Abstract
Many glucosamine residues of the pneumococcal peptidoglycan (PG) are not acetylated, which makes the PG resistant to lysozyme. A capsular type III mutant with an inactivated pgdA gene (encoding the peptidoglycan N-acetylglucosamine deacetylase A) became hypersensitive to exogenous lysozyme and showed reduced virulence in the intraperitoneal mouse model.
Streptococcus pneumoniae colonizes the human nasopharynx and can cause potentially life-threatening infections. The glycan strands in the cell wall peptidoglycan of this bacterium have been shown to contain a high percentage of nonacetylated glucosamine residues due to the action of the recently identified PgdA deacetylase (7), and the relative resistance of the pneumococcal peptidoglycan to lysozyme parallels this property. Chemical N acetylation of isolated peptidoglycan glycan strands or the isolated pneumococcal peptidoglycan greatly increased susceptibility of the peptidoglycan to degradation by lysozyme (7). Deacetylation of peptidoglycan is known to cause reduced susceptibility of several bacterial species to lysozyme (1, 2, 3, 8), a muramidase which cleaves the glycosidic bond between MurNAc and GlcNAc in the glycan strands.
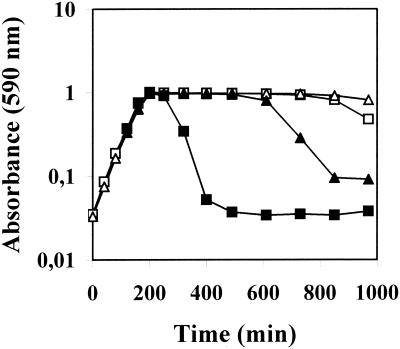
The mutant strain R36A::pPGDA has a nonfunctional pgdA gene and carries an erythromycin resistance marker due to insertion duplication mutagenesis using plasmid pPGDA (7). This strain produces fully acetylated peptidoglycan and is hypersensitive to lysozyme in the stationary phase of growth (7). The encapsulated mutant strain R36A::pPGDA(SIII) (capsular type III) was constructed by transformation of the nonencapsulated strain R36A::pPGDA with DNA from a capsular type III wild-type strain. The mucous colonies formed by the encapsulated transformants were clearly distinguishable (on the basis of their appearance on blood agar plates) from colonies of nonencapsulated pneumococci, and the correct capsular type (type III) of the transformants was confirmed by serotyping. The capsular phenotypes of R36A::pPGDA(SIII) and the encapsulated parental strain R36A(SIII) as well as the erythromycin resistance marker in the mutant were stable for at least 40 generations of growth in antibiotic-free C+Y medium (5). Also, the pgdA mutant cells expressing the type III capsule showed no morphological abnormalities when investigated by light microscopy and grew with the same generation time, 37 min, as R36A(SIII) in C+Y medium. Addition of 20 μg of lysozyme per ml to cultures of R36A::pPGDA(SIII) caused immediate rapid lysis in the stationary phase of growth (Fig. 1), indicating that the presence of the type III capsule did not diminish the higher sensitivity of the pgdA mutant towards exogenous lysozyme, an observation already described for the nonencapsulated strain R36A (7).
FIG. 1.
Increased lysozyme sensitivity of the type III pgdA mutant strain. R36A::pPGDA(SIII) (squares) and the parental strain R36A(SIII) (triangles) were grown in C+Y medium. At an optical density (590 nm) of 0.4, the cultures were divided, and to one part (closed symbols) 20 μg of lysozyme per ml was added. The control cultures (open symbols) received no lysozyme.
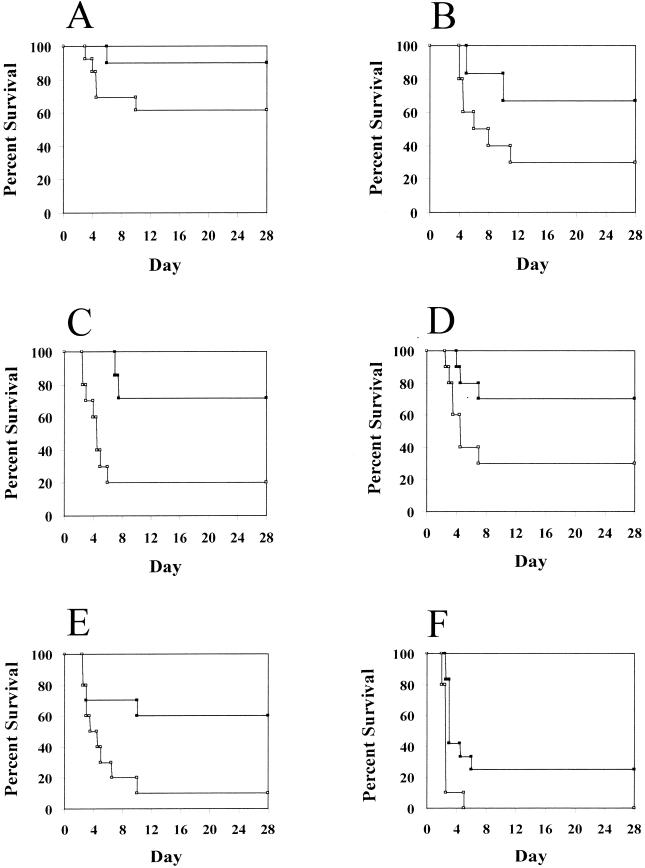
Cultures of R36A::pPGDA(SIII) and the parental strain R36A(SIII) were grown in cap medium (tryptic soy broth supplemented with 10 mg of glucose per ml, 2.5 mg of Difco yeast extract per ml, and 10% C+Y medium) to an optical density (590 nm) of 0.5. After centrifugation, the cells were resuspended in ice-cold nonpyrogenic 0.9% saline (Abbott Laboratories), and serial dilutions were made in the same solvent. Six groups of 8-week-old CD1 mice received 102, 103, 104, 105, 106, and 107 CFU of R36A::pPGDA(SIII) or R36A(SIII) injected into the peritoneal cavity. The survival of the mice was monitored for 28 days. The pgdA mutant was less virulent than the parental strain (Fig. 2). For all inocula, more mice which received the mutant strain survived and the mean survival time of these mice was higher. For example, in the group receiving 106 bacteria, 6 of 10 mice receiving the mutant strain survived, whereas only 1 of 10 mice receiving the parental strain survived. Statistical analysis using a stratified Wilcoxon/Mann-Whitney test confirmed the significantly lower virulence of the pgdA mutant strain (P < 0.005).
