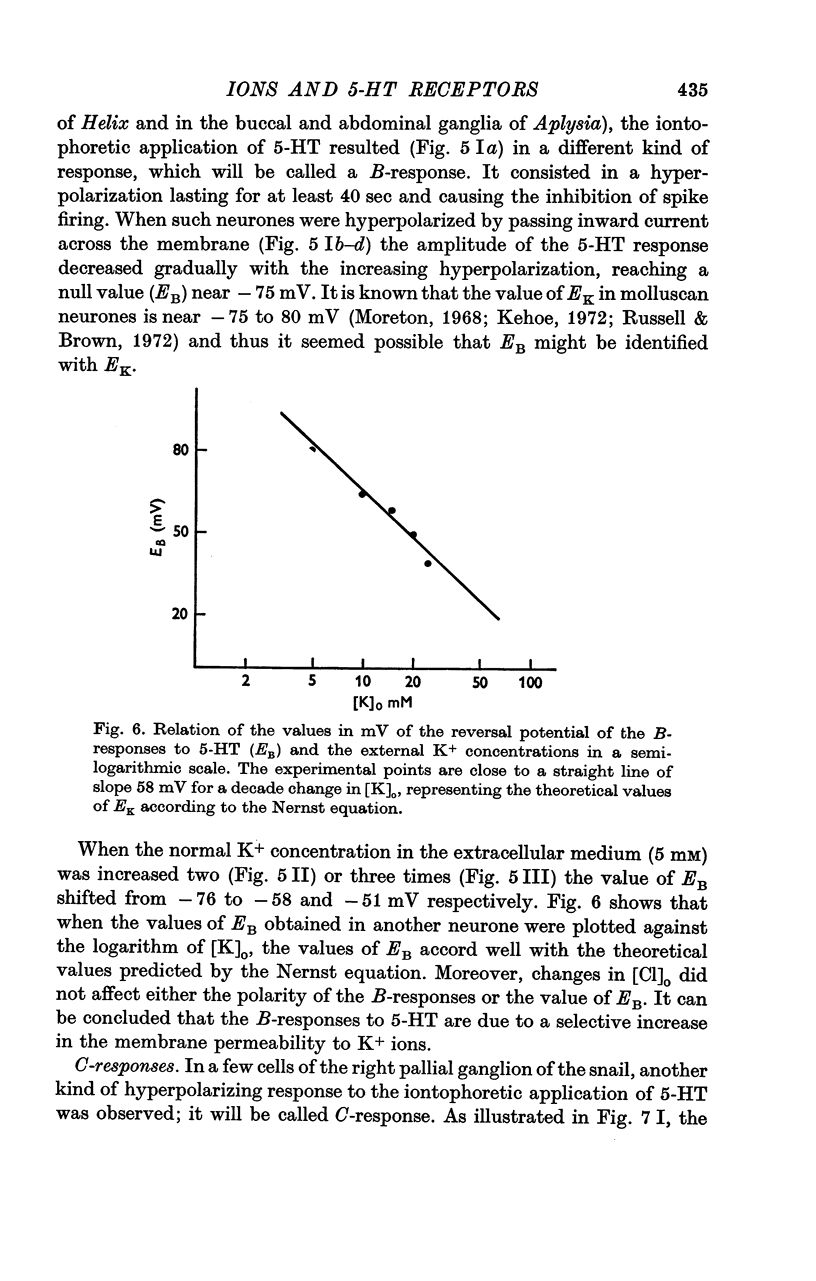
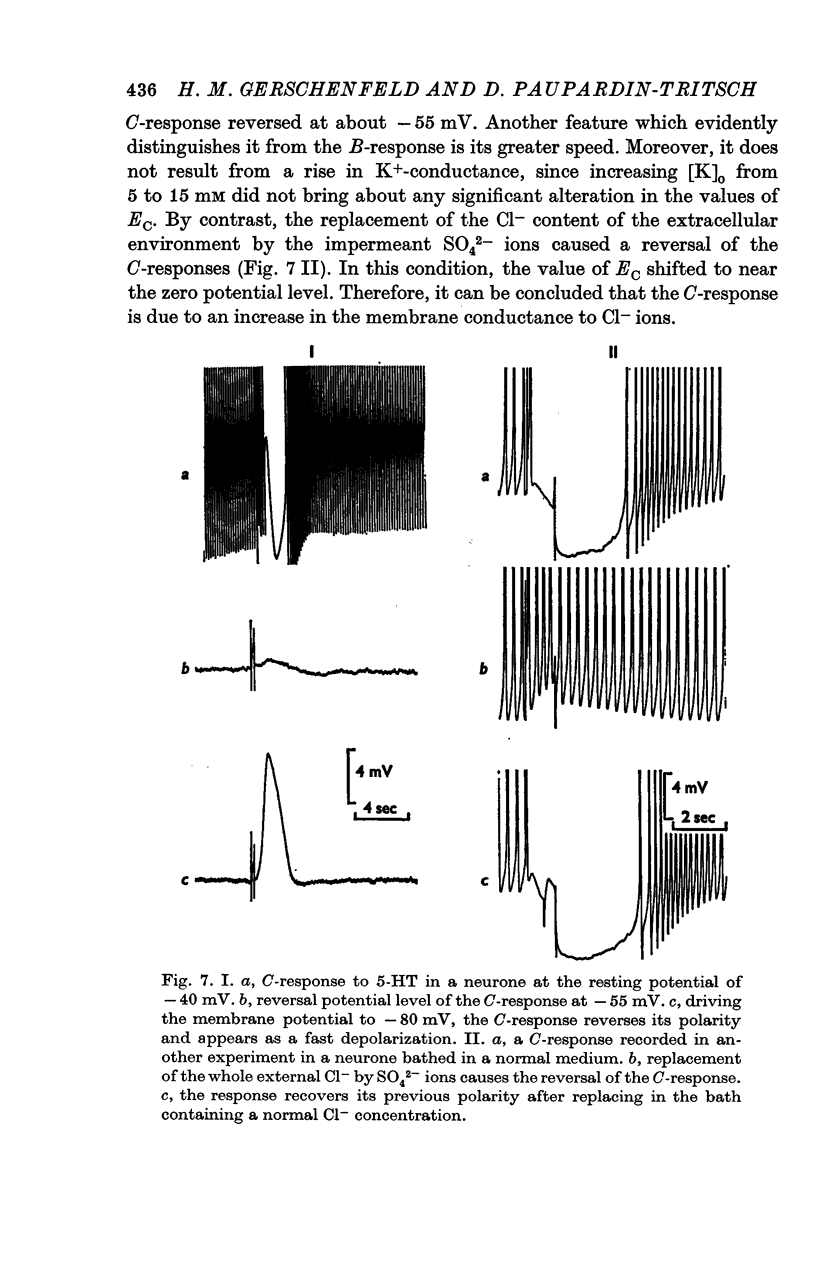
4. Both the B- and C-responses are inhibitory. The B-response is a `slow' hyperpolarization due to an increase in K+-conductance, the C-response is a fast hyperpolarization associated with an increase in Cl--conductance.
