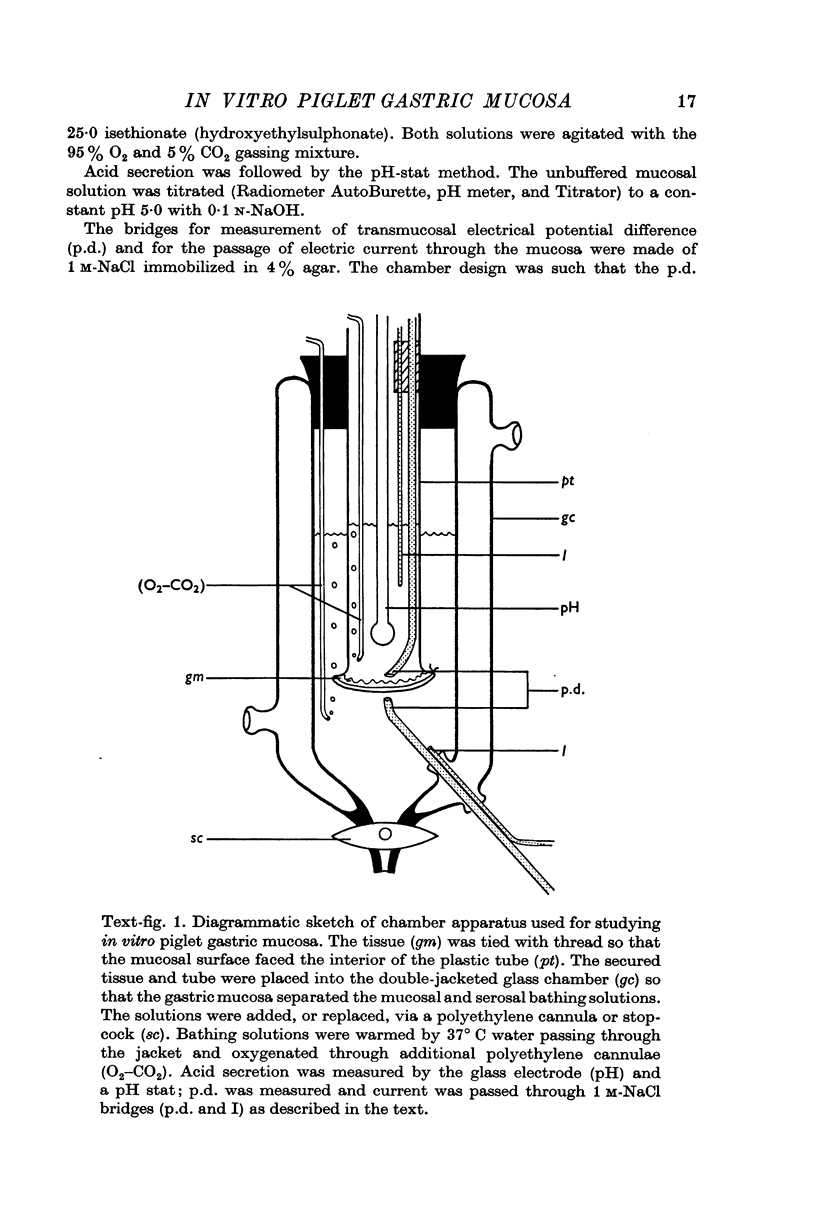
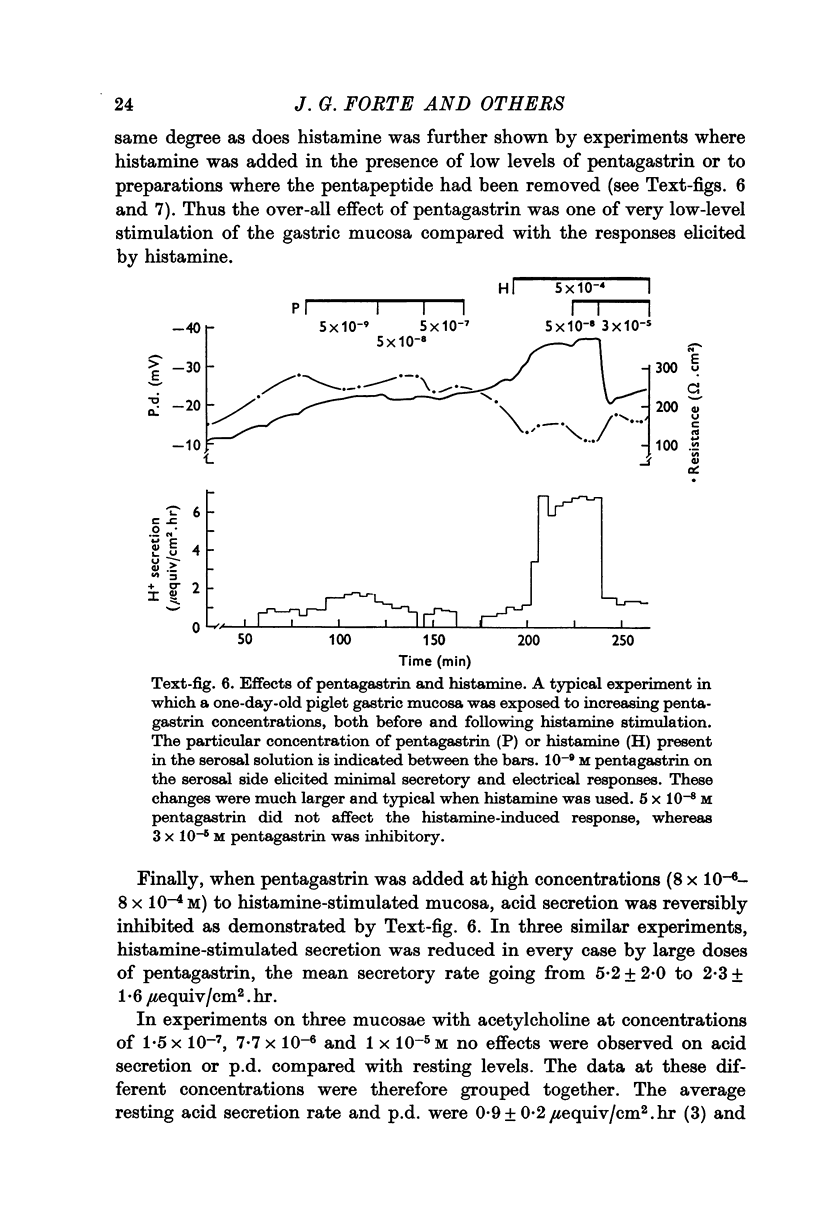
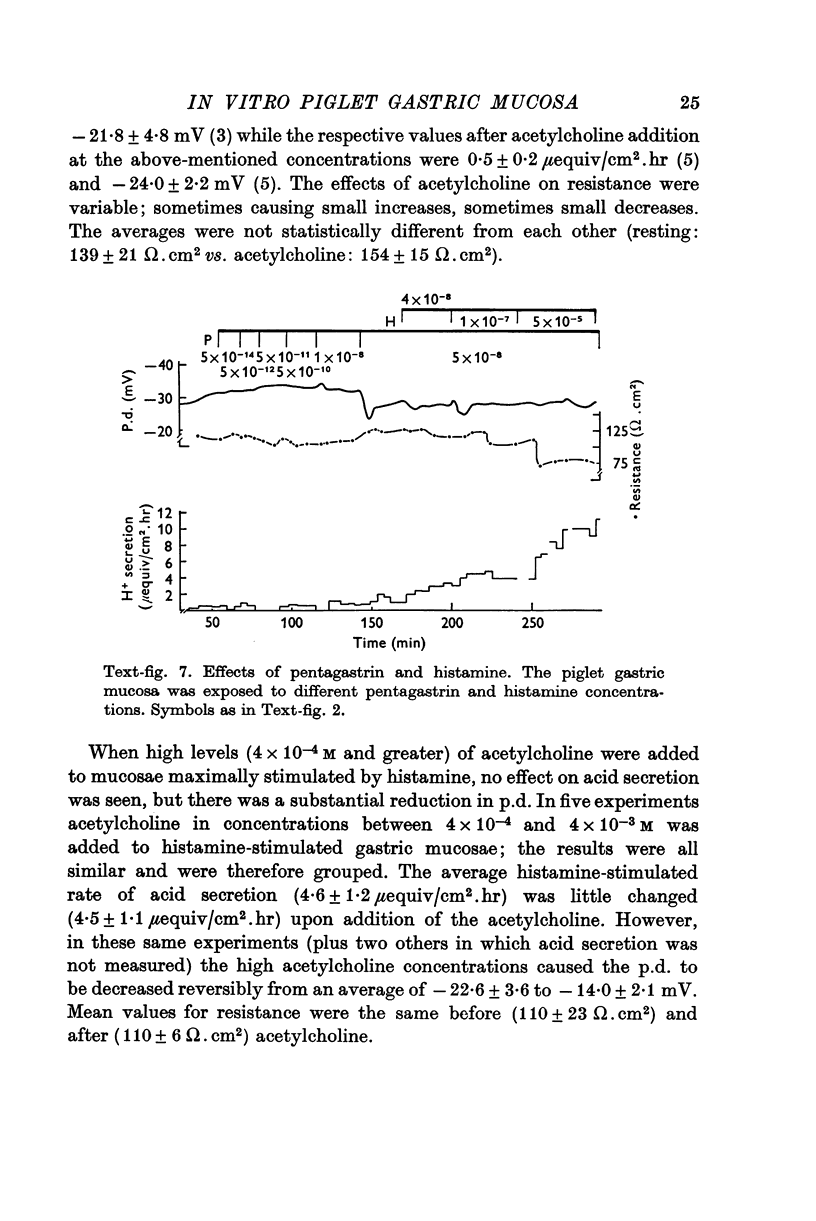
Abstract
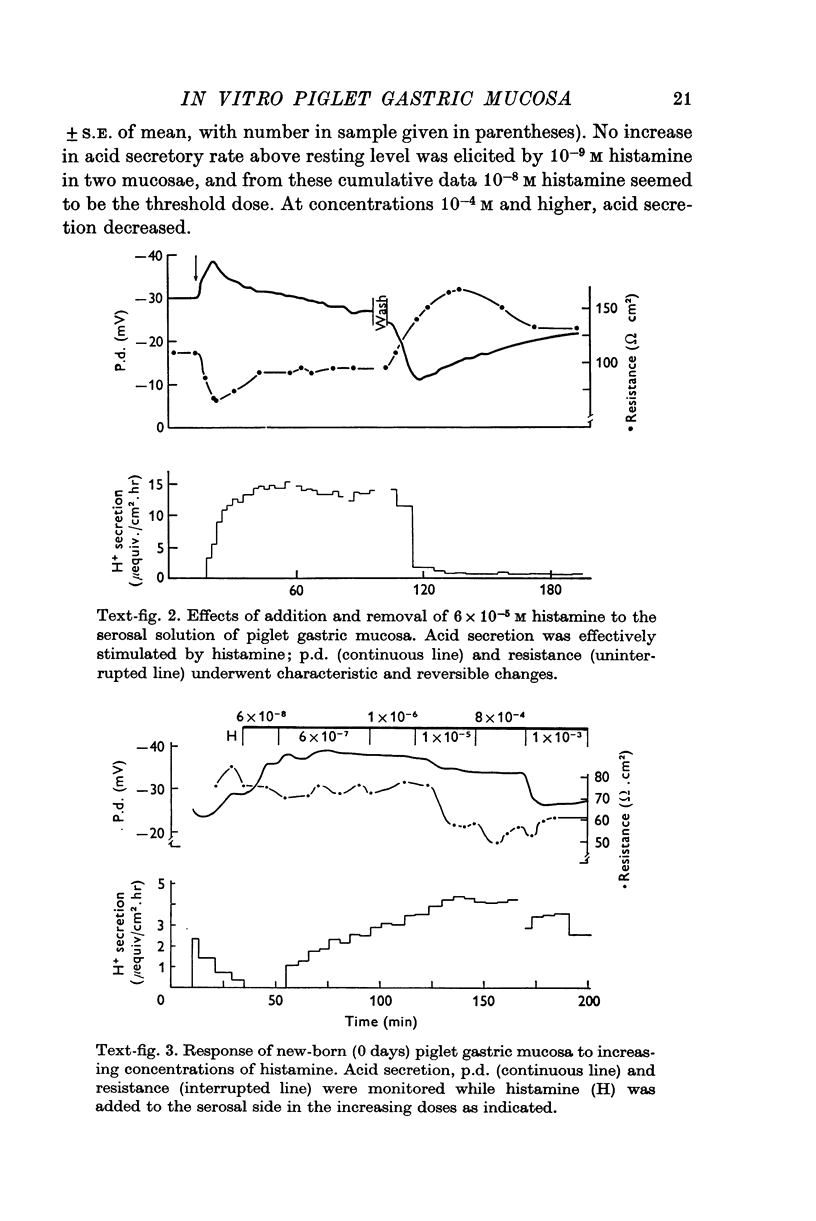
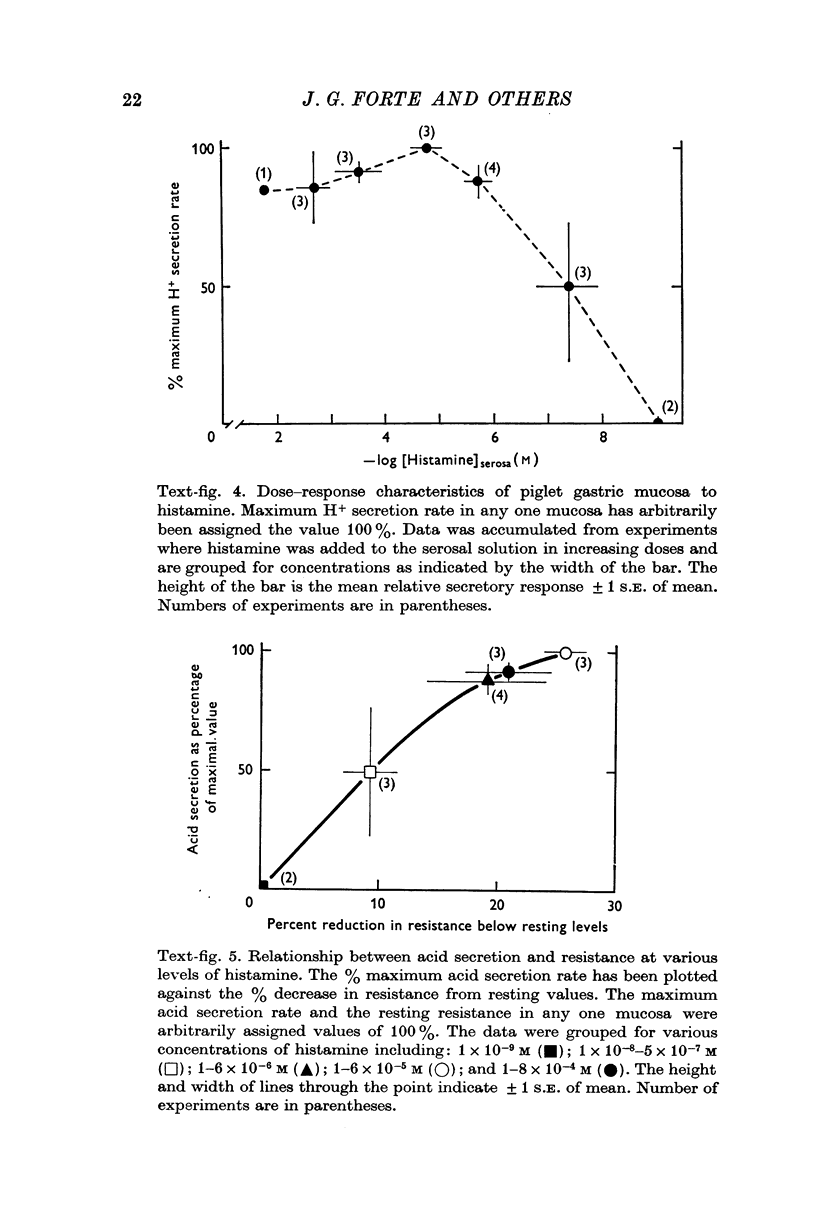
1. A new preparation of gastric mucosa isolated from new-born piglets is described. The piglet gastric mucosa was easily separated from the serosal muscle layers by a "blistering" technique which appeared to cause minimal trauma to the tissue and which allowed extended study in vitro in a suitable chamber. Normal resting p.d. was approximately minus 30 mV (mucosal side negative with respect to serosal side), resistance about 100 omega. cm-2 and H+ secretion was absent or occurred at very low rates (0-1mu-equiv/cm-2. hr). 2. Maximally stimulating doses of histamine (1-6 times 10-5 M) caused H+ secretion to increase (up to 15 muequiv/cm-2. hr), p.d. to increase and resistance to decrease. A close correlation was observed between the increase in H+ secretion and decrease in transmucosal resistance. The threshold dose of histamine appeared to be 10-8 M; concentrations 10-4 M and higher reduced H+ secretion somewhat. 