FIG. 2.
Decreased virulence of the pgdA mutant strain. Groups of 7 to 14 mice received an intraperitoneal injection of 102 (A), 103 (B), 104 (C), 105 (D), 106 (E), and 107 (F) CFU of either R36A::pPGDA(SIII) (closed squares) or the control strain R36A(SIII) (open squares). The survival of the mice is shown for the experimental period of 28 days.
The two observations described here, namely, the hypersensitivity of pgdA mutants to exogenous lysozyme and the reduced virulence of the mutant, suggest that pgdA may be a virulence determinant in S. pneumoniae. Lysozyme is part of the first-line defense mechanism in the human host against invading bacteria. Accumulation of lysozyme at high concentrations occurs in human meningeal disease (6) and was also experimentally demonstrated in the rabbit model of experimental meningitis after installation of pneumococci into the cerebrospinal fluid (4). The greatly increased sensitivity of pneumococcal cultures to exogenous lysozyme in the pgdA mutant is consistent with the full acetylation of the peptidoglycan in the mutants. Inactivation of pgdA also caused drastic reduction in the virulence of S. pneumoniae in the mouse model of intraperitoneal inoculation. One cannot exclude the possibility that the reduced virulence of the mutant bacteria may be an as-yet-undefined indirect consequence of the full acetylation of peptidoglycan on the pneumococcal surface. However, we favor a more direct mechanism. We propose that pgdA is a virulence determinant: deacetylation of the peptidoglycan by the activity of PgdA may provide pneumococci with increased resistance against the lysozyme of the human host, which translates to the decreased virulence of the mutant.
Acknowledgments
Partial support for this study was provided by grant RO1 AI37257 from the National Institutes of Health and by the Irene Diamond Foundation and by funds granted by the Charles H. Revson Foundation (to W.V.).
We thank biostatistician Knut Wittkowski (The Rockefeller University Hospital) for statistical analysis of the virulence study.
Editor: J. T. Barbieri
REFERENCES
- 1.Amano, K., H. Hayashi, Y. Araki, and E. Ito. 1977. The action of lysozyme on peptidoglycan with N-unsubstituted glucosamine residues. Isolation of glycan fragments and their susceptibility to lysozyme. Eur. J. Biochem. 76:299-307. [DOI] [PubMed] [Google Scholar]
- 2.Araki, Y., S. Fukuoka, S. Oba, and E. Ito. 1971. Enzymatic deacetylation of N-acetylglucosamine residues in peptidoglycan from Bacillus cereus cell walls. Biochem. Biophys. Res. Commun. 45:751-758. [DOI] [PubMed] [Google Scholar]
- 3.Atrih, A., G. Bacher, G. Allmaier, M. P. Williamson, and S. J. Foster. 1999. Analysis of peptidoglycan structure from vegetative cells of Bacillus subtilis 168 and role of PBP 5 in peptidoglycan maturation. J. Bacteriol. 181:3956-3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cottagnoud, P., and A. Tomasz. 1993. Triggering of pneumococcal autolysis by lysozyme. J. Infect. Dis. 167:684-690. [DOI] [PubMed] [Google Scholar]
- 5.Horne, D. S., and A. Tomasz. 1993. Possible role of a choline-containing teichoic acid in the maintenance of normal cell shape and physiology in Streptococcus oralis. J. Bacteriol. 175:1717-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klockars, M., S. Reitamo, T. Weber, and Y. Kerttula. 1978. Cerebrospinal fluid lysozyme in bacterial and viral meningitis. Acta Med. Scand. 203:71-74. [DOI] [PubMed] [Google Scholar]
- 7.Vollmer, W., and A. Tomasz. 2000. The pgdA gene encodes for a peptidoglycan N-acetylglucosamine deacetylase in Streptococcus pneumoniae. J. Biol. Chem. 275:20496-20501. [DOI] [PubMed] [Google Scholar]
- 8.Westmacott, D., and H. R. Perkins. 1979. Effects of lysozyme on Bacillus cereus 569: rupture of chains of bacteria and enhancement of sensitivity to autolysins. J. Gen. Microbiol. 115:1-11. [DOI] [PubMed] [Google Scholar]