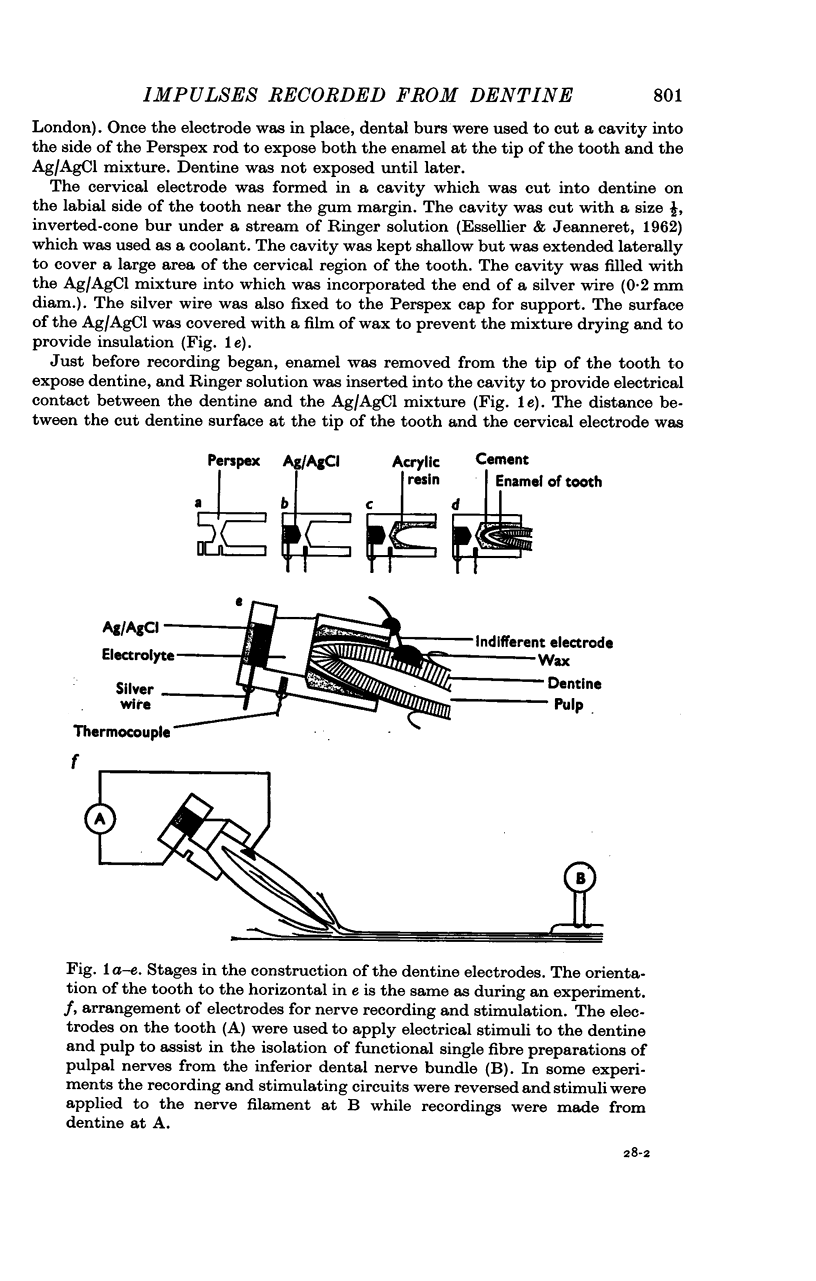
Abstract
1. Recordings have been made from dentine at the tip of the canine teeth of cats using a large Ag/AgCl electrode.
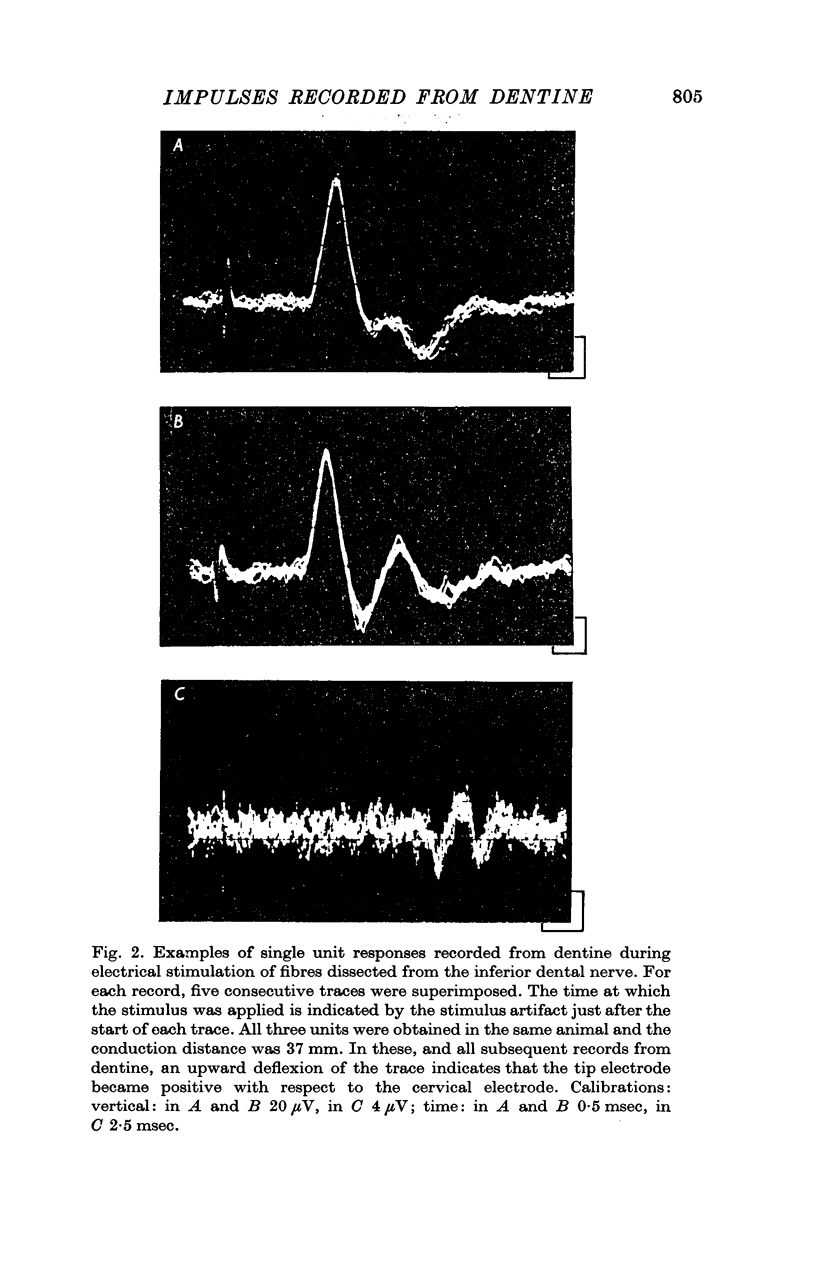
2. All-or-none action potentials with complex shapes were recorded when single nerve fibres from the dental pulp were stimulated electrically outside the tooth.