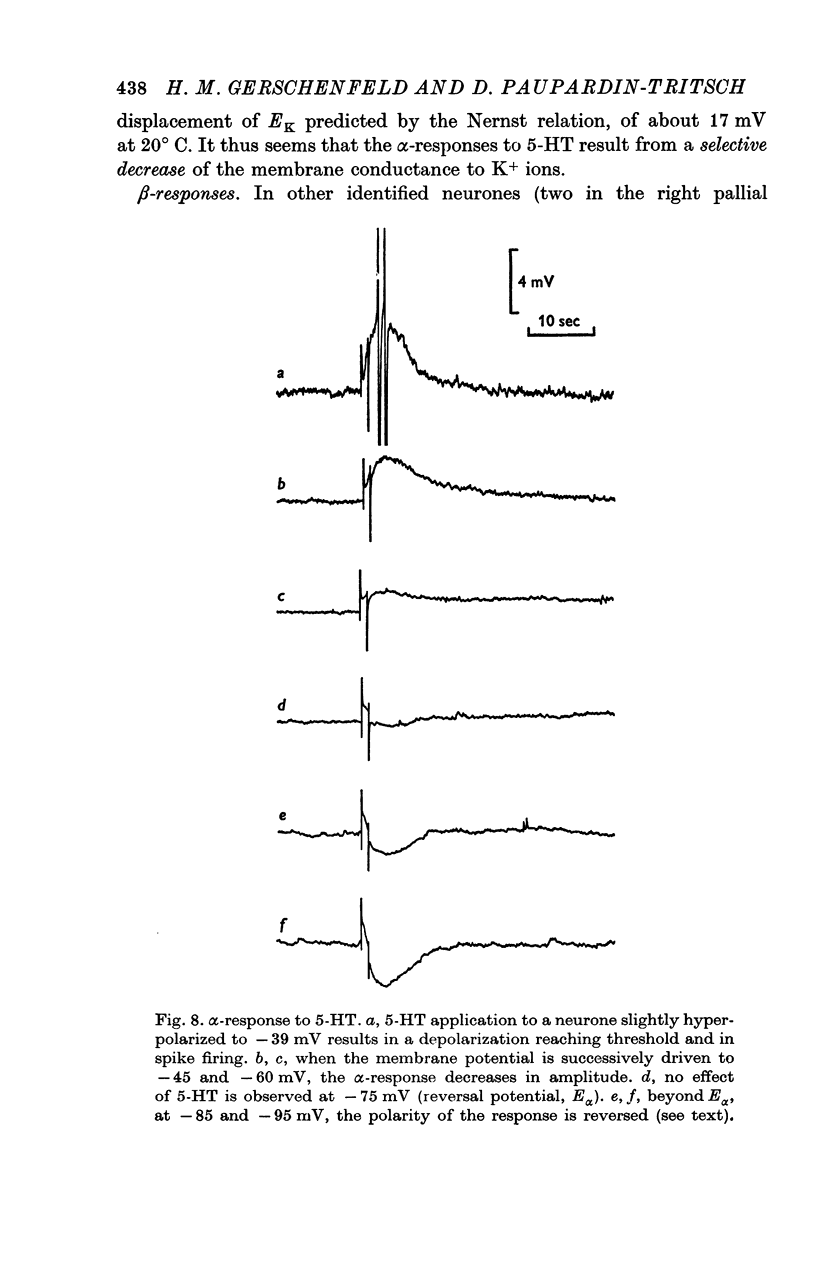
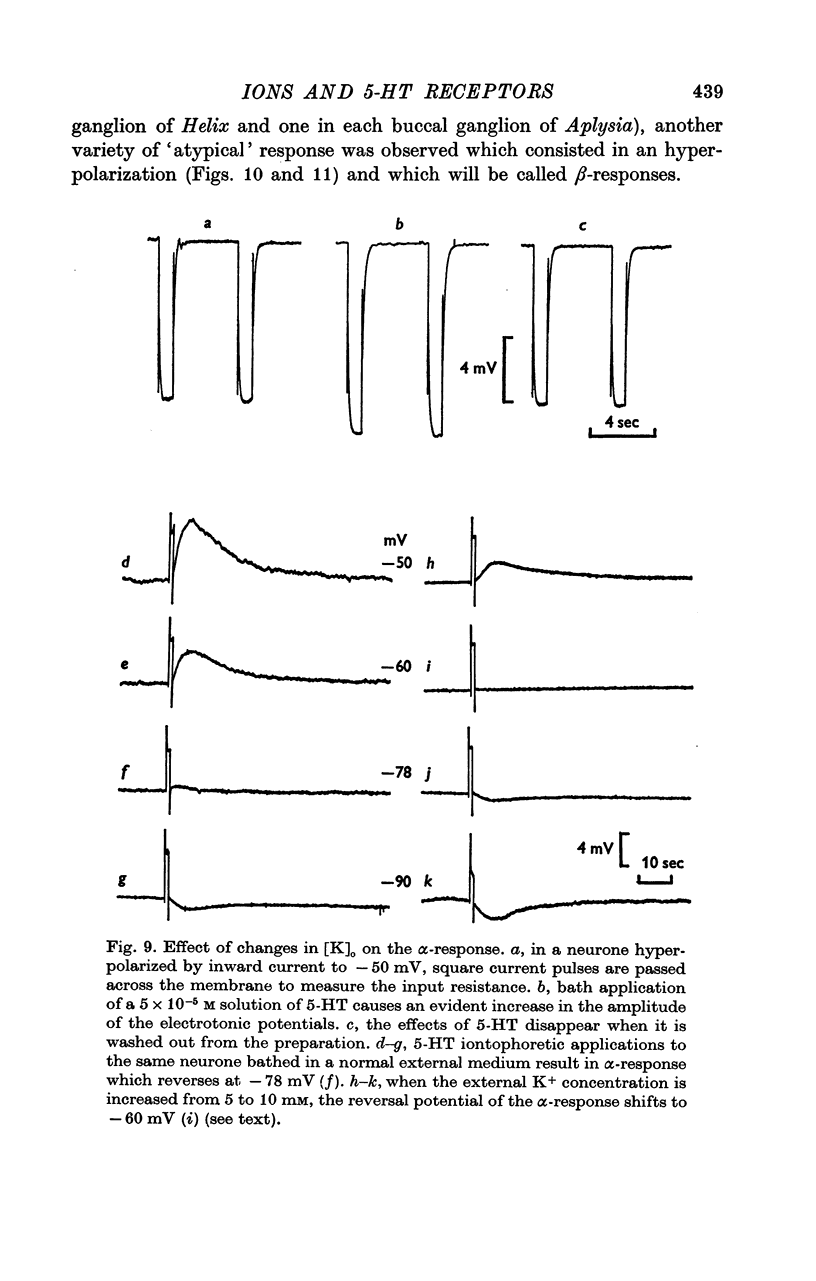
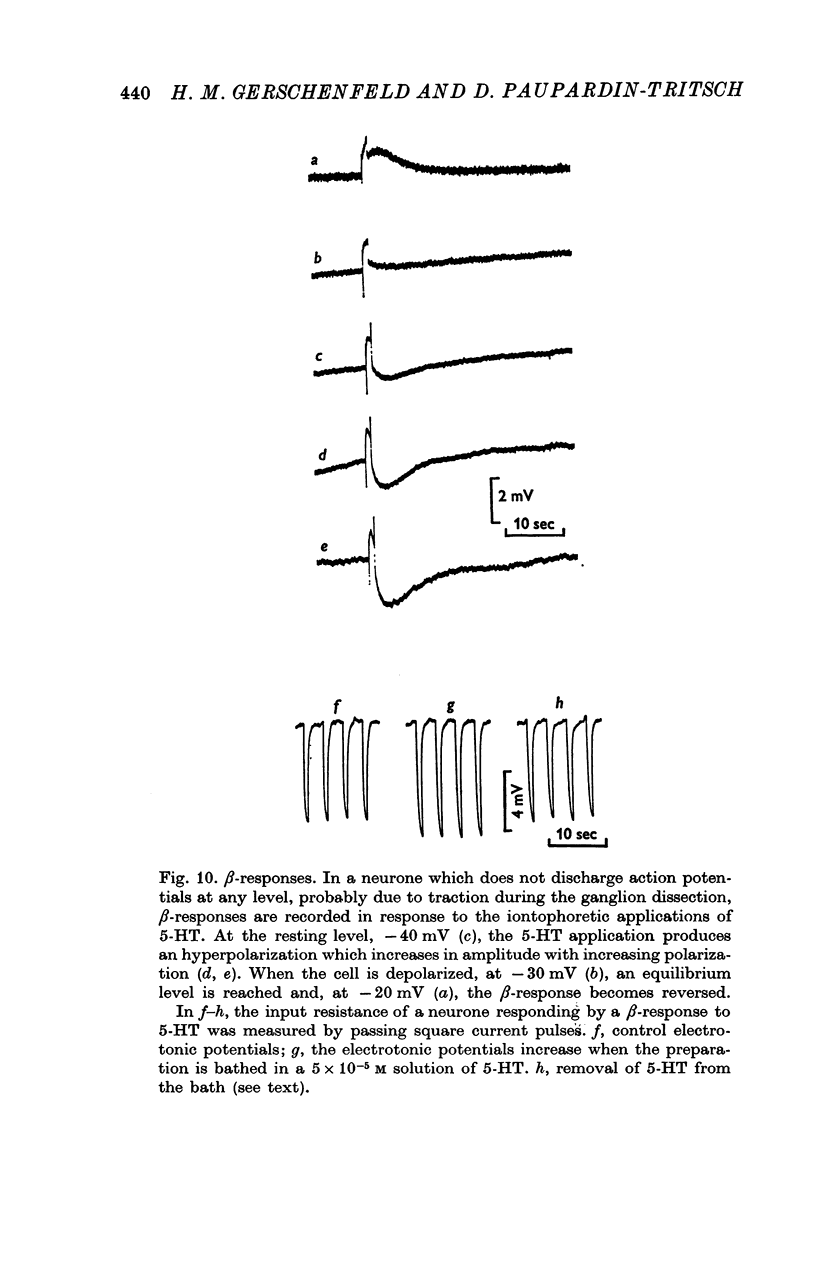
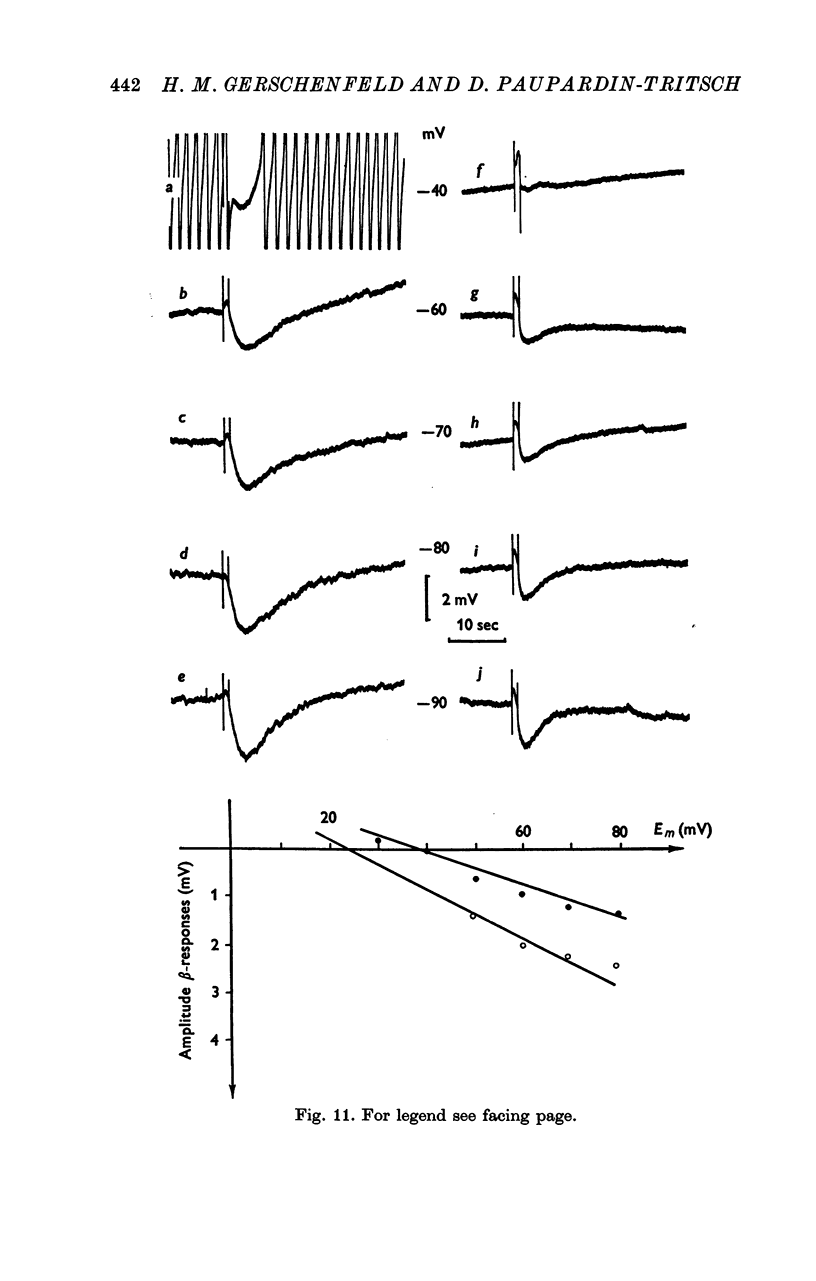
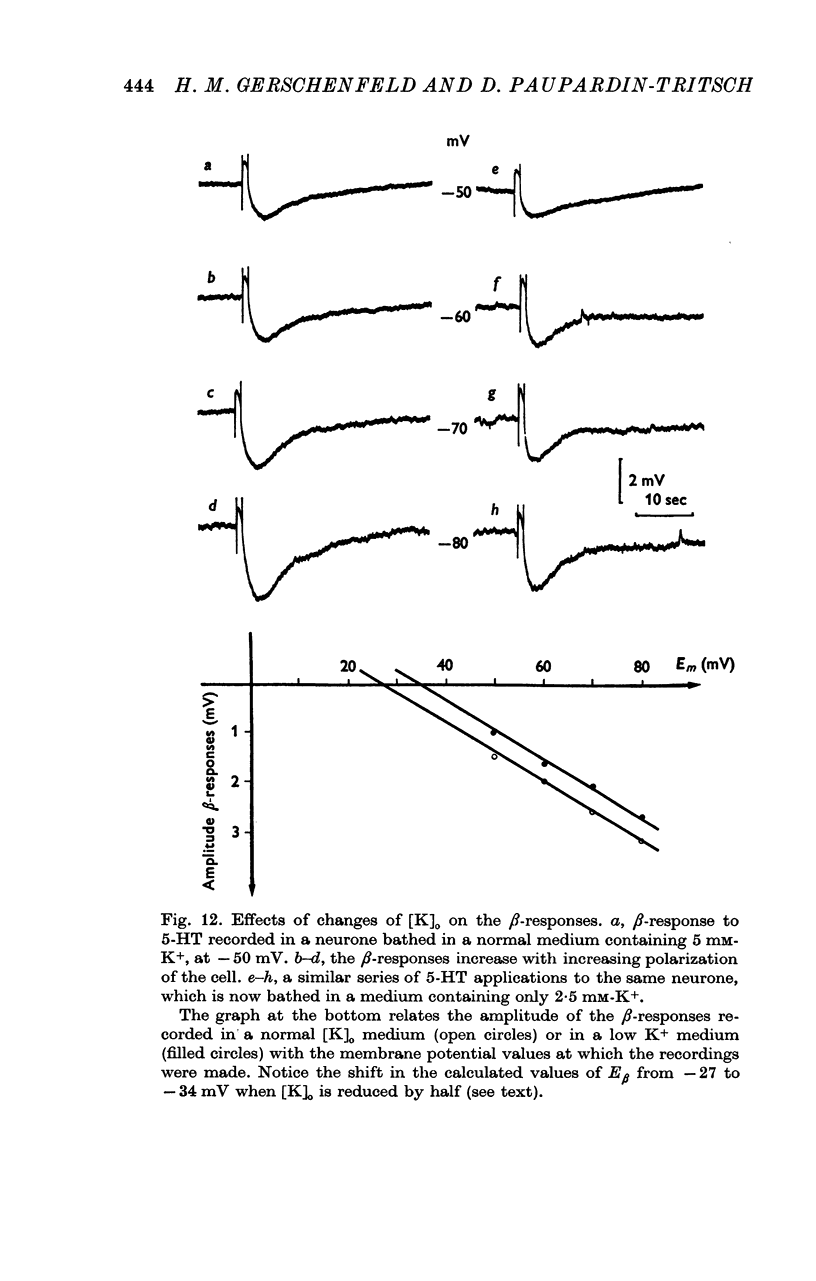
5. The α-response to 5-HT is a depolarization which becomes reduced in amplitude with cell hyperpolarization and reverses at -75 mV; it is caused by a decrease in K+-conductance. The β-response is an hyperpolarization which increases in amplitude with cell hyperpolarization and reverses at -20/-30 mV. It results from a decrease in conductance to both Na+ and K+ ions.