3. Pentagastrin ( 10-9-10-7 M) and acetylcholine (10-7-10-5 M) did not significantly stimulate the piglet gastric mucosa. Pentagastrin concentrations above 4 times 10-6 M reversibly inhibited H+ secretion of histamine-stimulated mucosa. High concentrations of acetylcholine (above 4 times 10-4 M) did not affect histamine-stimulated H+ secretion, but a significant reduction in p.d. was observed. 4. This investigation demonstrates the utility of the piglet gastric mucosa for in vitro studies of the mechanism H+ secretion and the action of secretagogues. From a consideration of such factors as the thinness of tissue and ease of preparation it is suggested that neonatal animals may represent a good source of in vitro mammalian gastric tissue.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CUMMINS J. T., VAUGHAN B. E. IONIC RELATIONSHIPS OF THE BIOELECTROGENIC MECHANISM IN ISOLATED RAT STOMACH. Biochim Biophys Acta. 1965 Jan 25;94:280–292. doi: 10.1016/0926-6585(65)90032-4. [DOI] [PubMed] [Google Scholar]
- DAVENPORT H. W., CHAVRE V. J. Acid secretion and oxygen consumption by mouse stomachs in vitro. Am J Physiol. 1953 Aug;174(2):203–208. doi: 10.1152/ajplegacy.1953.174.2.203. [DOI] [PubMed] [Google Scholar]
- DAVENPORT H. W., CHAVRE V. J. Conditions affecting acid secretion by mouse stomachs in vitro. Gastroenterology. 1950 Jul;15(3):467–480. [PubMed] [Google Scholar]
- Eaton M. D., Scala A. R. Further observations on the inhibitory action of ammonium chloride on influenza virus. Proc Soc Exp Biol Med. 1967 May;125(1):271–275. doi: 10.3181/00379727-125-32067. [DOI] [PubMed] [Google Scholar]
- Forte J. G., Machen T. E. Transport and electrical phenomena in resting and secreting piglet gastric mucosa. J Physiol. 1975 Jan;244(1):33–51. doi: 10.1113/jphysiol.1975.sp010783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forte T. M., Forte J. G. Definition of the extracellular space in secreting and nonsecreting oxyntic cells. J Cell Biol. 1970 Dec;47(3):782–786. doi: 10.1083/jcb.47.3.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEINZ E., DURBIN R. Evidence for an independent hydrogen-ion pump in the stomach. Biochim Biophys Acta. 1959 Jan;31(1):246–247. doi: 10.1016/0006-3002(59)90461-5. [DOI] [PubMed] [Google Scholar]