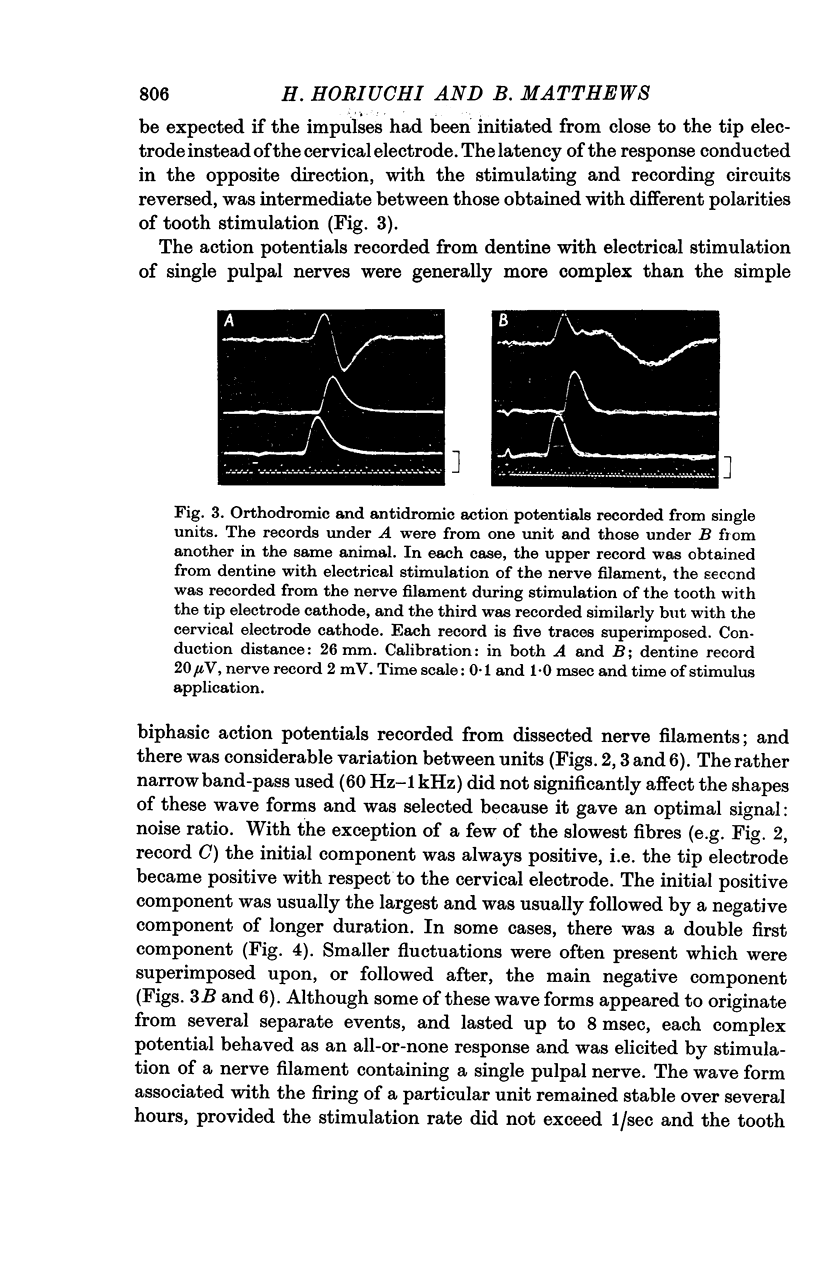
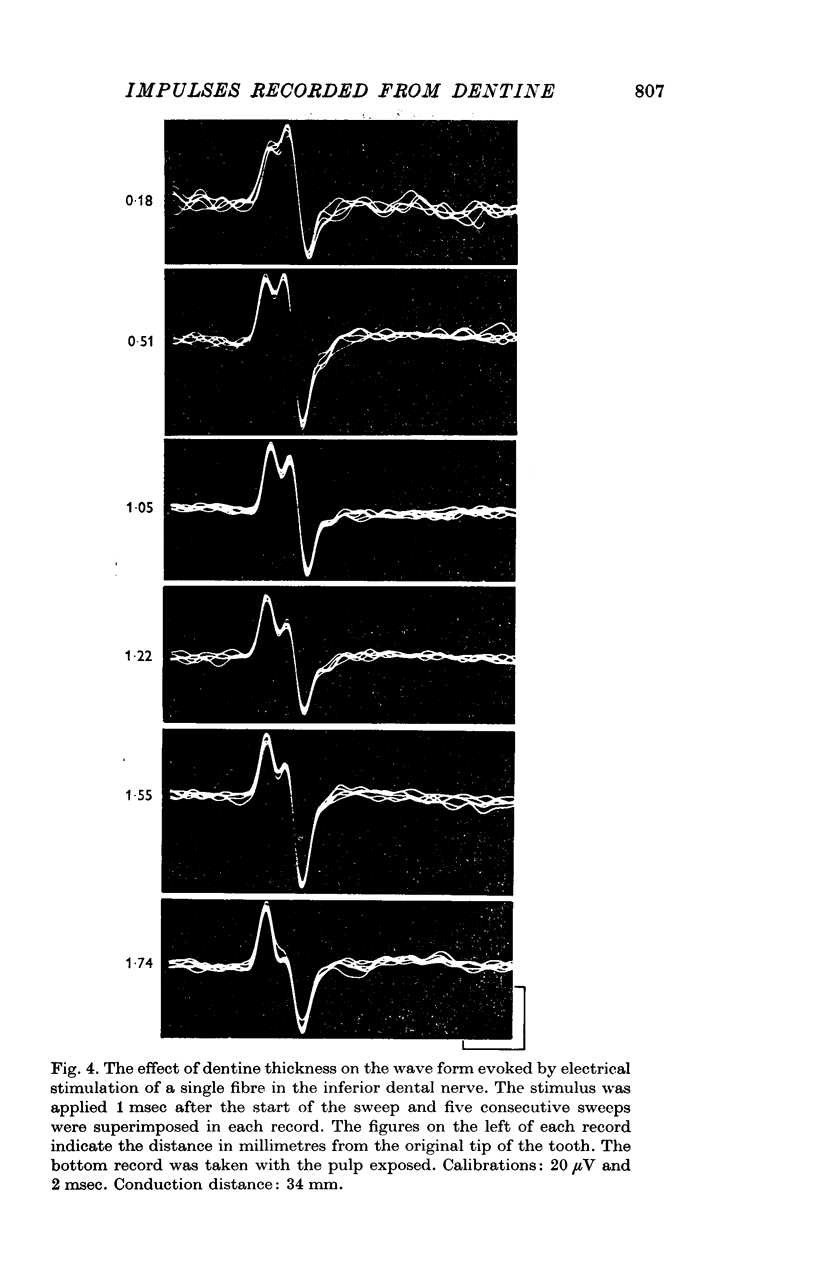
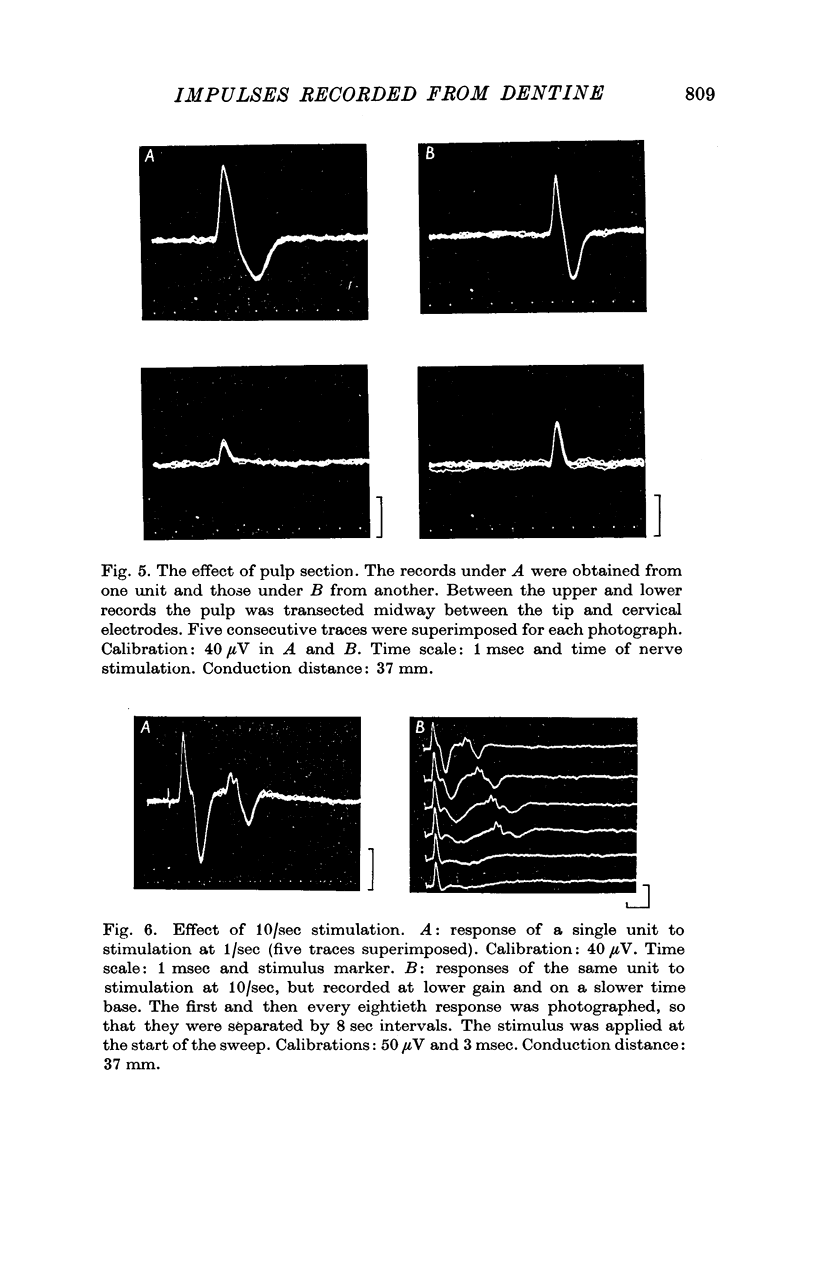
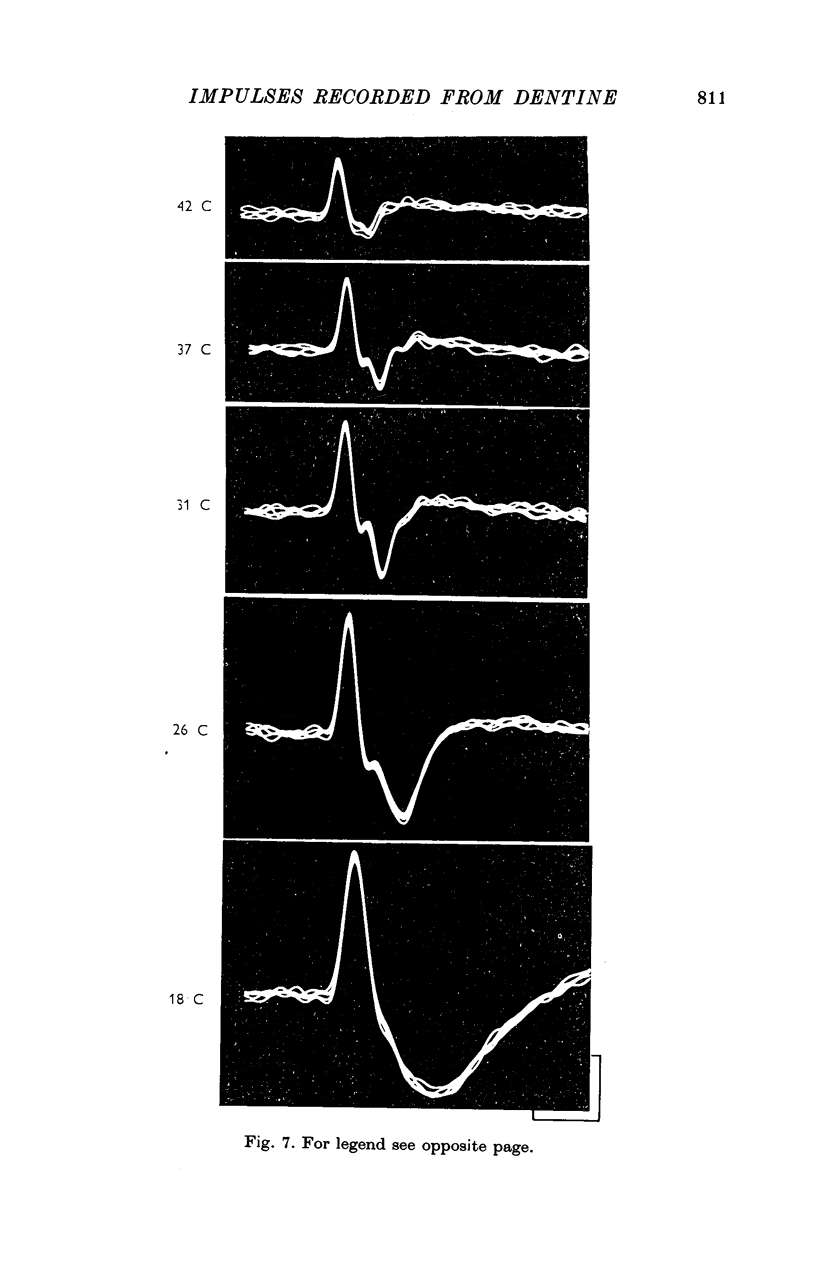
3. The wave forms of these action potentials changed when the stimulation rate was increased from 1/sec to 10/sec, when the temperature of the tooth surface was changed between 17 and 42° C, when the thickness of the dentine was reduced, and when local anaesthetic was applied to the dentine. Only a small, monophasic, positive potential remained after transection of the pulp in the crown of the tooth.