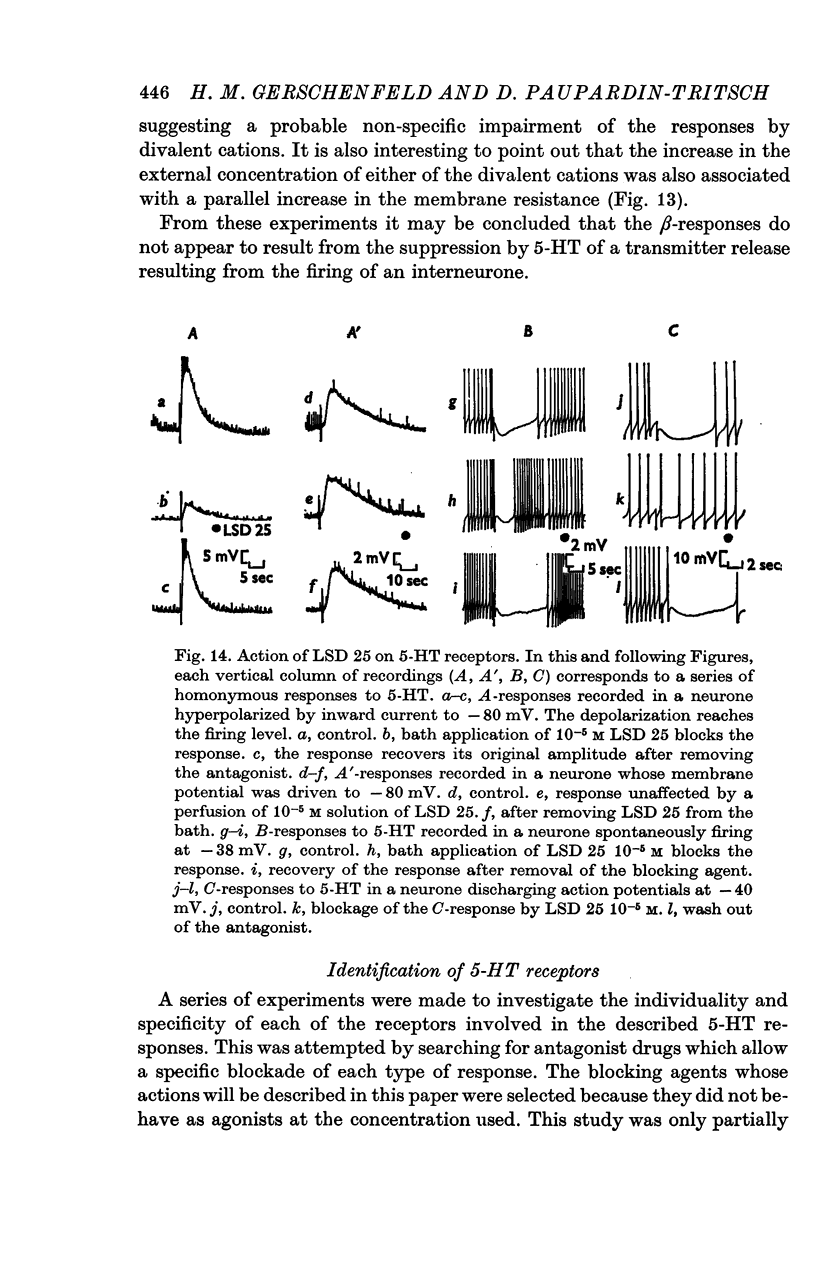
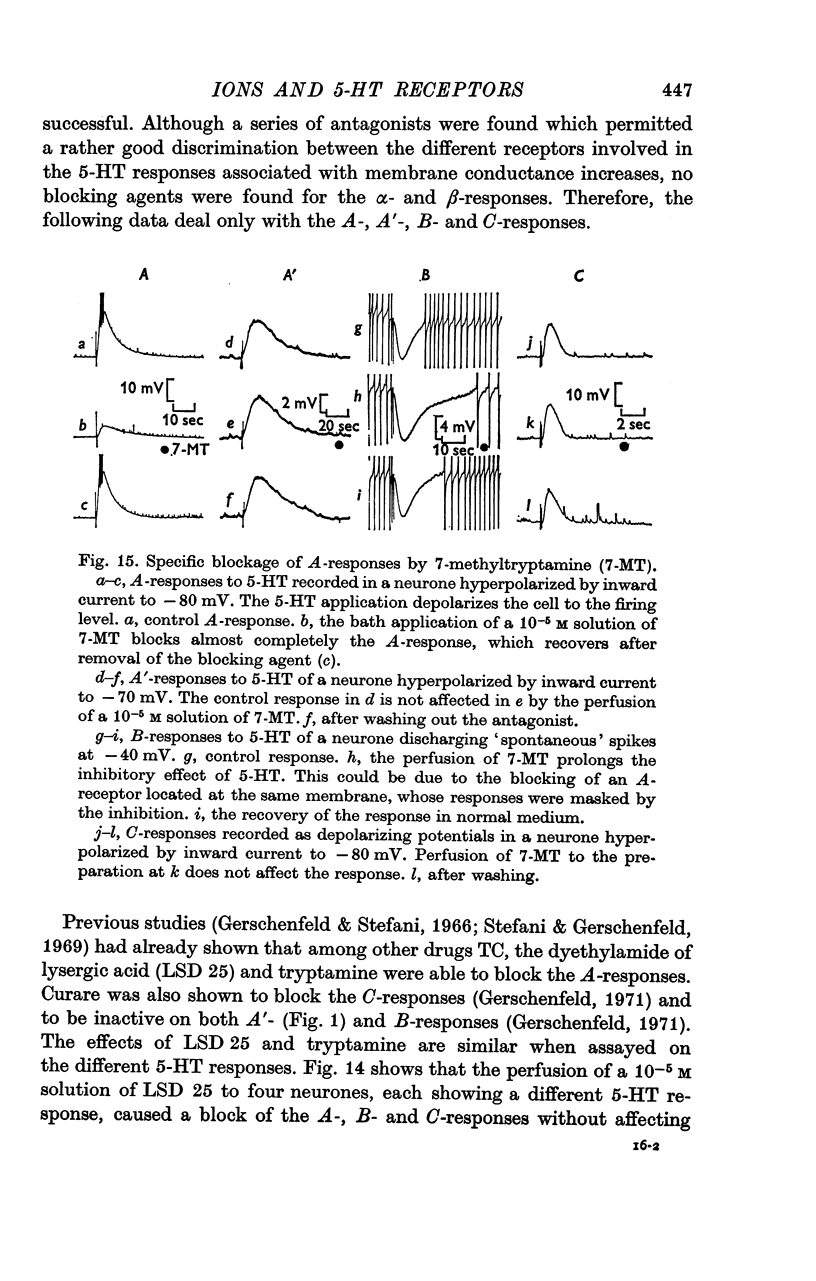
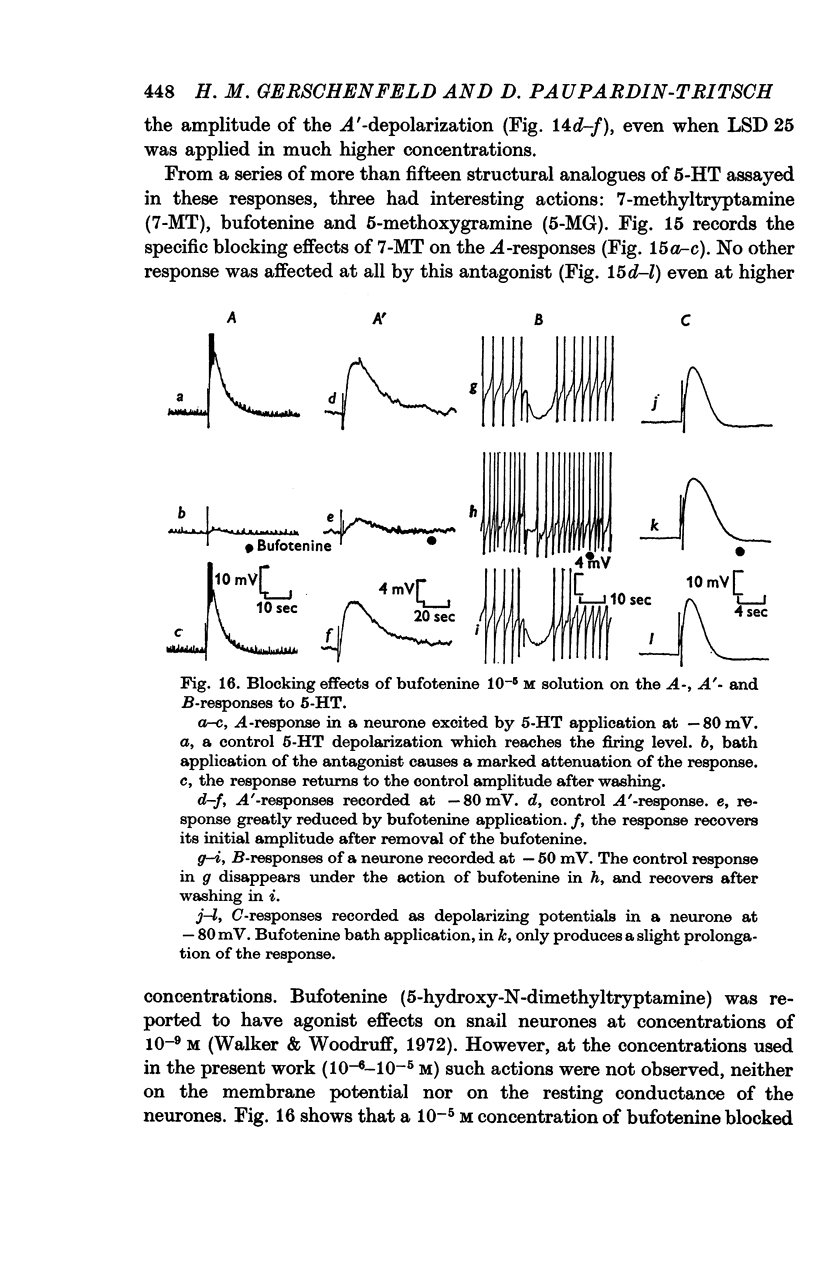
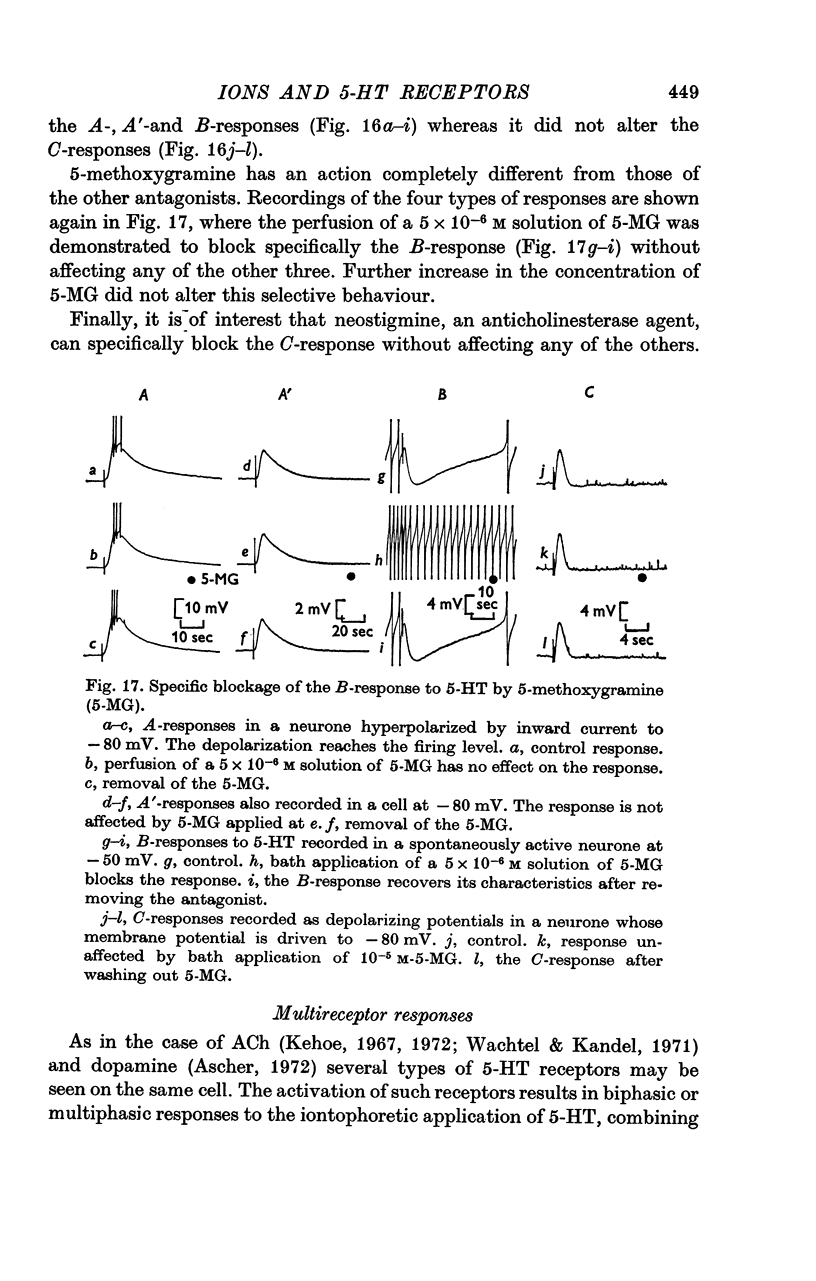
6. The receptors involved in the 5-HT responses associated with a conductance increase may be recognized by the action of specific antagonists: 7-methyltryptamine blocks only the A-receptors, 5-methoxygramine only the B-receptors and neostigmine only the C-receptors. Curare blocks the A- and C-receptors and bufotenine, the A-, A′- and B-receptors. No specific antagonists have yet been found for the 5-HT responses caused by a conductance decrease.
7. The significance of the multiplicity of receptors is discussed. Their functional significance at synapses is analysed in the following paper.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ascher P. Inhibitory and excitatory effects of dopamine on Aplysia neurones. J Physiol. 1972 Aug;225(1):173–209. doi: 10.1113/jphysiol.1972.sp009933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHIARANDINI D. J. A SALINE SOLUTION FOR PULMONATE MOLLUSCS. Life Sci. 1964 Dec;3:1513–1518. doi: 10.1016/0024-3205(64)90098-0. [DOI] [PubMed] [Google Scholar]
- Cervetto L., Piccolino M. Synaptic transmission between photoreceptors and horizontal cells in the turtle retina. Science. 1974 Feb 1;183(4123):417–419. doi: 10.1126/science.183.4123.417. [DOI] [PubMed] [Google Scholar]
- Cottrell G. A. Direct postsynaptic responses to stimulation of serotonin-containing neurones. Nature. 1970 Mar 14;225(5237):1060–1062. doi: 10.1038/2251060a0. [DOI] [PubMed] [Google Scholar]
- Cottrell G. A., Macon J. B. Synaptic connexions of two symmetrically placed giant serotonin-containing neurones. J Physiol. 1974 Jan;236(2):435–464. doi: 10.1113/jphysiol.1974.sp010445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling J. E., Ripps H. Effect of magnesium on horizontal cell activity in the skate retina. Nature. 1973 Mar 9;242(5393):101–103. doi: 10.1038/242101a0. [DOI] [PubMed] [Google Scholar]
- Engberg I., Marshall K. C. Mechanism of noradrenaline hyperpolarization in spinal cord motoneurones of the cat. Acta Physiol Scand. 1971 Sep;83(1):142–144. doi: 10.1111/j.1748-1716.1971.tb05061.x. [DOI] [PubMed] [Google Scholar]