- Kasbekar D. K., Ridley H. A., Forte J. G. Pentagastrin and acetylcholine relation to histamine in H+ secretion by gastric mucosa. Am J Physiol. 1969 Apr;216(4):961–967. doi: 10.1152/ajplegacy.1969.216.4.961. [DOI] [PubMed] [Google Scholar]
- Kidder G. W., 3rd Unstirred layers in tissue respiration: application to studies of frog gastric mucosa. Am J Physiol. 1970 Dec;219(6):1789–1795. doi: 10.1152/ajplegacy.1970.219.6.1789. [DOI] [PubMed] [Google Scholar]
- Kitahara S., Fox K. R., Hogben C. A. Acid secretion, Na+ absorption, and the origin of the potential difference across isolated mammalian stomachs. Am J Dig Dis. 1969 Apr;14(4):221–238. doi: 10.1007/BF02235951. [DOI] [PubMed] [Google Scholar]
- Knauf H. The minimum requirements for the maintenance of active sodium transport across the isolated salivary duct epithelium of the rabbit. Pflugers Arch. 1972;333(4):326–336. doi: 10.1007/BF00586212. [DOI] [PubMed] [Google Scholar]
- Makhlouf G. M., McManus J. P., Knill J. R. Quantitative aspects of synergism and inhibition of gastric acid secretion. Gastroenterology. 1968 Apr;54(4):532–537. [PubMed] [Google Scholar]
- REHM W. S. Acid secretion, resistance, short-circuit current, and voltage-clamping in frog's stomach. Am J Physiol. 1962 Jul;203:63–72. doi: 10.1152/ajplegacy.1962.203.1.63. [DOI] [PubMed] [Google Scholar]
- REHM W. S., DENNIS W. H., SCHLESINGER H. Electrical resistance of the mammalian stomach. Am J Physiol. 1955 May;181(2):451–470. doi: 10.1152/ajplegacy.1955.181.2.451. [DOI] [PubMed] [Google Scholar]
- REHM W. S. Effect of electric current on gastric hydrogen ion and chloride ion secretion. Am J Physiol. 1956 May;185(2):325–331. doi: 10.1152/ajplegacy.1956.185.2.325. [DOI] [PubMed] [Google Scholar]
- REHM W. S. Electrical resistance of resting and secreting stomach. Am J Physiol. 1953 Mar;172(3):689–699. doi: 10.1152/ajplegacy.1953.172.3.689. [DOI] [PubMed] [Google Scholar]
- REHM W. S., LEFEVRE M. E. EFFECT OF DINITROPHENOL ON POTENTIAL, RESISTANCE, AND H+ RATE OF FROG STOMACH. Am J Physiol. 1965 May;208:922–930. doi: 10.1152/ajplegacy.1965.208.5.922. [DOI] [PubMed] [Google Scholar]