4. The latency of the action potentials was not affected by these same procedures.
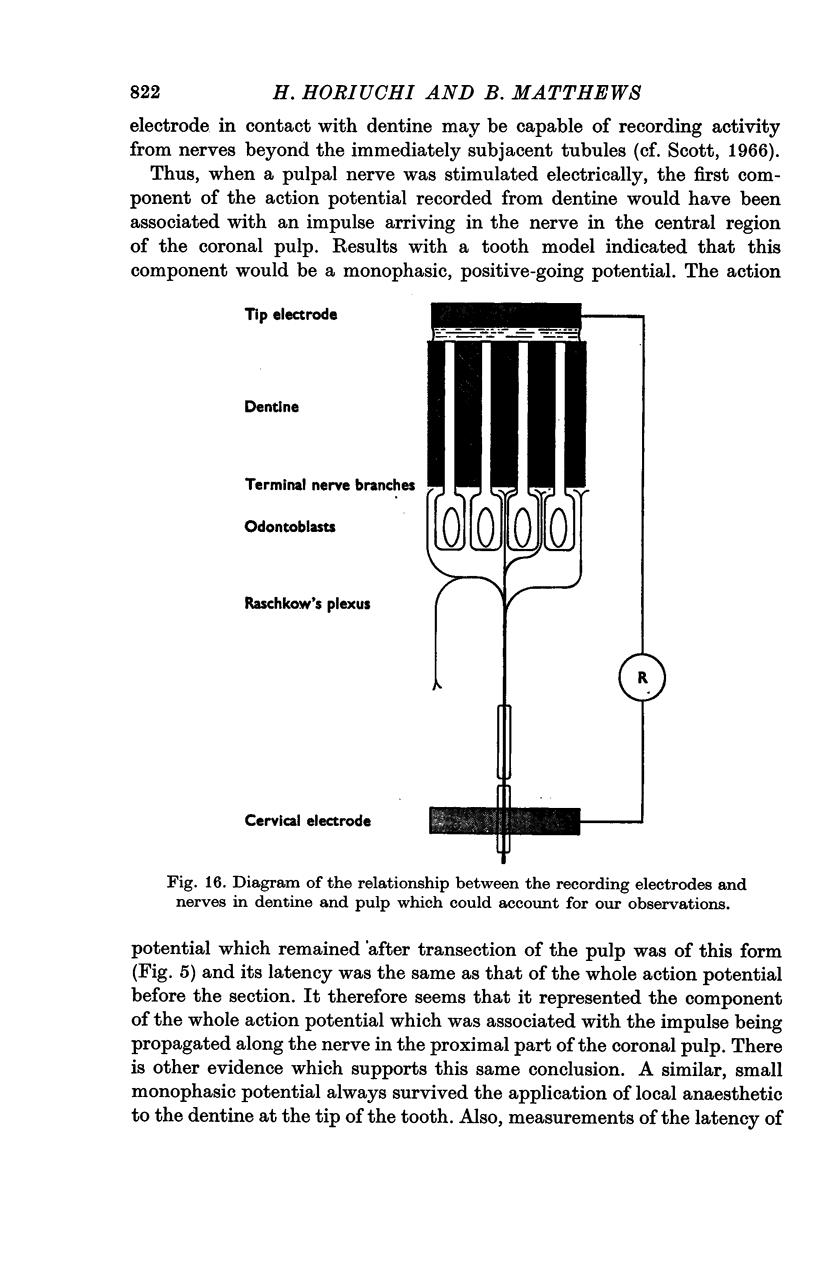
5. It is suggested that each wave form represented a compound action potential produced by impulses invading the main branches and terminals of a single nerve in the pulp. Some of the terminals may have penetrated the innermost layers of the dentine.