- FATT P., KATZ B. An analysis of the end-plate potential recorded with an intracellular electrode. J Physiol. 1951 Nov 28;115(3):320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. The effect of inhibitory nerve impulses on a crustacean muscle fibre. J Physiol. 1953 Aug;121(2):374–389. doi: 10.1113/jphysiol.1953.sp004952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERSCHENFELD H. M., LASANSKY A. ACTION OF GLUTAMIC ACID AND OTHER NATURALLY OCCURRING AMINO-ACIDS ON SNAIL CENTRAL NEURONS. Int J Neuropharmacol. 1964 Jul;3:301–314. doi: 10.1016/0028-3908(64)90022-x. [DOI] [PubMed] [Google Scholar]
- Gerschenfeld H. M. Chemical transmission in invertebrate central nervous systems and neuromuscular junctions. Physiol Rev. 1973 Jan;53(1):1–119. doi: 10.1152/physrev.1973.53.1.1. [DOI] [PubMed] [Google Scholar]
- Gerschenfeld H. M., Paupardin-Tritsch D. On the transmitter function of 5-hydroxytryptamine at excitatory and inhibitory monosynaptic junctions. J Physiol. 1974 Dec;243(2):457–481. doi: 10.1113/jphysiol.1974.sp010762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerschenfeld H. M. Serotonin: two different inhibitory actions on snail neurons. Science. 1971 Mar 26;171(3977):1252–1254. doi: 10.1126/science.171.3977.1252. [DOI] [PubMed] [Google Scholar]
- Gerschenfeld H. M., Stefani E. An electrophysiological study of 5-hydroxytryptamine receptors of neurones in the molluscan nervous system. J Physiol. 1966 Aug;185(3):684–700. doi: 10.1113/jphysiol.1966.sp008010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsborg B. L. Electrical changes in the membrane in junctional transmission. Biochim Biophys Acta. 1973 Nov 28;300(3):289–317. doi: 10.1016/0304-4157(73)90007-5. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe J. Ionic mechanisms of a two-component cholinergic inhibition in Aplysia neurones. J Physiol. 1972 Aug;225(1):85–114. doi: 10.1113/jphysiol.1972.sp009930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe J. Pharmacological characteristics and ionic bases of a 2 component postsynaptic inhibition. Nature. 1967 Sep 30;215(5109):1503–1505. doi: 10.1038/2151503b0. [DOI] [PubMed] [Google Scholar]