- Ridley H. A., Vaughan B. E., Cummins J. T. Parasympathetic action on gastric bioelectric potential in rats. Can J Physiol Pharmacol. 1967 Mar;45(2):281–290. doi: 10.1139/y67-030. [DOI] [PubMed] [Google Scholar]
- Sedar A. W. Fine structure of the stimulated oxyntic cell. Fed Proc. 1965 Nov-Dec;24(6):1360–1367. [PubMed] [Google Scholar]
- Sernka T. J., Hogben C. A. Active ion transport by isolated gastric mucosae of rat and guinea pig. Am J Physiol. 1969 Nov;217(5):1419–1424. doi: 10.1152/ajplegacy.1969.217.5.1419. [DOI] [PubMed] [Google Scholar]
- Shoemaker R. L., Sachs G., Hirschowitz B. I. Secretion by guinea pig gastric mucosa in vitro. Proc Soc Exp Biol Med. 1966 Dec;123(3):824–827. doi: 10.3181/00379727-123-31614. [DOI] [PubMed] [Google Scholar]
- WRIGHT G. H. Net transfers of water, sodium, chloride and hydrogen ions across the gastric mucosa of the rabbit foetus. J Physiol. 1962 Sep;163:281–293. doi: 10.1113/jphysiol.1962.sp006974. [DOI] [PMC free article] [PubMed] [Google Scholar]