6. There was no spontaneous discharge from pulpal nerves.
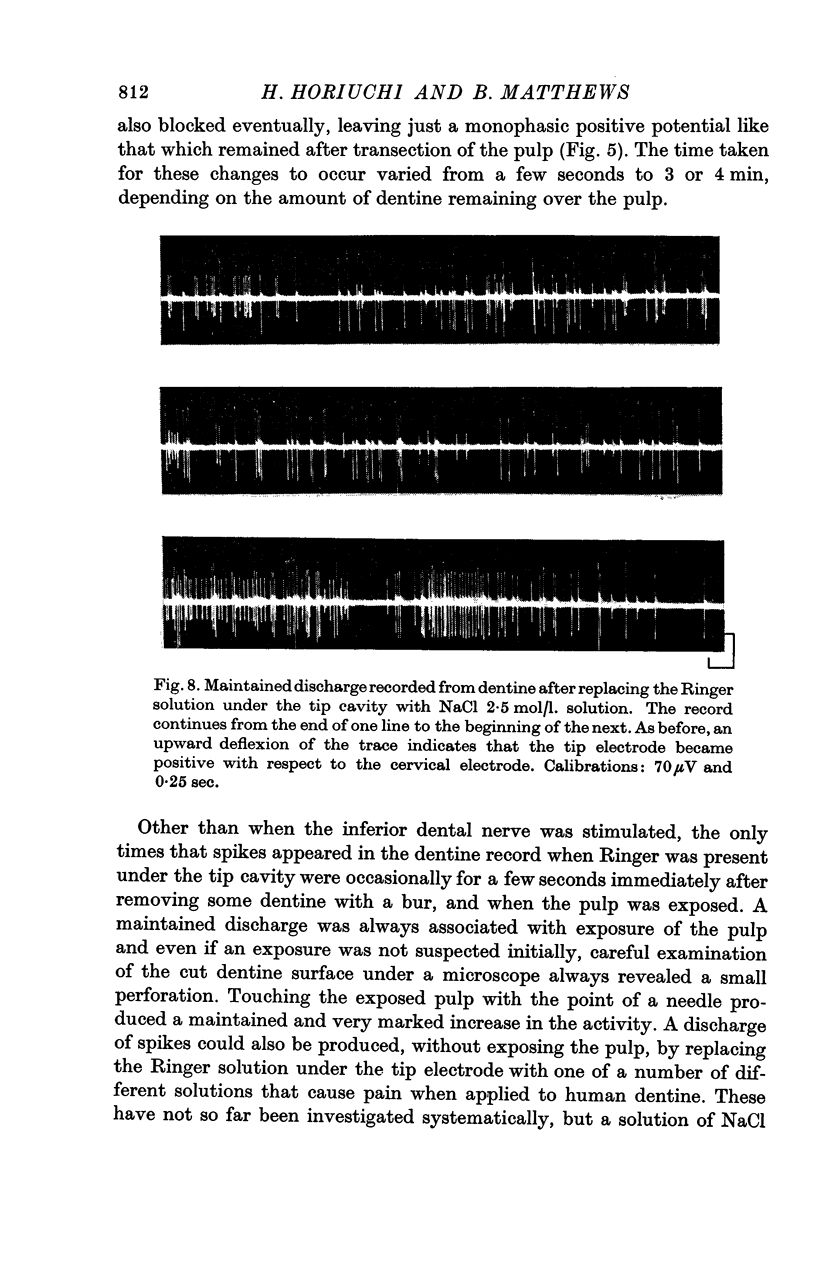
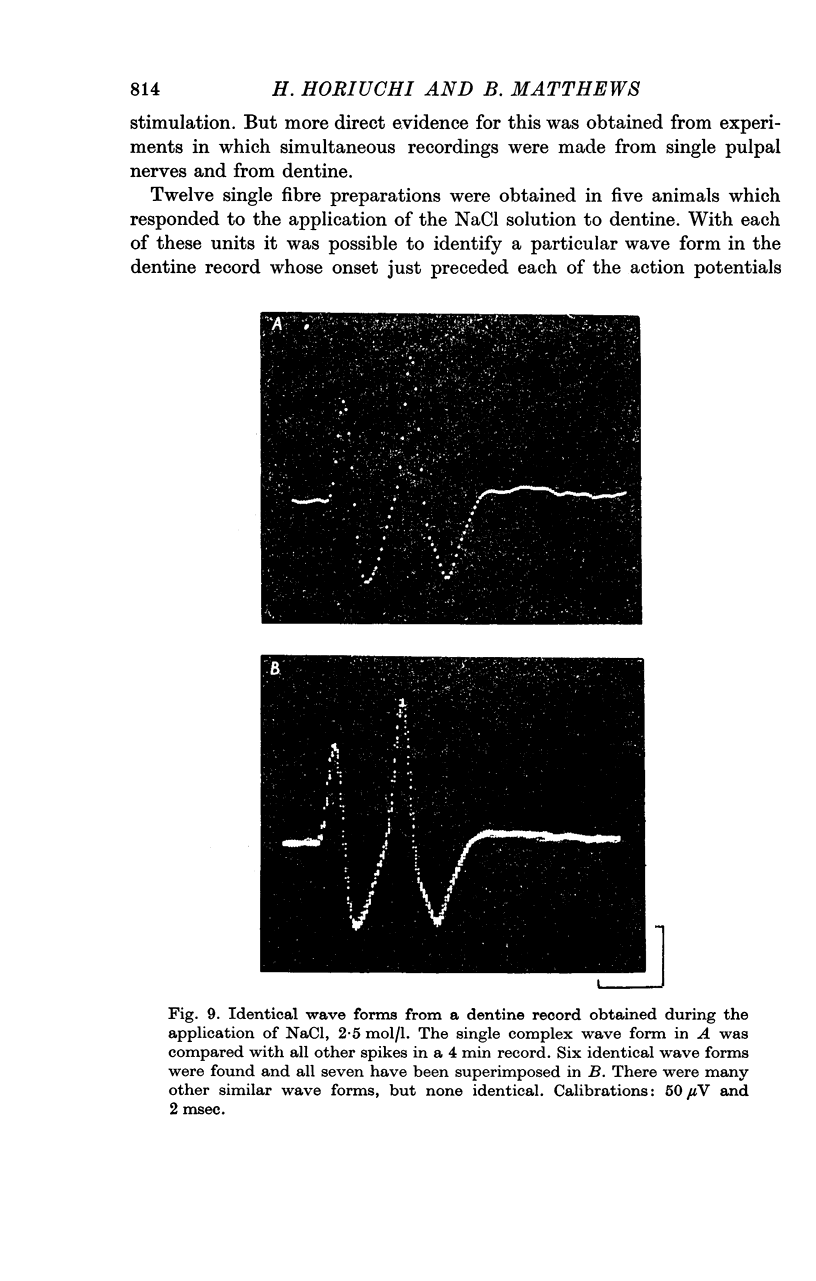
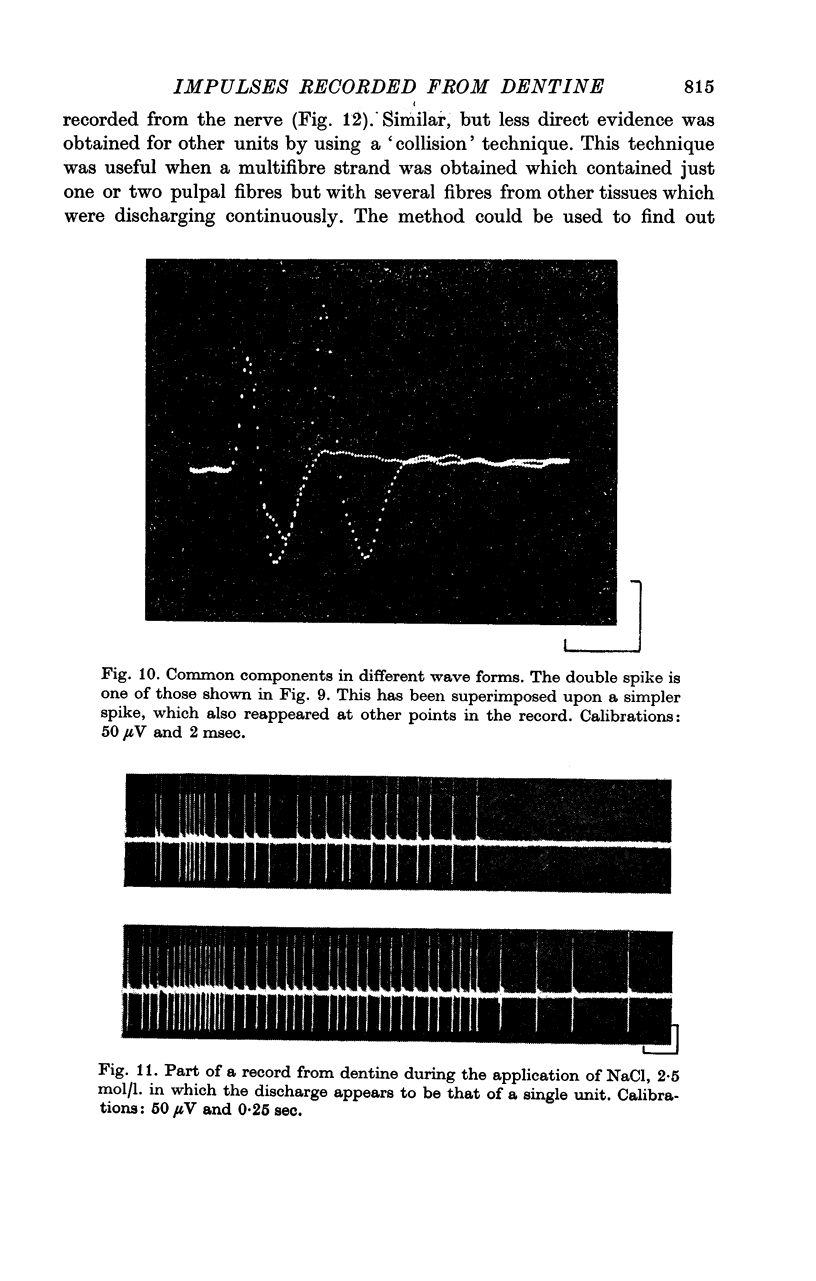
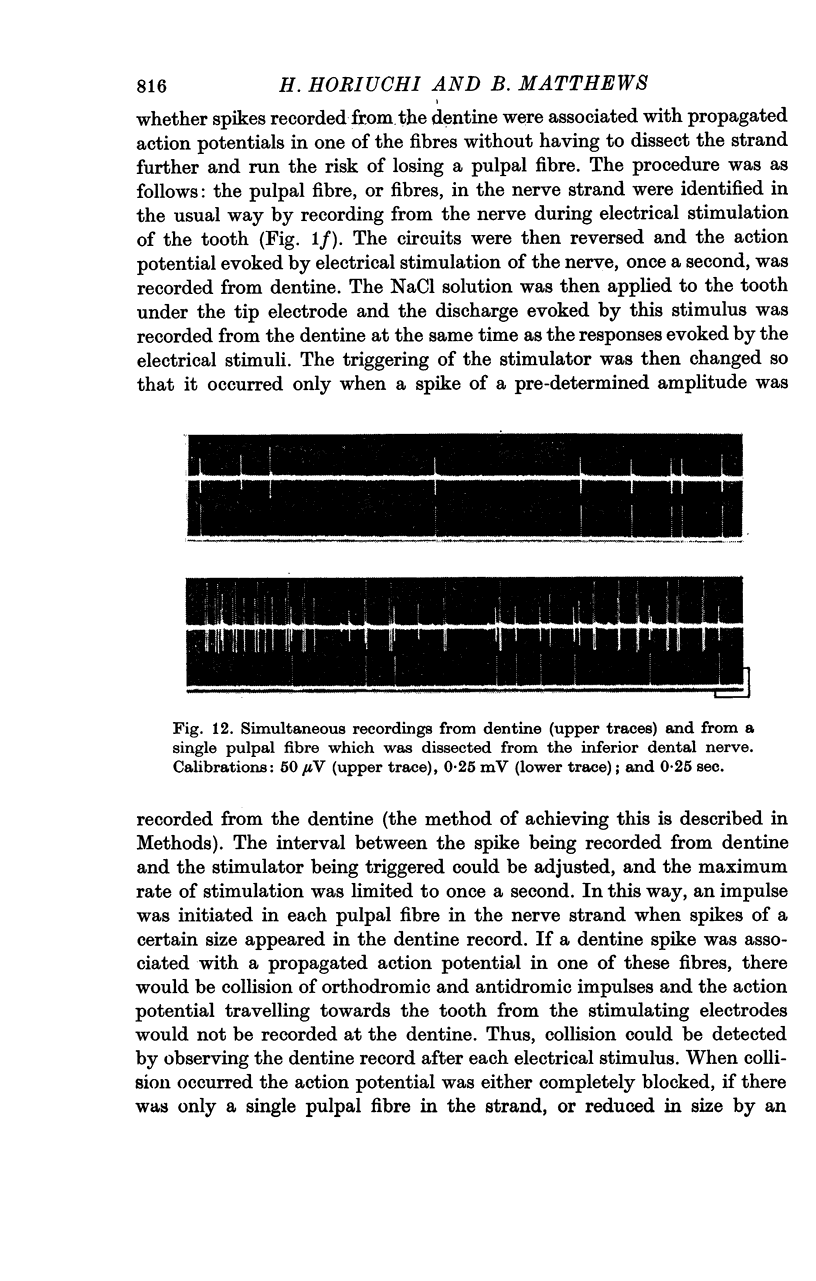
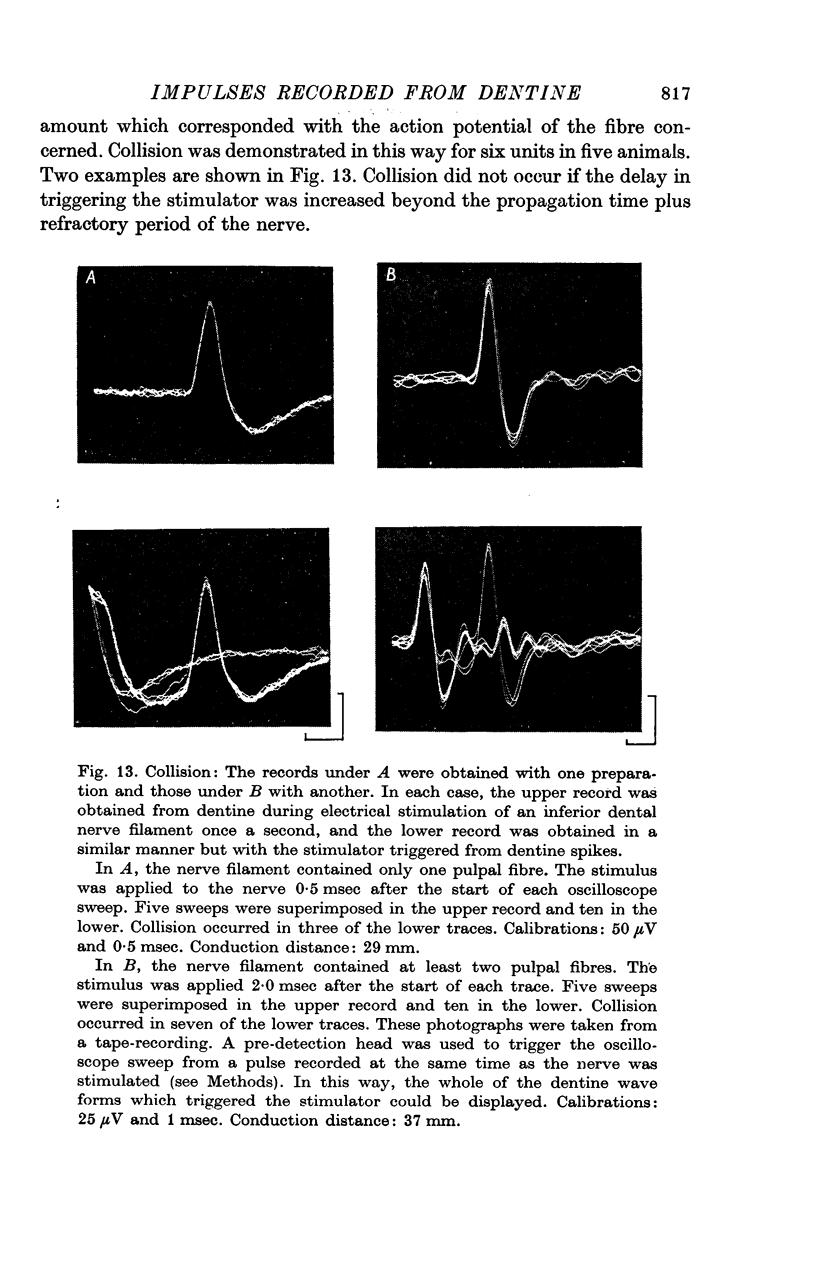
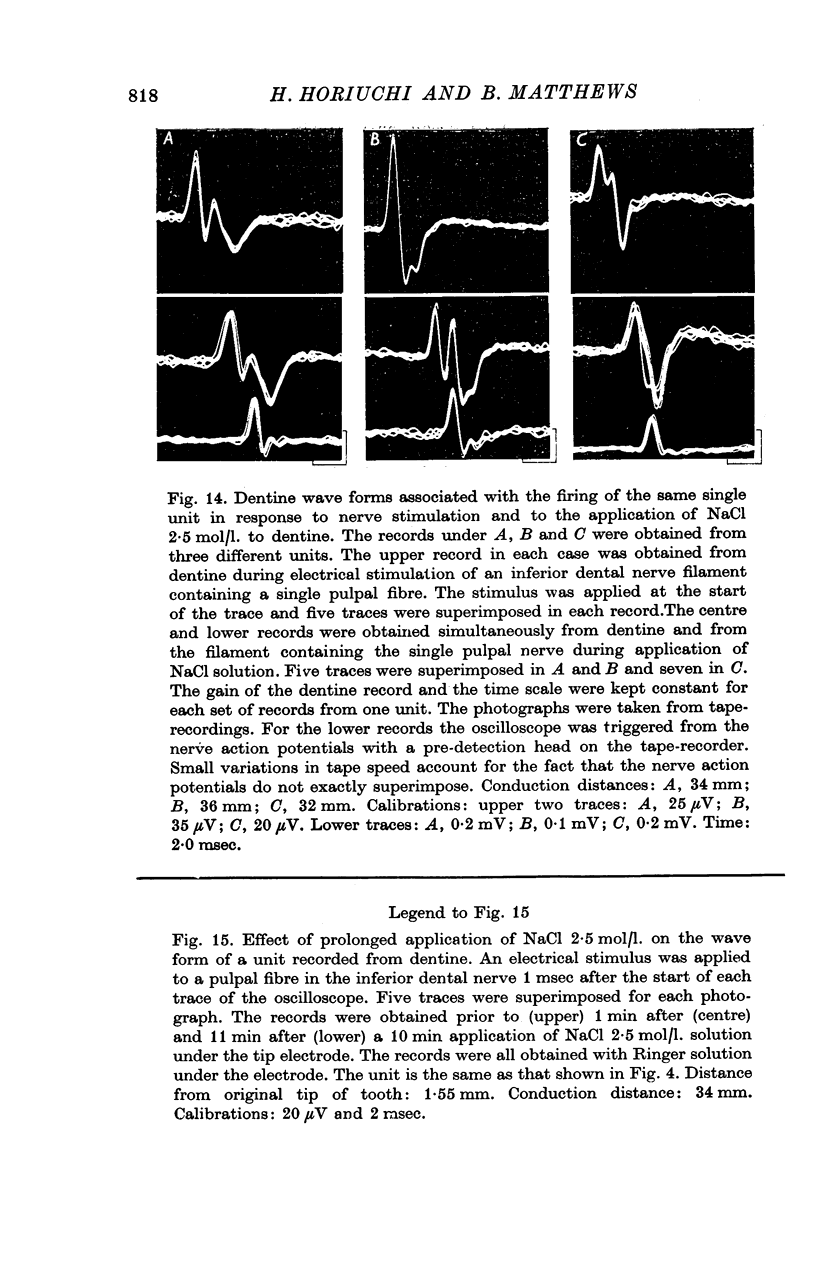
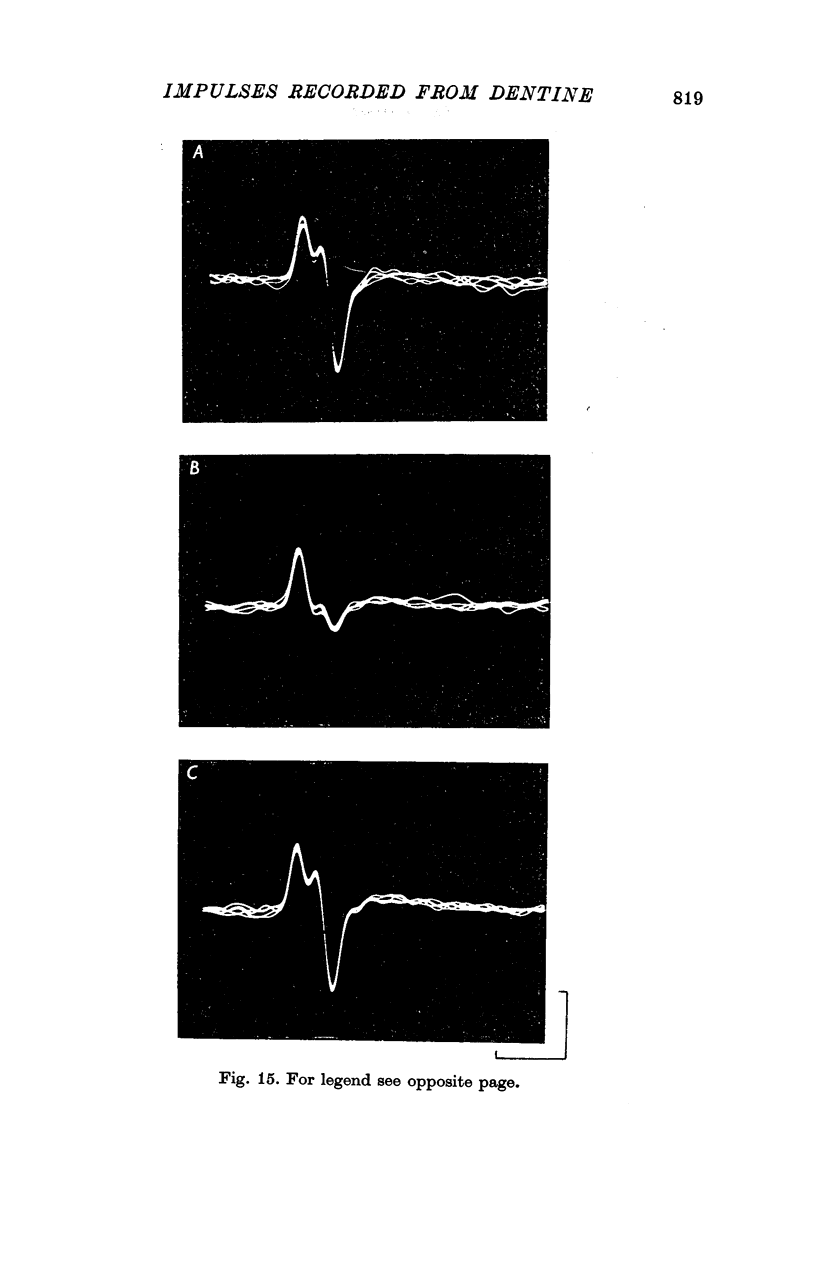
7. A discharge of impulses was recorded from dentine when 2·5 mol/l. NaCl was applied beneath the electrode at the tip of the tooth. By recording simultaneously from dentine and from single fibres from the tooth pulp, it was shown that impulses recorded from dentine were associated with propagated nerve action potentials.
Full text
PDF


















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. J., Hannam A. G., Mathews B. Sensory mechanisms in mammalian teeth and their supporting structures. Physiol Rev. 1970 Apr;50(2):171–195. doi: 10.1152/physrev.1970.50.2.171. [DOI] [PubMed] [Google Scholar]
- Anderson D. J., Matthews B. Osmotic stimulation of human dentine and the distribution of dental pain thresholds. Arch Oral Biol. 1967 Mar;12(3):417–426. doi: 10.1016/0003-9969(67)90227-0. [DOI] [PubMed] [Google Scholar]