- Krnjević K., Schwartz S. Some properties of unresponsive cells in the cerebral cortex. Exp Brain Res. 1967;3(4):306–319. doi: 10.1007/BF00237557. [DOI] [PubMed] [Google Scholar]
- Levitan H., Tauc L. Acetylcholine receptors: topographic distribution and pharmacological properties of two receptor types on a single molluscan neurone. J Physiol. 1972 May;222(3):537–558. doi: 10.1113/jphysiol.1972.sp009813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayeri E., Koester J., Kupfermann I., Liebeswar G., Kandel E. R. Neural control of circulation in Aplysia. I. Motoneurons. J Neurophysiol. 1974 May;37(3):458–475. doi: 10.1152/jn.1974.37.3.458. [DOI] [PubMed] [Google Scholar]
- Moreton R. B. An application of the constant-field theory to the behaviour of giant neurones of the snail, Helix aspersa. J Exp Biol. 1968 Jun;48(3):611–623. doi: 10.1242/jeb.48.3.611. [DOI] [PubMed] [Google Scholar]
- Nelson R. A comparison of electrical properties of neurons in Necturus retina. J Neurophysiol. 1973 May;36(3):519–535. doi: 10.1152/jn.1973.36.3.519. [DOI] [PubMed] [Google Scholar]
- Paupardin-Tritsch D., Gerschenfeld H. M. Neuronal responses to 5-hydroxytryptamine resulting from membrane permeability decreases. Nat New Biol. 1973 Aug 8;244(136):171–173. doi: 10.1038/newbio244171a0. [DOI] [PubMed] [Google Scholar]
- Paupardin-Tritsch D., Gerschenfeld H. M. Transmitter role of serotonin in identified synapses in Aplysia nervous system. Brain Res. 1973 Aug 30;58(2):529–534. doi: 10.1016/0006-8993(73)90027-9. [DOI] [PubMed] [Google Scholar]
- Russell J. M., Brown A. M. Active transport of potassium and chloride in an identifiable molluscan neuron. Science. 1972 Mar 31;175(4029):1475–1477. doi: 10.1126/science.175.4029.1475. [DOI] [PubMed] [Google Scholar]
- Siggins G. R., Oliver A. P., Hoffer B. J., Bloom F. E. Cyclic adenosine monophosphate and norepinephrine: effects on transmembrane properties of cerebellar Purkinje cells. Science. 1971 Jan 15;171(3967):192–194. doi: 10.1126/science.171.3967.192. [DOI] [PubMed] [Google Scholar]