- FEARNHEAD R. W. Histological evidence for the innervation of human dentine. J Anat. 1957 Apr;91(2):267–277. [PMC free article] [PubMed] [Google Scholar]
- FEARNHEAD R. W. The neurohistology of human dentine. Proc R Soc Med. 1961 Oct;54:877–884. [PMC free article] [PubMed] [Google Scholar]
- Flasterstein A. H. A general analysis of voltage fluctuations of metal-electrolyte interfaces. Med Biol Eng. 1966 Nov;4(6):589–593. doi: 10.1007/BF02474829. [DOI] [PubMed] [Google Scholar]
- Flasterstein A. H. Voltage fluctuations of metal-electrolyte interfaces in electrophysiology. Med Biol Eng. 1966 Nov;4(6):583–588. doi: 10.1007/BF02474828. [DOI] [PubMed] [Google Scholar]
- Frank R. M. Attachment sites between the odontoblast process and the intradentinal nerve fibre. Arch Oral Biol. 1968 Jul;13(7):833–834. doi: 10.1016/0003-9969(68)90104-0. [DOI] [PubMed] [Google Scholar]
- Franz D. N., Iggo A. Conduction failure in myelinated and non-myelinated axons at low temperatures. J Physiol. 1968 Dec;199(2):319–345. doi: 10.1113/jphysiol.1968.sp008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood F., Horiuchi H., Matthews B. Electrophysiological evidence on the types of nerve fibres excited by electrical stimulation of teeth with a pulp tester. Arch Oral Biol. 1972 Apr;17(4):701–709. doi: 10.1016/0003-9969(72)90196-3. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of temperature on the electrical activity of the giant axon of the squid. J Physiol. 1949 Aug;109(1-2):240–249. doi: 10.1113/jphysiol.1949.sp004388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIRALY J. K., KRNJEVIC K. Some retrograde changes in function of nerves after peripheral section. Q J Exp Physiol Cogn Med Sci. 1959 Jul;44:244–257. doi: 10.1113/expphysiol.1959.sp001397. [DOI] [PubMed] [Google Scholar]
- King J. S., Jewett D. L., Sundberg H. R. Differential blockade of cat dorsal root C fibers by various chloride solutions. J Neurosurg. 1972 May;36(5):569–583. doi: 10.3171/jns.1972.36.5.0569. [DOI] [PubMed] [Google Scholar]
- MURRAY R. W. The initiation of cutaneous nerve impulses in elasmobranch fishes. J Physiol. 1961 Dec;159:546–570. doi: 10.1113/jphysiol.1961.sp006826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCOTT D., Jr, TEMPEL T. R. NEUROPHYSIOLOGICAL RESPONSE OF SINGLE RECEPTOR UNITS IN THE TOOTH OF THE CAT. J Dent Res. 1965 Jan-Feb;44:20–27. doi: 10.1177/00220345650440011701. [DOI] [PubMed] [Google Scholar]
- Scott D., Jr, Stewart G. G. Excitation of the dentinal recptor of the cat by heat and chemical agents. Oral Surg Oral Med Oral Pathol. 1965 Dec;20(6):784–794. doi: 10.1016/0030-4220(65)90142-8. [DOI] [PubMed] [Google Scholar]
- TATEDA H., MORITA H. Initiation of spike potentials in contact chemosensory hairs of insects. I. The generation site of the recorded spike potentials. J Cell Comp Physiol. 1959 Oct;54:171–176. doi: 10.1002/jcp.1030540207. [DOI] [PubMed] [Google Scholar]
- WINTER H. F., BISHOP J. G., DORMAN H. L. Transmembrane potentials of odontoblasts. J Dent Res. 1963 Mar-Apr;42:594–598. doi: 10.1177/00220345630420020701. [DOI] [PubMed] [Google Scholar]