- Stefani E., Gerschenfeld H. M. Comparative study of acetylcholine and 5-hydroxytryptamine receptors on single snail neurons. J Neurophysiol. 1969 Jan;32(1):64–74. doi: 10.1152/jn.1969.32.1.64. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. On the permeability of end-plate membrane during the action of transmitter. J Physiol. 1960 Nov;154:52–67. doi: 10.1113/jphysiol.1960.sp006564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAUC L., GERSCHENFELD H. M. Cholinergic transmission mechanisms for both excitation and inhibition in molluscan central synapses. Nature. 1961 Oct 28;192:366–367. doi: 10.1038/192366a0. [DOI] [PubMed] [Google Scholar]
- Takeuchi A., Takeuchi N. On the permeability of the presynaptic terminal of the crayfish neuromuscular junction during synaptic inhibition and the action of gamma-aminobutyric acid. J Physiol. 1966 Mar;183(2):433–449. doi: 10.1113/jphysiol.1966.sp007875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. Intracellular sodium activity and the sodium pump in snail neurones. J Physiol. 1972 Jan;220(1):55–71. doi: 10.1113/jphysiol.1972.sp009694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T. Electrophysiological study of the mechanisms subserving color coding in the fish retina. Cold Spring Harb Symp Quant Biol. 1965;30:559–566. doi: 10.1101/sqb.1965.030.01.054. [DOI] [PubMed] [Google Scholar]
- Wachtel H., Kandel E. R. Conversion of synaptic excitation to inhibition at a dual chemical synapse. J Neurophysiol. 1971 Jan;34(1):56–68. doi: 10.1152/jn.1971.34.1.56. [DOI] [PubMed] [Google Scholar]
- Weight F. F., Padjen A. Acetylcholine and slow synaptic inhibition in frog sympathetic ganglion cells. Brain Res. 1973 May 30;55(1):225–228. doi: 10.1016/0006-8993(73)90506-4. [DOI] [PubMed] [Google Scholar]
- Weight F. F., Padjen A. Slow synaptic inhibition: evidence for synaptic inactivation of sodium conductance in sympathetic ganglion cells. Brain Res. 1973 May 30;55(1):219–224. doi: 10.1016/0006-8993(73)90505-2. [DOI] [PubMed] [Google Scholar]
- Weight F. F., Votava J. Slow synaptic excitation in sympathetic ganglion cells: evidence for synaptic inactivation of potassium conductance. Science. 1970 Nov 13;170(3959):755–758. doi: 10.1126/science.170.3959.755. [DOI] [PubMed] [Google Scholar]


